Abstract
Research to improve and expand treatment options for motor impairment after stroke remains an important issue in rehabilitation as the reduced ability to move affected limbs is still a limiting factor in the selection of training content for stroke patients. The combination of action observation and peripheral nerve stimulation is a promising method for inducing increased excitability and plasticity in the primary motor cortex of healthy subjects. In addition, as reported in the literature, the use of action observation and motor imagery in conjunction has an advantage over the use of one or the other alone in terms of the activation of motor-related brain regions. The aim of the pilot study was thus to combine these findings into a multimodal approach and to evaluate the potential impact of the concurrent application of the three methods on dexterity in stroke patients. The paradigm developed accordingly was tested with 10 subacute patients, in whom hand dexterity, thumb-index pinch force and thumb tapping speed were measured for a baseline assessment and directly before and after the single intervention. During the 10-min session, patients were instructed to watch a repetitive thumb-index finger tapping movement displayed on a monitor and to imagine the sensations that would arise from physically performing the same motion. They were also repeatedly electrically stimulated at the wrist on the motorically more affected body side and asked to place their hand behind the monitor for the duration of the session to support integration of the displayed hand into their own body schema. The data provide a first indication of a possible immediate effect of a single application of this procedure on the dexterity in patients after stroke.
Similar content being viewed by others
Introduction
Stroke is one of the most common causes of disability in adults1, resulting in a significant need for medical rehabilitation. This remains an important issue in rehabilitation research2 although substantial efforts have been devoted to improve functional recovery3,4. A number of randomized-controlled studies and experimental trials on the effectiveness, for instance of virtual reality rehabilitation, with promising results have been found5. A major impairment after a stroke can be the loss of dexterity as upper limb skills are essential in everyday activities that require fine movements for handling and manipulating a variety of objects. The consequences of a persistent, non-recovered upper limb function impairment are immense and often directly lead to a reduced quality of life6. In the attempt to foster recovery, the measures taken to support motor improvement depend on a number of central nervous system processes referred to as neuroplasticity7.
Two well established interventions to support neuroplastic changes in patients with a limited capacity to physically practice are action observation and motor imagery8. For upper limb rehabilitation after stroke, action observation (AO) was found beneficial in improving motor function and dependence in activities of daily living but its clinical relevance is unclear9. AO is a cognitive process involving the perception and comprehension of an action performed by another individual. It typically involves visually observing someone else's actions, which can activate similar neural networks in the observer's brain as if they were performing the action themselves10. On the other hand, Motor Imagery (MI) is a cognitive process in which an individual mentally simulates or rehearses a specific motor action without physically executing it11. During MI, individuals vividly imagine themselves performing a movement, engaging sensory and motor representations in the brain. When added to rehabilitation interventions, it can result in improvements in upper limb function and movement, while there is no evidence for a beneficial effect of MI alone compared to conventional treatment12. However, evidence exists of plastic changes alongside behavioral improvements during MI13.
In neurophysiological research using Electroencephalography (EEG) or Functional magnetic resonance imaging (fMRI), it was found that the combined application of AO and MI (AOMI) can increase brain activity involving cortical areas more comprehensively compared to applying them independently. Neuroimaging studies on action observation combined with motor imagery (AOMI) suggest more robust activation of motor-related brain regions compared to conditions involving only AO or only MI, as demonstrated using fMRI14,15,16,17, EEG18,19,20, Transcranial magnetic stimulation21,22,23,24 or Functional near-infrared spectroscopy25,26. For both, a shared neural network was proposed, but with differences in the brain activity overlap compared with physically performed movements27. AOMI was therefore suggested to potentially entail a wider overlap with motor execution28.
There are several available studies that have focused on the immediate effects of synchronous AO + MI instructions on neurophysiological and behavioural parameters for reviews, see Eaves et al., 2016a; Emerson et al., 2018; McNeill et al., 2020; Vogt et al., 2013; Wright et al., 2021). A recent paper reported two meta-analyses to quantify changes in corticospinal excitability and motor skill performance during combined, simultaneous AOMI compared to AO, MI and control conditions supporting the effectiveness of AOMI as an alternative intervention to AO and MI28. Based on these neurophysiological and behavioral findings, AOMI was accordingly suggested to be a beneficial approach for motor rehabilitation purposes29. In stroke recovery, a few studies reported on improvements in motor performance and cortico-motor involvement measures in patients who received AOMI30,31,32. Choi et al. found a positive effect in both corticospinal excitability and upper-limb function in stroke patients in a randomized controlled trial32 and, in terms of the upper-limb function measured using the Fugl-Meyer Assessment, improvements were also reported by Robinson-Bert and Woods30. Clinically relevant benefits of synchronous AOMI treatment in a group of stroke survivors compared with asynchronous AO and MI treatment highlight the potential benefit of synchronous AOMI practice31. For an overview of the variety of AOMI practices, see Eaves et al.29.
