Abstract
Although pesticide-free techniques have been developed in agriculture, pesticides are still routinely used against weeds, pests, and pathogens worldwide. These agrochemicals pollute the environment and can negatively impact human health, biodiversity and ecosystem services. Acetamiprid, an approved neonicotinoid pesticide in the EU, may exert sub-lethal effects on pollinators and other organisms. However, our knowledge on the scope and severity of such effects is still incomplete. Our experiments focused on the effects of the insecticide formulation Mospilan (active ingredient: 20% acetamiprid) on the peripheral olfactory detection of a synthetic floral blend and foraging behaviour of buff-tailed bumblebee (Bombus terrestris) workers. We found that the applied treatment did not affect the antennal detection of the floral blend; however, it induced alterations in their foraging behaviour. Pesticide-treated individuals started foraging later, and the probability of finding the floral blend was lower than that of the control bumblebees. However, exposed bumblebees found the scent source faster than the controls. These results suggest that acetamiprid-containing Mospilan may disrupt the activity and orientation of foraging bumblebees. We hypothesize that the observed effects of pesticide exposure on foraging behaviour could be mediated through neurophysiological and endocrine mechanisms. We propose that future investigations should clarify whether such sub-lethal effects can affect pollinators’ population dynamics and their ecosystem services.
Similar content being viewed by others
Introduction
Pesticides are used to protect seeds and crops from weeds, herbivore pests and pathogens in agricultural production. In 2021, the total amount of active ingredients of pesticides used in agriculture reached 3.54 million tonnes worldwide, double the amount utilised in 19901. However, due to processes such as (surface) runoff and drift, the vast majority of administered agrochemicals reach organisms other than their targets2,3, and pesticides thus often act as environmental pollutants, threatening human health4, ecosystem services5 and biodiversity6. Intensive agriculture inevitably relies on pesticide use to achieve global food security. Still, the continuous monitoring of their environmental impacts is essential to ensure that the application does not jeopardize the viability and functioning of affected (agro)ecosystems7.
Acetamiprid is an N-cyanoamidine neonicotinoid, which has widely been used as an insecticide in increasing quantity in both small gardens and large cultivations8 after the regulation change in the EU banned the more potent active ingredients of this group of compounds9,10. This substance is reported to be non-toxic to pollinators due to its rapid metabolisation11, yet recent investigations suggest that in high doses, it can induce various sub-lethal effects and thus pose a threat to pollinators12,13, natural predators of pests14,15 and other non-target organisms including vertebrates16. Previous investigations indicate that the field-relevant concentrations of acetamiprid can vary widely with specific environmental conditions and locations17,18, and even the concentrations of residues in flowers following application may range within several orders of magnitude (from 0.1 to 3360 ppb19,20,21), probably depending on the mode of application and crop type. Due to the apparent uncertainty associated with the exposure levels to this substance (including the exposure of humans to this neonicotinoid22), the sub-lethal effects of acetamiprid applied in agricultural production are likely to remain in the focus of ecotoxicology, conservation biology and health science research in the coming years.
Experimental results suggest that neonicotinoids may exert sub-lethal effects on insects by impairing olfactory detection and processing, thus subsequently affecting individual foraging behaviour. For instance, Andrione et al.23 found that acute imidacloprid treatment can disrupt honeybees’ odour coding and discrimination ability by reducing odour-induced calcium response in the antennal lobes. Antennal selectivity to common floral volatiles is also impacted by larval exposure to thiacloprid in this species24. Furthermore, clothianidin-exposed individuals show reduced antennal sensitivity in two bee species (Osmia bicornis and Bombus terrestris), with associated detrimental changes in foraging behaviour including a reduced number of flowers visited per flight and increased searching time in O. bicornis25. Similarly, exposure to imidacloprid reduces the activity of olfactory neurons in Drosophila melanogaster, and changes individuals’ relative preference for odour sources26. In B. terrestris, the same neonicotinoid negatively affects the motivation to initiate foraging bouts and reduces the amount of nectar collected27. However, different neonicotinoids can differentially influence bumblebee behaviour28, so it is unclear whether acetamipridinduced reductions in foraging performance can be attributed to impairments in peripheral olfactory detection, failure of information processing in the central nervous system, or linked to an overall activity-related deficiency (as suggested by29).
