Abstract
Stroke is the second leading cause of death worldwide, and China has the highest stroke incidence in the world. The systemic inflammatory response index (SIRI), systemic inflammatory response index (SIRI), systemic immune-inflammatory index (SII), neutrophil-to-high-density lipoprotein ratio (NHR), neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR) and monocyte-to-lymphocyte ratio (MLR) have clinical in predicting the prognosis of acute ischaemic stroke (AIS) patients. No studies have compared the predictive value of these six composite inflammatory markers. This study included 516 AIS patients with AIS symptoms for < 24 h. The short-term prognosis of AIS patients at 30 days was assessed using the modified Rankin scale (mRS), an mRS score > 2 defining poor prognosis. The results of the univariate analysis showed that all six composite inflammatory indices, SIRI, SII, NHR, NLR, PLR and MLR, were associated with a poor prognosis in patients with AIS. All six composite inflammatory indicators correlated with the short-term prognosis of AIS patients. The six composite inflammation indicators were included in the binary logistic regression, and the results showed that SIRI, NLR and PLR were found to be independent risk factors for poor short-term prognosis in AIS patients. Among the six inflammatory markers, SIRI, NLR and PLR were the most clinically valuable for predicting the short-term prognosis of patients with AIS. Peripheral blood indices are easy to obtain clinically and can provide important clinical value for early prognosis and treatment adjustment.
Similar content being viewed by others
Introduction
A Global Burden of Disease study showed that stroke is the second leading cause of death and the third leading cause of disability worldwide1. In 2019, there were 12.2 million new strokes and 6.55 million deaths from stroke. The overall lifetime risk of stroke in China is 39.9%, which is the highest in the world, meaning that approximately two out of every five Chinese people will suffer a stroke. In addition, stroke is the number one cause of disease-induced years of life lost in China2. AIS caused by cerebral artery blockage accounts for approximately 87% of all strokes3,4. Most patients with cerebral infarction have serious complications, causing heavy social and family burdens1,5. Therefore, there is an urgent need to find novel biological markers to predict disease progression and functional prognosis to improve the current status of acute stroke patient diagnosis and treatment.
AIS patients can be treated with tissue fibrinogen activator, intravenous thrombolytic agents, or mechanical thrombectomy (using a stent retriever or endovascular therapy) to reduce brain tissue damage through reperfusion within the therapeutic time window6,7. Due to the strict time constraints and range of intraoperative and postoperative complications, only a small proportion of patients benefit from mechanical thrombectomy and intravenous thrombolysis8. Based on their clinical status, most clinical patients still choose conservative medical treatment. To improve the poor prognosis of AIS patients, targeting neuroinflammation to alleviate this poor prognosis has become a research hotspot in recent years.
Ischaemic stroke is characterised by multiple highly correlated neuropathological processes in which an intense and persistent inflammatory response plays a crucial role in worsening brain damage7,9. A large body of evidence suggests that postischemic inflammatory responses are associated with acute blood‒brain barrier (BBB) disruption, vasogenic oedema, haemorrhagic transformation, and severe neurological consequences10. Cell counts and their composite inflammatory indices, such as the NLR11, NHR12, PLR13, MLR14, SII15, and SIRI16, are regarded as valuable biological predictors of prognosis in AIS patients. These cells are readily available in peripheral blood and are considered classical haematological markers of systemic inflammation. Furthermore, all six composite inflammatory markers can predict the prognosis of AIS patients. No studies have compared the precision of several composite inflammation indicators in predicting the prognosis of stroke.
We collected clinical data from 516 patients with acute cerebral infarction and statistically analysed the clinical value of the above six composite inflammatory indicators for predicting the short-term prognosis of AIS patients. The experimental design is shown in Fig. 1.
Study flow chart. AIS: acute ischaemic stroke; mRS: modified Rankin scale; NLR: neutrophil/lymphocyte ratio; NHR: neutrophil/high-density lipoprotein cholesterol ratio; PLR: platelet-to-lymphocyte ratio; MLR: monocyte-to-lymphocyte ratio; SII: systemic immune-inflammatory index; SIRI: systemic inflammatory response index.