Another method to achieve an enhanced facilitatory effect of AO is to combine it with peripheral nerve stimulation (PNS). In two studies with healthy participants, a stimulation protocol was used that consisted of a combination of watching a video showing repetitive thumb-index tapping movements and concurrent PNS33,34. The concurrent AO-PNS was found to induce plasticity in the primary motor cortex, which only occurred in combination but not when applied alone. The effect was remarkably present for a duration of at least 45 min after a single, brief intervention33.
Based on these findings, it seemed worthwhile to collect first data on the impact of the combination of the described intervention options with stroke patients. Therefore, the aim of the study was to assess whether a combined, concurrent application of Action Observation, Motor Imagery and Peripheral Nerve Stimulation would result in a measurable improvement in dexterity using functional tests in patients after stroke.
Methods
Patients
Patients after their first ischemic or hemorrhagic stroke, who were able to sit independently, scored higher than 19 in the Montreal Cognitive Assessment35 and higher than 1 in the Box-and-Block Test36 could be enrolled in the study. Since the level of neuroplasticity and thus the recovery-related processes post-stroke are time-dependent37,38, patients were included if they were still in the pre-chronic phase (< 6 months). The patients further needed to satisfactorily score in two out of three of the following motor imagery ability instruments: (a) Kinesthetic and Visual Imagery Questionnaire score of 30/5039, (b) Mental rotation > 75%40, and (c) Mental chronometry ratio of 1 ± 0.2539. Exclusion criteria were visual impairment, epileptic seizures in the past six months, other neurological, metabolic, or mental disorders, a pacemaker, or a metal implant in the hand or forearm of the affected side. The study protocol was approved by the local ethics committee for Northwest and Central Switzerland (Project-ID: 2022-00815) and conformed to the declaration of Helsinki. All patients provided written informed consent before the start of the data collection.
Intervention procedure
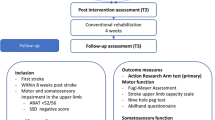
The patients were requested to sit relaxed and observe a computer screen showing a lower arm and hand performing repetitive thumb-index finger-tapping movements. Corresponding to the affected side of the body, the right- or left-hand video was selected. The patients were also asked to place their affected hand behind the screen so that the depicted hand and part of the forearm on the screen would appear to be their own (Fig. 1). This was done to facilitate the incorporation of the virtual hand into the individual's body schema41, which is the brain's representation of the body42, by closely matching the spatial orientation of their own hand. Regarding this part of the experimental approach, it must be noted that there is still limited literature concerning the effects of stroke on embodiment. Studies on this topic have uncovered both similarities and differences in the experiences of stroke survivors and healthy individuals. Borrego et al. found that both groups experienced a sense of embodiment and presence in a virtual environment, although to a lesser extent in stroke survivors43. Slightly deviating from that, using the well-established Rubber Hand Illusion paradigm, researchers found an even significantly stronger sense of body ownership and agency in patients compared to healthy individuals after stroke44. Here, the authors suggested that an increased dominance of visual input over proprioception may have contributed to the observed results44. However, using this approach seemed worthwhile for the current experiment. The patients were also instructed not to perform the observed movement themselves.