Bumblebees are known to provide essential pollination service in natural and agricultural ecosystems of temperate regions30, with implications for biodiversity and quality food production31,32. The foraging ranges of some Bombus species, such as B. terrestris, often include long-distance bouts, by which bumblebees can buffer against the adverse effects of heterogeneity amongst foraging patches and flowering crops33. If patch quality varies, chemosensory dysfunction in these species may substantially compromise foraging success, and due to the small colonies and annual life cycle characteristic of most bumblebees, the associated sub-lethal effects may have severe fitness consequences34.
In this study, we investigated whether a 3-week-long exposure to the insecticide formulation Mospilan (active ingredient: 20% acetamiprid) can disrupt olfactory detection and affect foraging decisions in B. terrestris workers by conducting choice tests on control and exposed individuals and subsequent electrophysiological recordings of their antennal response. As a floral scent source, we used the synthetic odour blend of the white mustard (Sinapis alba), an annual plant of Mediterranean origin belonging to the family Brassicaceae. This species is in part pollinated by bumblebees, cultivated worldwide and has a significant agronomic value due to its wide range of applications (see35). Blend composition and the ratios of the components were determined following Saunier et al.36. We predicted that the applied pesticide treatment might exert adverse sub-lethal effects by hindering exposed individuals (1) from detecting the volatile blend by reducing antennal sensitivity, i.e. low antennal response, (2) from orienting toward the blend source, i.e. decreased success or increased latency of locating the scent source, or (3) from initiating a foraging bout, i.e. increased latency to start foraging. We also assumed that these potential influences are not mutually exclusive and may manifest simultaneously.
Results
Foraging behaviour
We performed behavioural tests to investigate how control and exposed individuals differ in their orientation toward a mineral oil- and a synthetic blend-containing scent source in a wind tunnel. At the start of the trials, a higher percentage of bees left the releasing cage immediately (i.e., with no latency) in the control group than in the pesticide-treated group (χ21 = 3.88, P = 0.049; Fig. 1a). Nevertheless, when individuals left the cage with latency, these latencies were similar in the two treatment groups (pesticide-treated [mean ± SD]: 13.55 ± 12.60 s; control: 13.50 ± 14.27 s). The starting time of trial did not affect this latency measure. The proportion of the blend-containing scent source as the first choice was significantly higher in the control than in the pesticide-treated group, indicating that untreated bumblebees located the blend first more successfully than pesticide-treated individuals (pesticide-treated: 16 out of 30 bumblebees, control: 23 out of 30 bumblebees; χ21 = 3.87, P = 0.049; Fig. 1b). This difference also meant a significant deviation from the 50% expected by chance in the control (probability of success ± SE: 0.80 ± 0.08, z-ratio = 2.66, P = 0.016) but not in the pesticide-treated individuals (0.54 ± 0.11, z-ratio = 0.38, P = 0.707). The starting time of trial was also positively related to this measure (χ21 = 4.76, P = 0.029), implying that the odds of choosing the blend-containing scent source increased over time (most likely due to increasing hunger) irrespective of the pesticide treatment. Pesticide-treated bees, however, approached the first scent source significantly sooner than control individuals did when it contained the synthetic blend (χ21 = 4.13, P = 0.042; Fig. 1c). The latency to approach the first scent source when it contained only mineral oil did not differ between treatment groups. The starting time of trial had no significant effect on this measure at either scent source (Table 1).
Behavioural responses of bumblebees to the present scent sources. (a) The number of focal individuals (blue: control; red: pesticide-treated) that left the releasing cage at the beginning of the behavioural trial without latency. Values on the top of the bars represent the number of individuals as a percentage of the total number of tested bees in each treatment group. (b) The proportion of focal individuals that approached the blend-containing (green) and the mineral oil-containing (grey) scent sources in the two treatment groups. (c) Kaplan–Meier curves for the cumulative incidences of the first scent source (upper panel: mineral oil; lower panel: floral blend) in the two treatment groups (blue: control; red: pesticide-treated). Curves are shown with 95% confidence intervals. For graphical presentation only, we used the survfit function of the ‘survival’ R package72,73 without including the random term.
Antennal detection
We determined the impact of pesticide treatments on bumblebees’ floral volatile detection by examining the antennal electrophysiological responses of bumblebees’ to the synthetic floral blend after the wind tunnel test. The antennal responses of bumblebees were found to be dose-dependent (χ24 = 3308.80, P < 0.001; Table 1; Fig. 2), with EAG amplitudes being the highest at the highest dose level. All doses significantly differed from the control (i.e., mineral oil) and from each other (including consecutive doses; Table 2). Pesticide treatment, however, had no significant effect on this measure either by itself or in interaction with the stimulus dose (both P ≥ 0.141).