Methods
Subject population
This study included a total of 516 patients with AIS who were hospitalised at Nantong Third People's Hospital between September 2019 and february 2024.
Inclusion criteria:
-
1.
Age 18 years or older.
-
2.
First stroke, with a diagnosis of AIS within 24 h of onset (the diagnosis of ischaemic stroke was confirmed by the use of noncontrast computed tomography and computed tomography angiography).
-
3.
Head MRI completed within 48 h.
-
4.
Standardised treatment for acute and secondary prevention of ischaemic stroke.
Exclusion criteria:
-
1.
Previous history of AIS or cerebral haemorrhage;
-
2.
Early selection for reperfusion therapy in the IS;
-
3.
Pregnancy or breastfeeding;
-
4.
Severe cardiac, pulmonary, hepatic, renal, neoplastic or autoimmune disease or combined severe infection;
-
5.
Automatic withdrawal from the study or loss to follow-up.
The study strictly adhered to the Declaration of Helsinki and was reviewed and approved by the Ethics Committee of the Third People's Hospital of Nantong (Ethics No: YJ2022034). All patients voluntarily enrolled in this study and signed an informed consent form. For privacy purposes, the subjects' personally identifiable information was anonymized.
Data collection
A complete history, neurological examination and baseline data (basic information (age, sex and body mass index), vascular risk factors (hypertension, diabetes mellitus, dyslipidaemia, atrial fibrillation, history of smoking and alcohol consumption, history of previous stroke, peripheral arterial disease and coronary arterial disease), and history of medication use (antiplatelets, anticoagulants, statins, etc.) were documented by a specialised neurologist for all the patients within four hours of admission to hospital. Electrocardiography, chest X-ray, brain CT or MRI and carotid ultrasound were completed in all patients within 24 h of admission.
The mRS score was used to define short-term outcomes at 30 days, an mRS score > 2 defining adverse outcomes.
Blood sampling and calculation of complex inflammatory indicators
Blood routine values, including high-density lipoprotein (HDL), low-density lipoprotein (LDL), C-reactive protein (CRP), total cholesterol, monocyte, neutrophil, and lymphocyte data, were collected within one hour of admission. Peripheral blood cells were counted using an automated analyser (XT-1800i, Sysmex, Kobe, Japan) and used to calculate the compound inflammation ratio.
NLR = neutrophil/lymphocyte ratio; NHR = neutrophil count/high-density lipoprotein cholesterol ratio; PLR = platelet count/lymphocyte ratio; MLR = monocyte/lymphocyte ratio; SII = platelet count × neutrophil count/lymphocyte count; SIRI = monocyte × neutrophil/lymphocyte.
Statistical analysis
The Kolmogorov‒Smirnov test was used to test the normality of the distribution of the data. The mean ± standard deviation was used to describe continuous variables with a normal distribution, and the median (quartiles [Q25, Q75]) was used to describe continuous variables with a nonnormal distribution. The independent-sample t tests and Mann‒Whitney U tests were used to analyse between-group differences in continuous variables. Categorical variables are presented as n (%), and differences between the groups were analysed with the chi-squared test.
A subject operating characteristic (ROC) curve was drawn by plotting the sensitivity versus 1-specificity and calculating the area under the curve (AUC). Optimal thresholds were calculated through Youden's J test. Binary logistic regression was used to test the independent effects of the 6 composite inflammation ratios on the short-term prognosis (30 days) of AIS patients. The data were visualised with SPSS Statistics 25.0 software (IBM Analytics) and GraphPad (Prism 8). p < 0.05 was the threshold for statistical significance.
Results
Baseline data
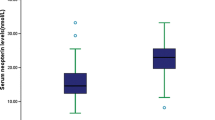
A total of 516 patients with acute stroke were included in this study. To eliminate data bias, after excluding endovascular therapy and thrombolytic therapy, standard medical treatment remained in 306 patients. As shown in Table 1, 205 (67%) of the 306 patients with AIS had a good prognosis, and 101 (33%) had a poor prognosis. The percentage of males, proportion of cardiac embolism type (CE, TOAST classification), age, percentage of patients with coronary artery disease, percentage of patients with atrial fibrillation, white blood cell count, percentage of neutrophils, apolipoprotein A1 (ApoA1), acid glycoprotein, PLR, NHR, NLR, MLR, SII, and SIRI were significantly greater in the poor prognosis group than in the good prognosis group (P < 0.05). ApoB/ApoA1 and the lymphocyte percentage in the poor prognosis subgroup were significantly lower than those in the good prognosis subgroup. The remaining baseline information was not significantly different.