Patients watched a video sequence showing continuous, repetitive thumb-index tapping of either the right or left hand, depending on the impaired side of each participant, with the affected hand positioned behind the monitor (Fig. 1). On the screen, only a hand and part of the forearm were visible against a dark background. The experimental procedure was conducted in a darkened room, so that participants could only see and better concentrate on what was shown on the screen. They were asked to concurrently imagine the sensations of the thumb and index finger that would occur during the execution of the movement without actually performing it, which corresponds to the kinesthetic mode. The intervention/the video lasted for 10 min with the depicted tapping movement executed at a frequency of 2 Hz33. Also concurrently, while observing the movement, electrical stimuli were applied to the median nerve at the wrist. These stimuli were triggered approximately every 4 s at the end of a closing phase of thumb-index tapping movement, corresponding to every 8th thumb-index finger-tapping movement. The closing phase was chosen according to Bisio et al., who reported a higher excitability in the primary motor cortex during AO in this phase33. Furthermore, a nearly identical approach was chosen to ensure that the study participants were indeed attentive to the screen and did not shift their focus elsewhere. Thus, to enhance attention to the visual stimuli, the video was interrupted five times, each time displaying a black screen with a white cross for 5 s. The participants were instructed beforehand to count the number of occurrences.
Electrical stimuli were applied through a bipolar electrode connected to a Digitimer (DS7A, Welwyn Garden City, UK) constant current stimulator, using square wave pulses (duration 1 ms) at an intensity of three times the perceptual threshold, able to evoke a small twitch in the abductor pollicis brevis muscle which was tolerated by the patients. The presentation of the video sequence including the accurate timing of the trigger signals for the electrical stimulation was created using Psychopy345.
The intervention was carried out once per patient, while dexterity of the affected side was assessed three times: 3–7 days before the intervention (baseline), and immediately before (pre) and after (post). For this, the following three different assessments were used at each of the three time points. (1) Gross manual dexterity: Box-and-Block Test36 performed once each time, (2) Thumb-index pinch force performed three times at each time point to determine the respective average. We used a hydraulic pinch gauge (North Coast Medical Inc., Morgan Hill, CA) to measure the maximum force between the thumb and index finger of the affected hand. (3) Thumb tapping speed: A hand held counter was used to determine the number of thumb movements within 10 s. This was also performed three times at each time point to obtain the mean values of the maximum possible tapping speed.
We conducted one-tailed paired t-tests on the different assessments results, but did not perform an additional analysis given the limited data. Due to the exploratory nature of the study, no adjustment of the alpha-level was conducted. Furthermore, to assess the strength of the results, we carried out a post-hoc power analysis.
Results
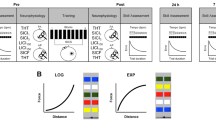
In total, ten patients (mean age of 63.4 ± 15.5 years, see Table 1 for all characteristics) could be included. Separate QQ-plots (Fig. 2) of all three data sets were used to assess normality. Despite slight deviations, they indicated a tendency for the data to follow a normal distribution.
The one-tailed paired t-test showed that the difference (Fig. 3) between pre (mean 33.6 blocks, SD = 17.8) and post (mean = 37.0, SD = 19.0) BBT results was significant (t(9) = − 3.9, P = 0.002) with an effect size of Cohen's d = 1.23. For the pinch force assessment, data revealed no significant difference (t(9) = − 1.53, P = 0.08) between pre (mean = 3.4 kg, SD = 1.1) and post (mean = 3.8, SD = 1.3) with d = 0.48. This was also the case for the data from the thumb-tapping tests (t(9) = − 1.66, P = 0.06) between pre (mean = 22.2 repetitions, SD = 13.3) and post (mean = 24.1, SD = 14.4) with d = 0.53.
The post-hoc power analysis unveiled that the attained statistical power for the BBT data was 0.84. For the pinch force data and thumb tapping data, the statistical power was 0.27 and 0.30, respectively.
Discussion
The aim of the present study was to investigate the potential effect of a combined application of action observation, motor imagery and electrical peripheral nerve stimulation on dexterity in patients after their first-ever stroke. The used paradigm extended the AO-PNS approach of Bisio et al. with healthy individuals, which showed a positive effect on corticospinal excitability33. We attempted to potentially enhance this effect by adding two further components. (1) The experimental setup was modified to facilitate the integration of the observed hand into the patient's individual body schema. (2) The patients were asked to imagine how the observed thumb-index movements would feel.
We found that a brief AOMI-PNS intervention may have induced a transient improvement in dexterity in the affected hand of the included patients. However, data also suggest that a single AOMI-PNS session is at least partly not sufficient to clearly improve the scores of the assessments used.