Electroantennographic dose responses of the focal individuals to the synthetic blend (N = 30 in both treatment groups). Boxplots show the median and interquartile range, whiskers indicate values within 1.5-fold of the interquartile range, and dots represent individual data points. The zero dose denotes the mineral oil control; we measured the EAG responses to this stimulus before and after the blend dose series and averaged the two measurements for each individual. Categories on the x-axis indicate the amount of volatile blend loaded into the stimulus cartridge.
Discussion
Chemosensory perception is crucial for all insects to locate food37. Thus, pesticide-induced changes in this process may have far-reaching consequences on food web relationships with plants and the provided ecosystem services in bumblebees38,39. We found no evidence that chronic exposure to an acetamiprid-containing formulation would influence antennal sensitivity to floral volatiles in buff-tailed bumblebee workers; however, we demonstrated several pesticide-induced alterations in their foraging behaviour. Specifically, we showed that a lower proportion of treated individuals started foraging immediately during the trials (6.7% vs. 30% of the tested bumblebees) and approached first the blend-containing scent source than the controls (53.3% vs. 76.7%). However, when the pesticide-treated bumblebees choose the floral blend first, they approach this source faster than the untreated animals (with the mean latency being 81.21 vs. 126.19 s). The observed behavioural changes imply that exposure to the Mospilan formulation affects both activity and orientation. Similar to other studies27,40, pesticide-exposed bumblebees were less motivated to initiate food searches but then reached the blend-containing scent source sooner; a faster initial flight speed in neonicotinoid-exposed bumblebees was also found by Kenna et al.41. On the other hand, treated individuals were not more attracted to the blend than to the odourless source (visual cues were present at both scent sources). These findings suggest that chronic exposure to this neonicotinoid can potentially reduce the resource-exploiting capacities of bumblebees by impairing their ability to locate high-quality patches signalled by concentrated floral scent.
We observed no adverse effect of the pesticide exposure on the olfactory detection of bumblebees despite previous findings showing strong support for reduced antennal sensitivity induced by other neonicotinoids23,24,25,26. However, the lack of effect is likely true only for the peripheral olfactory system. Neonicotinoids are known to act as potent agonists of the nicotinic acetylcholine receptors42, disturbing acetylcholine receptor signalling in the insect nervous system. This interference also occurs in the antennal lobes, which are the first odour-processing centres of the insect brain23. Moreover, experimental evidence indicates that Kenyon cells, major neuronal components of the mushroom bodies, may also become non-functional or unresponsive to excitatory synaptic input if neonicotinoids modulate the activity of nicotinic acetylcholine receptors43. Such effects, in turn, can lead to significant impairment of cognitive functions that are associated with these regions, including multisensory integration, spatial orientation and olfactory learning44,45,46. This mode of action would explain why pesticide-exposed bumblebees were not attracted to the blend source in our experiment but had the same sensitivity in the peripheral olfactory system.
As an alternative to the above mechanism or in parallel with it, the applied pesticide treatment may also alter foraging behaviour through other pathways. Neonicotinoids can act like endocrine-disrupting chemicals47, so low doses of these substances may modulate natural hormonal activities and interfere with gene expression48. Such disrupting effects of chronic acetamiprid exposure have been described in bumblebees related to locomotor activity: neurological symptoms such as abnormal stance, slow to no movements, and termination of normal daily activities like foraging29. This mechanism would explain, for instance, why treated bumblebees in this study were initially less motivated to start foraging than controls (as 4.5 times more bees started foraging without latency in the control group). As all observed behavioural alterations cannot be attributed to a single process, acetamiprid, like all other neonicotinoids, is likely to exert sub-lethal effects on different foraging parameters through several (neuro)physiological pathways, leading to varied and seemingly unrelated trait shifts.