Correlation analysis of the complex inflammation ratio and short-term prognosis
Pearson's correlation coefficients are shown in Fig. 2. All six inflammation indicators, PLR, NHR, NLR, MLR, SII, and SIRI, were positively correlated with the mRS score. The correlation coefficients of the SIRI, PLR, NHR, NLR, MLR, SII, and MLR were 0.304, 0.262, 0.222, 0.302 , 0.308 and 0.277, respectively (P < 0.0001).
Multifactor analysis of AIS patients with short-term prognosis
After correcting for confounders, binary logistic regression analysis revealed that SIRI (OR 5.912, 95% CI 1.653 to 21.14, P = 0.006), NLR (OR 1.334, 95% CI 1.038 to 1.715, P = 0.025) and PLR (OR 1.02, 95% CI 1.006 to 1. 034, P = 0.005) were independent risk factors for poor short-term prognosis (30 days) in patients with AIS (Table 2, Fig. 3).
Clinical value of SIRI, NLR, and PLR in predicting poor outcomes in AIS patients
The subject ROC curves (Fig. 4) were drawn based on sensitivity versus 1-specificity and area under the curve, and the AUC of SIRI for predicting poor prognosis in patients with acute ischaemic stroke was 0.748 (95% CI: 0.688 to 0.807), with a sensitivity of 63.4% and specificity of 82%. The positive predictive value of SIRI was 81.95%(168/205), and the negative predictive value was 62.38%(63/101). SIRI was at the optimal cut-off value of 1.778 × 109/L. NLR predicted poor prognosis in patients with acute ischaemic stroke. The AUC for NLR for predicting poor prognosis in patients with acute ischaemic stroke was 0.745(95% CI = 0.686–0.804), and when NLR was at the optimal cut-off value of 2.689 × 109/L, the sensitivity was 83.2% and the specificity was 60.5%. The positive predictive value of NLR was 87.94%(124/141), and the negative predictive value was 50.91%(84/165). Similarly, the area under the curve (AUC) for PLR for predicting poor prognosis in patients with acute ischaemic stroke was 0.676(95% CI = 0.613–0.739); when PLR was at the optimal cut-off value of 134.149 × 109/L, the sensitivity was 63.4% and the specificity was 69.3%. The positive predictive value of PLR was 79.12% (144/182), and the negative predictive value was 50.81% (63/124).
Discussion
In this study, for the first time, we evaluated the clinical value of six composite inflammation ratios (NLR, NHR, SII, SIRI, MLR, and PLR) at admission for predicting 30-day prognosis in patients with AIS. Our results showed that all six inflammatory indicators were associated with poor short-term prognosis in patients with AIS. Among them, the NLR, SIRI and PLR were more advantageous for predicting AIS prognosis. Our study provides basic clinical evidence for the relationship between inflammation and AIS. Most importantly, our study suggests a potentially simple method to predict and improve unfavourable functional outcomes in AIS patients through the use of peripheral-blood inflammatory markers.
Stroke is caused by an obstruction of a cerebral artery or its branches, and the formation of an embolism reduces the blood supply to the brain, damaging neurons. Inflammation, oxidative stress, immune cell recruitment and microglial activation contribute to the development of stroke, causing irreversible damage to brain tissue17. Among these factors, inflammation is considered a key factor in all stages of stroke development, from occlusion in the acute phase to postischemic repair18. Under normal conditions, inflammation is a defence response that removes toxic substances and limits their harmful effects6. A large body of evidence suggests that some inflammatory responses play an unfavourable role in the development of stroke19. BBB destruction is an important pathophysiological process in AIS that leads to severe malignant brain oedema and haemorrhagic transformation. Rapid activation of immune cells plays a key role in BBB destruction after ischaemic stroke. Immune cells (e.g., neutrophils, monocytes, and lymphocytes) that infiltrate brain tissue can increase BBB permeability by secreting inflammatory factors and affecting microvessels. Curiously, they play opposite roles in the later stages of ischaemic stroke, when they can promote BBB repair and angiogenesis20,21.