In this preliminary study, only a limited sample size was used and no control group included, which limits the interpretability of the data. Further, the possibility of a short-term learning effect cannot completely be ruled out, although we made efforts to mitigate its influence by adjusting the methodology accordingly. The thumb tapping speed test and pinch force test were executed six times each prior to the intervention event, and the final three were utilized to calculate the pre-intervention average. This procedure presumably did not entirely eradicate the learning effect, but it certainly minimized its impact. Further, on occasion, study patients reported experiencing minor motor fatigue as a result of the assessments conducted immediately before the intervention and also of the PNS itself, which might even have a reduction effect on the scores of the post measurements and counteract possible gains.
As we did not apply TMS and investigated a different population, it cannot necessarily be assumed that the stimulation protocol (AOMI-PNS) applied in this study also resulted in an increase of the M1 excitability as reported by Bisio et al. using AO-PNS33. The latter did not test for dexterity changes, however, evidence of a connection between increased M1 excitability and improved manual dexterity can be found in the literature46,47. Sun et al. found clinically relevant improvements in chronic stroke patients both in measures of motor performance and in cortico-motor involvement following synchronous AOMI treatment alongside physical rehabilitation31. In these patients, the synchronous AOMI treatment obviously had a distinct advantage over asynchronous AOMI. Capozio and colleagues, on the other hand, could not find an improvement in dexterity after a combined application of transcutaneous electrical stimulation of the spinal cord and MI48. However, they reported an acute effect of both applied in conjunction on cortical neural excitability assessed using transcranial magnetic stimulation.
Indeed, it could be argued that the requirements for the simultaneous and coordinated performance of AO and MI tasks might be too demanding from a cognitive point of view for some patients after a stroke. In order to avoid a bias due to cognitive overload, we carefully tested patients prior to study inclusion by using the Montreal Cognitive Assessment and three different assessments to evaluate their individual MI ability. Testing for an adequate MI performance was also applied by Sun et al., who at least used the short version of kinesthetic and visual imagery questionnaire31. All patients tested in the current study prior to a possible enrolment met the cognitive requirements.
In light of the results, the partially low statistical power and the existing literature, a next step would be to apply this intervention in an expanded cohort of stroke patients including a control group and an additional assessment of the neural activity at cortical level.
Conclusion
In conclusion, this study demonstrates that applying the combination of the different described therapeutic approaches is possible with patients after stroke and provides an initial indication of a potential positive effect on dexterity.
Data availability
The dataset used and analyzed during the current study is available from the corresponding author upon reasonable request.
References
GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age–sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 385, 117–171 (2015).
Lencioni, T. et al. A randomized controlled trial on the effects induced by robot-assisted and usual-care rehabilitation on upper limb muscle synergies in post-stroke subjects. Sci. Rep. 11, 5323 (2021).
Pollock, A. et al. Interventions for improving upper limb function after stroke. Cochrane Database Syst. Rev. 11, CD010820 (2014).
Lieshout, E. C. C., van de Port, I. G., Dijkhuizen, R. M. & Visser-Meily, J. M. A. Does upper limb strength play a prominent role in health-related quality of life in stroke patients discharged from inpatient rehabilitation?. Top. Stroke Rehabil. 27, 525–533 (2020).
Anwer, S. et al. Rehabilitation of upper limb motor impairment in stroke: A narrative review on the prevalence, risk factors, and economic statistics of stroke and state of the art therapies. Healthcare (Switzerland). 10 (2022).
Murphy, M. A., Willén, C. & Sunnerhagen, K. S. Kinematic variables quantifying upper-extremity performance after stroke during reaching and drinking from a glass. Neurorehabil. Neural Repair 25, 71–80 (2011).
Nudo, R. J. Recovery after brain injury: Mechanisms and principles. Front. Hum. Neurosci. 7, 887 (2013).
Bisio, A., Bassolino, M., Pozzo, T. & Wenderoth, N. Boosting action observation and motor imagery to promote plasticity and learning. Neural Plast. 2018, 1–3 (2018).
Borges, L. R., Fernandes, A. B., Melo, L. P., Guerra, R. O. & Campos, T. F. Action observation for upper limb rehabilitation after stroke. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD011887.pub2 (2018).
Rizzolatti, G. & Craighero, L. The mirror-neuron system. Annu. Rev. Neurosci. 27, 169–192 (2004).
Jeannerod, M. Neural simulation of action: A unifying mechanism for motor cognition. Neuroimage 14, S103–S109 (2001).