Our study has two important limitations. On the one hand, we used colonies from the same batch, so our experimental design did not allow us to investigate inter-colony variation in antennal detection and scent source choice. In natural circumstances, colonies may differ profoundly in various behavioural parameters29. Therefore, the impact of these differences needs to be understood before we can generalise the observed effects of pesticide exposure on the entire species. On the other hand, we exposed bumblebees to 750–950 ppb concentrations of an acetamiprid-containing formulation for three weeks. Although this range represents a high but field-relevant dose following application20,49, continuous exposure to such concentrations is unlikely to occur under natural circumstances due to the expected degradation of the substance. Previous findings suggest that the post-application concentration of this neonicotinoid varies substantially depending on extrinsic factors, including the spraying technique and climatic conditions or crop type19,20,21. Besides, acetamiprid is known to degrade over time in soil and water due to, among others, microbial activity50,51, even though its concentration in aqueous solutions may remain unchanged for at least 5 days52. Not surprisingly, we also have very limited information on how the concentration of this substance changes over time in the nectar and pollen of flowering crops, which is the most relevant source of exposure to all pollinating insects. We propose that exposure levels similar to those applied in our study may nevertheless arise if bees feed from freshly sprayed flower patches for an extended period of time or if resources contaminated to this extent are stored in the colonies’ storage pots and get exploited frequently by colony members.
There is currently limited information on how acetamiprid affects olfactory perception and individual foraging behaviour in bumblebees53. Previously, acetamiprid was shown to increase antennal sensitivity; however, this investigation was conducted on honeybees following acute exposure54. In recent work, Tóth & Kovács55 found that acetamiprid treatments did not reduce syrup consumption but mediated how tested individuals exploited a second available food patch. Other studies focused only on this neonicotinoid’s effect on microcolonies’ overall syrup and pollen consumption56,57,58. The presented experimental findings are thus the first to imply that a field-relevant exposure to the Mospilan formulation can alter some aspects of foraging performance in bumblebees, likely by attenuating olfactory perception-related information processing in the central nervous system. Besides, pesticide-treated individuals also showed deviations from the locomotor activity of controls, probably due to an endocrine-disrupting effect of the pesticide. Similar works coupling individual- and colony-level sub-lethal effects could help to clarify whether chronic exposure to acetamiprid can jeopardise colony growth, population persistence or pollination service in this species. Such investigations are especially relevant as acetamiprid is expected to be used in large quantities8,53, leading to prolonged exposure of pollinators to this substance in agricultural areas59. Furthermore, neonicotinoid-substitute active ingredients, such as sulfoxaflor and flupyradifurone, should also deserve the attention of researchers because these substances may have similarly covert detrimental effects on pollinating insects60.
Materials and methods
Animal husbandry
We purchased 10 super-mini (containing > 40 workers) Biobest® hives of B. terrestris on 31st August 2023 (Árpád Biokontroll 2003 Ltd). Upon our specific request, bees in the 10 hives originated from the same batch to avoid any substantial confounding colony-level effects on the behavioural measurements29. Colonies remained in their original brood box, with the top of the cardboard outer box left partly open for better ventilation. Bumblebees fed through an adjacent bottle containing sugar solution provided by the supplier (Biogluc®). In addition, we provisioned organic flower pollen (ApiLand SRL, Baia Mare, Romania) to the animals ad libitum. We randomly assigned the colonies to one of two treatment groups, control and pesticide-treated, and arranged the colonies randomly on the laboratory shelves concerning treatment. The ambient temperature was 24.4 ± 0.57 °C, and the relative humidity was 57.3 ± 2.77. Red light (Philips LED red bulbs; Philips International BV, Amsterdam, The Netherlands) has been used to illuminate the laboratory during the daytime (in a 13.5:10.5 h L:D).
Preparation of floral volatiles
Based on Saunier et al.36, a synthetic floral volatile blend consisting of 10 synthetic compounds has been prepared. We combined the individual, synthetic, neat compounds in the following proportions: 2% of (Z)-3-hexen-1-ol (CAS: 928-96-1), 50% of benzaldehyde (CAS: 100-52-7), 8% of (Z)-3-hexenyl acetate (CAS: 3681-71-8), 1.4% of benzyl alcohol (CAS: 100-51-6), 0.2% of phenylacetaldehyde (CAS: 122-78-1), 3% of β-ocimene (CAS: 13877–91-3), 1.4% of acetophenone (CAS: 98-86-2), 2.8% of methyl salicylate (CAS: 119-36-8), 30% of anisaldehyde (CAS: 123-11-5) and 1.2% of β-caryophyllene (CAS: 87-44-5). For the electroantennogram (EAG) measurements, we dissolved the blend in mineral oil (CAS: 8042-47-5) and applied 10 µl of each dilution (0.125, 1.25, 12.5, and 125 µg/µl) to a filter paper disk (cotton liner, diameter: 12.7 mm; Carl Roth GmbH, Karlsruhe, Germany). Subsequently, the treated disk was inserted into a Pasteur pipette, serving as a stimulus cartridge. For the behavioural observations, we loaded 1–1 ml of the neat blend (as in36) and mineral oil as control stimulus in brown vial-wick dispensers61 and used these as two different types of scent source in the trials.