Given that inflammatory cells play an important role in the development of IS, researchers have begun to explore ways to improve the prognosis of stroke patients by targeting inflammatory cells to alleviate brain tissue damage. In their mouse model of a tethered middle cerebral artery, Hu et al. found that suppressing the number of neutrophils in the ischaemic region of acute ischaemic stroke minimised cell death and promoted functional recovery of the nervous system22; Huang et al. found that microglial IL-1RA ameliorates brain injury after ischaemic stroke by inhibiting astrocytic CXCL1-mediated neutrophil recruitment23. Al Ruwaili et al. demonstrated that inhibition of phosphodiesterase 5 (PDE5) promotes neurological recovery in AIS patients by modulating the brain cyclic adenosine monophosphate (cAMP)/cyclic guanosine monophosphate (cGMP)/nitric oxide (NO) pathways24. Studies on reducing the severity of AIS and promoting neurological recovery by alleviating neuroinflammation have been carried out in mouse models, and there is an urgent need for additional researchers to increase their efforts.
Our findings showed that all six inflammatory indicators tested were negatively associated with short-term prognosis in patients with AIS, providing valuable clinical evidence for further basic experiments. Our study does have some limitations. First, it was a retrospective study conducted at a single centre and therefore suffered from selection bias. Second, the sample was small, and the findings need to be validated in other larger populations. Third, we analysed only peripheral-blood data at admission and should have tracked the dynamic changes in these values. A multicentre prospective study will be considered in the future to further explore the relationship between the compound inflammation ratio and AIS incidence.
Conclusion
SIRI, PLR and NLR are important predictors of early functional outcomes in AIS patients and can provide a reference for early diagnosis and treatment by clinicians. Further studies dynamically monitoring SIRI, PLR and NLR are needed to better understand the predictive value of these parameters in larger cohorts. Therefore, AIS patients with elevated SIRI, PLR, and NLR should be closely monitored, as these values may suggest new therapeutic strategies to improve poor outcomes in AIS patients.
Data availability
All the original data are real and reliable and are uploaded in the supplementary information file.
Abbreviations
- AIS:
-
Acute ischaemic stroke
- mRS:
-
Modified Rankin scale
- BBB:
-
Blood‒brain barrier
- NLR:
-
Neutrophil–lymphocyte ratio
- NHR:
-
Neutrophil–high-density lipoprotein cholesterol ratio
- PLR:
-
Platelet–to-lymphocyte ratio
- SII:
-
Systemic immune–inflammatory index
- SIRI:
-
Systemic inflammatory response index
- MLR:
-
Monocyte–to-lymphocyte ratio
- HDL:
-
High-density lipoprotein
- LDL:
-
Low-density lipoprotein
- CRP:
-
C-reactive protein
- ROC:
-
The subject operating characteristic curve
- AUC:
-
Area under the curve
- ApoA1:
-
Apolipoprotein A1
References
Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 20(10), 795–820 (2021).
Roth, G. A. et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: Update from the GBD 2019 study. J. Am. Coll. Cardiol. 76(25), 2982–3021 (2020).
Meschia, J. F. et al. Guidelines for the primary prevention of stroke: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 45(12), 3754–3832 (2014).
Ajoolabady, A. et al. Targeting autophagy in ischemic stroke: From molecular mechanisms to clinical therapeutics. Pharmacol. Ther. 225, 107848 (2021).
Mohebi, R. et al. Cardiovascular disease projections in the United States based on the 2020 census estimates. J. Am. Coll. Cardiol. 80(6), 565–578 (2022).
Cao, Y. et al. Neuroinflammation and anti-inflammatory therapy for ischemic stroke. Heliyon 9(7), e17986 (2023).
Li, J. et al. The role of protein glycosylation in the occurrence and outcome of acute ischemic stroke. Pharmacol. Res. 191, 106726 (2023).