Barclay, R. E., Stevenson, T. J., Poluha, W., Semenko, B. & Schubert, J. Mental practice for treating upper extremity deficits in individuals with hemiparesis after stroke. Cochrane Database Syst. Rev. 2020 (2020).
Yoxon, E. & Welsh, T. N. Motor system activation during motor imagery is positively related to the magnitude of cortical plastic changes following motor imagery training. Behav. Brain Res. 390, 112685 (2020).
Macuga, K. L. & Frey, S. H. Neural representations involved in observed, imagined, and imitated actions are dissociable and hierarchically organized. Neuroimage 59, 2798–2807 (2012).
Nedelko, V., Hassa, T., Hamzei, F., Schoenfeld, M. A. & Dettmers, C. Action imagery combined with action observation activates more corticomotor regions than action observation alone. J. Neurol. Phys. Ther. 36, 182–188 (2012).
Taube, W. et al. Brain activity during observation and motor imagery of different balance tasks: An fMRI study. Cortex 64, 102–114 (2015).
Villiger, M. et al. Enhanced activation of motor execution networks using action observation combined with imagination of lower limb movements. PLoS One 8, e72403 (2013).
Berends, H. I., Wolkorte, R., Ijzerman, M. J. & Van Putten, M. J. A. M. Differential cortical activation during observation and observation-and-imagination. Exp. Brain Res. 229, 337–345 (2013).
Eaves, D. L., Riach, M., Holmes, P. S. & Wright, D. J. Motor imagery during action observation: A brief review of evidence, theory and future research opportunities. Front. Neurosci. https://doi.org/10.3389/fnins.2016.00514 (2016).
Neuper, C., Scherer, R., Wriessnegger, S. & Pfurtscheller, G. Motor imagery and action observation: Modulation of sensorimotor brain rhythms during mental control of a brain–computer interface. Clin. Neurophysiol. 120, 239–247 (2009).
Sakamoto, M., Muraoka, T., Mizuguchi, N. & Kanosue, K. Combining observation and imagery of an action enhances human corticospinal excitability. Neurosci. Res. 65, 23–27 (2009).
Tsukazaki, I., Uehara, K., Morishita, T., Ninomiya, M. & Funase, K. Effect of observation combined with motor imagery of a skilled hand-motor task on motor cortical excitability: Difference between novice and expert. Neurosci. Lett. 518, 96–100 (2012).
Wright, D. J., Williams, J. & Holmes, P. S. Combined action observation and imagery facilitates corticospinal excitability. Front. Hum. Neurosci. 8, 951 (2014).
Wright, D. J. et al. Corticospinal excitability is facilitated by combined action observation and motor imagery of a basketball free throw. Psychol. Sport Exerc. 39, 114–121 (2018).
Holper, L. et al. Testing the potential of a virtual reality neurorehabilitation system during performance of observation, imagery and imitation of motor actions recorded by wireless functional near-infrared spectroscopy (fNIRS). J. Neuroeng. Rehabil. 7, 1–13 (2010).
Holper, L. et al. Trial-to-trial variability differentiates motor imagery during observation between low versus high responders: A functional near-infrared spectroscopy study. Behav. Brain Res. 229, 29–40 (2012).
Hardwick, R. M., Caspers, S., Eickhoff, S. B. & Swinnen, S. P. Neural correlates of action: Comparing meta-analyses of imagery, observation, and execution. Neurosci. Biobehav. Rev. https://doi.org/10.1016/j.neubiorev.2018.08.003 (2018).
Chye, S. et al. The effects of combined action observation and motor imagery on corticospinal excitability and movement outcomes: Two meta-analyses. Neurosci. Biobehav. Rev. 143, 104911 (2022).
Eaves, D. L., Hodges, N. J., Buckingham, G., Buccino, G. & Vogt, S. Enhancing motor imagery practice using synchronous action observation. Psychol. Res. https://doi.org/10.1007/s00426-022-01768-7 (2022).
Robinson-Bert, K. & Woods, A. B. Effectiveness of synchronous action observation and mental practice on upper extremity motor recovery after stroke. Occup. Ther. Health Care https://doi.org/10.1080/07380577.2022.2138675 (2022).
Sun, Y., Wei, W., Luo, Z., Gan, H. & Hu, X. Improving motor imagery practice with synchronous action observation in stroke patients. Top. Stroke Rehabil. 23, 245–253 (2016).