Pesticide treatment and synthetic blend training
The pesticide exposure period took 21 days for each colony. We ensured the exact duration of exposure for all colonies by randomly appointing one control and one pesticide-treated colony to one of five exposure-starting days. Bumblebees from these pairs of colonies participated in the experimental tests on the same day (see below). We prepared a 0.4 g/L stock solution of Mospilan® 20 SG (20% acetamiprid content; Nippon Soda Co. Ltd., Japan; more details of the purchased formulation product can be found in the Supplementary Materials). As this formulation is water-soluble, we diluted 0.2 g of the granulate in 0.5 L of RO-filtered water (following the maximum recommended label rate). Then, we stored the stock solution at 4 ℃. On each exposure-starting day, we measured the volume of the Biogluc® syrup provided with the control and pesticide-treated colonies assigned to that given day. Then, we added the necessary volume of stock solution to create a syrup that contained a nominal concentration of 750 ppb acetamiprid for the pesticide-treated colony and added the same amount of filtered water (instead of the stock solution of the formulation) to the syrup for the control colony. When preparing the pesticide-treated Biogluc® syrup, we pipetted the appropriate amount of the stock solution (dependent on the volume of the syrup) into the syrup (also water-soluble) and homogenised the final solution. We administered the stock solution to the Biogluc® syrup only at the beginning of the 3-week-long exposure period. We performed a residual analysis using an Agilent (Santa Clara, California, USA) 5977C GC/MSD system to verify the concentration of acetamiprid in the prepared pesticide-containing syrup and to examine how it changed over time. For that, we sampled the syrup of each colony on the first and last day of the exposure period. This analysis confirmed that the administration of acetamiprid to the Biogluc® syrup was successful but also revealed that its concentration slightly increased over time, likely due to evaporation (mean (± s.d.) concentrations were 727.0 ± 115.31 ppb at the beginning and 927.12 ± 86.36 ppb at the end of the experiment). Thus, although the initial concentration of acetamiprid was within the range previously found in different crops after spraying20,21, representing a high but field-relevant dose, continuous exposure to such a concentration may rarely occur under natural circumstances. For more details on the residue analysis, see the Supplementary Materials.
We also pipetted 20 μl of the neat blend of synthetic floral volatiles into the Biogluc® syrup three times during the exposure period; thus, bumblebees could associate the scent with the nutritional reward.
Behavioural observations
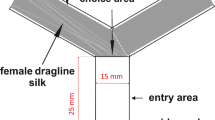
Behavioural trials took place in a wind tunnel (110 cm long × 30 cm wide × 30 cm high; Fig. 3), into which charcoal-filtered air at 22–23 °C and 59–65% RH was pushed through a fine-mesh screen at 0.1 m sec−1 airflow from the direction of scent sources toward the test animals. An exhaust expelled the wind tunnel air outside the building on the tunnel’s far end. The scent sources were two vial-wick dispensers sunk into the bottom of the wind tunnel (made of fibreboard); these dispensers were 8.5 cm from the sidewalls and 15 cm from each other. To facilitate orientation to the scent sources by visual cues, round disks (diameter: 2.4 cm) cut out from blue plastic sheets were attached below the black caps of the dispensers. The appointed pair of colonies was moved into the preparation room next to the wind tunnel one hour before the experiment. During a trial, we first placed the scent sources into their respective, randomly determined positions in the wind tunnel and then put a haphazardly selected individual from one of the two colonies (we determined the order in which the colonies were tested randomly) into an upside-down wire mesh cylinder (‘releasing cage’ henceforward; length: 5.5 cm, internal diameter [ID]: 3 cm) and left the focal individual in the cage for five minutes; the distance between the scent sources and the releasing cage was 65 cm. The room was lit only by a red LED light source during this period. After acclimatisation, the observer turned on the light (similar to daylight [5500–6500 K], provided by Osram Sylvania Luxline Plus light tubes), turned the releasing cage in a lateral position so the focal bee could leave the cage, and then left the room. During the following 10 min, we recorded the behaviour of the focal bee at the releasing cage and near the scent sources with two Panasonic HC-V380 video cameras from above. After 10 min, we turned off the light, so only a red light illuminated the premises again. We removed the focal individual from the wind tunnel in its releasing cage and put it aside for the EAG measurements. After each trial, the observer cleaned the releasing cage, the disks of the dispensers, and the wind tunnel using 70% ethanol and wiped with paper towels; the continuous airflow between trials ensured that no traces of the synthetic blend or alcohol were left in the tunnel.