Van Der Ende, N. A. M. et al. Symptomatic intracranial hemorrhage after endovascular stroke treatment: External validation of prediction models. Stroke 54(2), 476–487 (2023).
Jenny, N. S. et al. Inflammatory cytokines and ischemic stroke risk: The REGARDS cohort. Neurology 92(20), e2375–e2384 (2019).
Zeng, J. et al. The mechanism of microglia-mediated immune inflammation in ischemic stroke and the role of natural botanical components in regulating microglia: A review. Front. Immunol. 13, 1047550 (2022).
Zhu, F. et al. Correlations between NLR, NHR, and clinicopathological characteristics, and prognosis of acute ischemic stroke. Medicine 102(24), e33957 (2023).
Zhang, R. et al. Neutrophil to high-density lipoprotein ratio is associated with hemorrhagic transformation in patients with acute ischemic stroke. J. Inflamm. Res. 15, 6073–6085 (2022).
Wang, C. et al. GNRI, PLR and stroke-associated pneumonia: From association to development of a web-based dynamic nomogram. Clin. Interv. Aging 18, 1893–1904 (2023).
Jiang, F. et al. The role of the monocyte-to-lymphocyte ratio in acute ischemic stroke patients with acute kidney injury. Mediators Inflamm. 2022, 7911033 (2022).
Hou, D. et al. Systemic immune-inflammation index (SII) but not platelet-albumin-bilirubin (PALBI) grade is associated with severity of acute ischemic stroke (AIS). Int. J. Neurosci. 131(12), 1203–1208 (2021).
Ma, F. et al. The relationship between systemic inflammation index, systemic immune-inflammatory index, and inflammatory prognostic index and 90-day outcomes in acute ischemic stroke patients treated with intravenous thrombolysis. J. Neuroinflammation 20(1), 220 (2023).
Stoll, G. & Nieswandt, B. Thrombo-inflammation in acute ischaemic stroke—implications for treatment. Nat. Rev. Neurol. 15(8), 473–481 (2019).
Maida, C. D. et al. Neuroinflammatory mechanisms in ischemic stroke: Focus on cardioembolic stroke, background, and therapeutic approaches. Int. J. Mol. Sci. 21(18), 6454 (2020).
Huang, Y. et al. Edaravone dexborneol downregulates neutrophil extracellular trap expression and ameliorates blood-brain barrier permeability in acute ischemic stroke. Mediators Inflamm. 2022, 3855698 (2022).
Qiu, Y. M. et al. Immune cells in the BBB disruption after acute ischemic stroke: Targets for immune therapy?. Front. Immunol. 12, 678744 (2021).
Bonaventura, A. et al. Update on inflammatory biomarkers and treatments in ischemic stroke. Int. J. Mol. Sci. 17(12), 1967 (2016).
Hu, R. et al. Gasdermin D inhibition ameliorates neutrophil mediated brain damage in acute ischemic stroke. Cell Death Discov. 9(1), 50 (2023).
Huang, X. et al. Microglial IL-1RA ameliorates brain injury after ischemic stroke by inhibiting astrocytic CXCL1-mediated neutrophil recruitment and microvessel occlusion. Glia 71(7), 1607–1625 (2023).
Alruwaili, R. et al. The potential therapeutic effect of phosphodiesterase 5 inhibitors in the acute ischemic stroke (AIS). Mol. Cell Biochem. 479, 1267 (2023).
Acknowledgements
We would like to thank the doctors in the Department of Neurology of the Third People's Hospital of Nantong City for assisting us in diagnosing diseases and collecting data.
Funding
This study was supported by the Project of Nantong Municipal Health Commission (MB2020034).
Author information
Authors and Affiliations
Contributions
Y.J. contributed to the conception of the study and provided statistical analysis. F.Z. and Z.H. contributed to the analysis and wrote the manuscript. J.S. collected the data and helped perform the analysis with constructive discussions.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhu, F., Wang, Z., Song, J. et al. Correlation analysis of inflammatory markers with the short-term prognosis of acute ischaemic stroke. Sci Rep 14, 17772 (2024). https://doi.org/10.1038/s41598-024-66279-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-66279-4
- Springer Nature Limited