Choi, J. B., Yang, S. W. & Ma, S. R. The effect of action observation combined with motor imagery training on upper extremity function and corticospinal excitability in stroke patients: A randomized controlled trial. Int. J. Environ. Res. Public Health 19, 12048 (2022).
Bisio, A. et al. Observing and perceiving: A combined approach to induce plasticity in human motor cortex. Clin. Neurophysiol. https://doi.org/10.1016/j.clinph.2014.08.024 (2015).
Bisio, A., Avanzino, L., Biggio, M., Ruggeri, P. & Bove, M. Motor training and the combination of action observation and peripheral nerve stimulation reciprocally interfere with the plastic changes induced in primary motor cortex excitability. Neuroscience https://doi.org/10.1016/j.neuroscience.2017.02.018 (2017).
Nasreddine, Z. S. et al. The montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. https://doi.org/10.1111/j.1532-5415.2005.53221.x (2005).
Mathiowetz, V., Volland, G., Kashman, N. & Weber, K. Adult norms for the Box and Block Test of manual dexterity. Am. J. Occup. Ther. Off. Publ. Am. Occup. Ther. Assoc. 39, 386–391 (1985).
Kwakkel, G., Kollen, B. J., Van der Grond, J. V. & Prevo, A. J. H. Probability of regaining dexterity in the flaccid upper limb: Impact of severity of paresis and time since onset in acute stroke. Stroke 34, 2181–2186 (2003).
van der Vliet, R. et al. Predicting upper limb motor impairment recovery after stroke: A mixture model. Ann. Neurol. 87, 383–393 (2020).
Kobelt, M., Wirth, B. & Schuster-Amft, C. Muscle activation during grasping with and without motor imagery in healthy volunteers and patients after stroke or with Parkinson’s disease. Front. Psychol. https://doi.org/10.3389/fpsyg.2018.00597 (2018).
Sharma, N., Jones, P. S., Carpenter, T. A. & Baron, J. C. Mapping the involvement of BA 4a and 4p during motor imagery. Neuroimage https://doi.org/10.1016/j.neuroimage.2008.02.009 (2008).
Poeck, K. & Orgass, B. The concept of the body schema: A critical review and some experimental results. Cortex 7, 254–277 (1971).
Matsumiya, K. Multiple representations of the body schema for the same body part. Proc. Natl. Acad. Sci. U. S. A. 119, e2112318119 (2022).
Borrego, A., Latorre, J., Alcañiz, M. & Llorens, R. Embodiment and presence in virtual reality after stroke. A comparative study with healthy subjects. Front. Neurol. 10, 476598 (2019).
Llorens, R. et al. Body schema plasticity after stroke: Subjective and neurophysiological correlates of the rubber hand illusion. Neuropsychologia 96, 61–69 (2017).
Peirce, J. et al. PsychoPy2: Experiments in behavior made easy. Behav. Res. Methods https://doi.org/10.3758/s13428-018-01193-y (2019).
Kidgell, D. J., Goodwill, A. M., Frazer, A. K. & Daly, R. M. Induction of cortical plasticity and improved motor performance following unilateral and bilateral transcranial direct current stimulation of the primary motor cortex. BMC Neurosci. 14, (2013).
Karok, S., Fletcher, D. & Witney, A. G. Task-specificity of unilateral anodal and dual-M1 tDCS effects on motor learning. Neuropsychologia 94, 84–95 (2017).
Capozio, A., Ichiyama, R. & Astill, S. L. The acute effects of motor imagery and cervical transcutaneous electrical stimulation on manual dexterity and neural excitability. Neuropsychologia 187, 108613 (2023).
Acknowledgements
We wish to thank all the patients who participated in this study.
Author information
Authors and Affiliations
Contributions
Study concept and design: F.B., S.S.; data acquisition: S.S.; data analysis and interpretation: S.S., F.B., J.W., C.S.A., L.H.B., K.P., H.U.G.; statistical analyses: J.W., F.B., S.S.; manuscript drafting: F.B., S.S.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Seitz, S., Schuster-Amft, C., Wandel, J. et al. Effect of concurrent action observation, peripheral nerve stimulation and motor imagery on dexterity in patients after stroke: a pilot study. Sci Rep 14, 14858 (2024). https://doi.org/10.1038/s41598-024-65911-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-65911-7
- Springer Nature Limited