Schematic drawing of the wind tunnel. The releasing cage was 65 cm downwind from the scent sources (floral blend and mineral oil). Black filled circles were randomly placed on both inner sides of the wind tunnel providing visual cues for orientation. Video cameras recorded the scent sources and the releasing cage from above.
Antennal detection
To determine the impact of pesticide treatments on bumblebees’ floral volatile detection, we conducted electroantennography (EAG) recordings using the synthetic floral blend. All bumblebees were tested after their behavioural trial (i.e., within two hours on the same day). EAG recordings quantify the depolarisation amplitude of all responsive olfactory sensory neurons when exposed to a stimulus62. For the EAG recordings, the antenna of a B. terrestris worker was excised, half of the last (distal) segment was cut and both sides of the antenna were inserted into two glass electrodes (ID: 1.17 mm, Syntech, Kirchzarten, Germany), which were filled with Ringer solution63. The antennal signal underwent a pre-amplification of tenfold, was then converted into a digital signal using a DC amplifier interface (IDAC-2, Syntech), and subsequently recorded utilising GC-EAD software (GC-EAD 2014, version 1.2.5, Syntech). Antennae were stimulated for 0.5 s using a Stimulus Controller (CS-55, Syntech). The stimulation air (2 l/min) was directed into a consistently humidified and charcoal-filtered air stream (2 l/min). Mineral oil, the solvent of the floral volatiles, served as the control stimulus. Before and after each stimulus session, we measured the antennal responses of the focal individual to the mineral oil; within sessions, the four concentrations of the synthetic blend were applied in ascending order. We also counted the antennal segments after the EAG measurement to confirm that the tested individual was a worker.
Video analysis
We analysed the video files with the event recorder BORIS 7.7.364. To avoid observer bias, we renamed each video file to an identification number and analysed the recordings in a random order. We determined the following behavioural measures from the video recordings: time to leave the releasing cage after the observer turned the releasing cage in a lateral position and time to approach the first scent source. This latter was defined as an event when the focal individual gets so close to a scent source that its head crosses the edge of the blue disk attached to the dispenser or when the animal stops at the edge of the blue disk and starts cleaning its antennae (two instances).
Statistical analysis
We tested 62 bumblebee workers, of which 31 were control and 31 were pesticide-treated individuals. We excluded two bees (one control and one pesticide-treated) from the subsequent analyses because they did not approach either scent source during the behavioural trial and had unusually low antennal responses even at the 12.5 µg dose in the EAG test. The EAG amplitudes of the mineral oil control stimulus measured before and after each stimulus session were averaged. In four instances, the antennal response of the animals to the 1.25 µg dose was lower than the averaged EAG amplitudes to the control; we replaced these values with the latter (i.e., allowed no lower response than to the control in the analysis). We obtained essentially identical results if we excluded these data points instead. All tests were performed in R 4.2.0.65. We used a linear mixed-effect model (LMM) with an autocorrelation-moving average correlation structure (p = 0, q = 2; ‘nlme’ R package66) to investigate how stimulus dose and pesticide treatment (both included as factors) affected the measured EAG amplitudes. The dependent variable was log-transformed (log(x + 0.1)) to improve its fit to normal distribution. We included ‘Individual identity’ nested within ‘Colony’ nested within ‘Day’ in this model as a random term. We applied a zero-inflated-Gamma generalised linear mixed model (GLMM) using Template Model Builder (‘glmmTMB’ R Package67) to examine how the starting time of trial (decimal time as a covariate) and pesticide treatment (as a factor) affected bumblebee’s latency to leave the releasing cage in the behavioural trial. By using a zero-inflated model, we were also able to take into account the frequent zero-valued observations. We fitted a GLMM with binomial error distribution to investigate how the type of the first-approached scent source (with the value of 1 for ‘blend’ and 0 for ‘mineral oil’) was affected by the starting time of trial and pesticide treatment. To check compliance with the assumptions of the (G)LMMs, we applied residual plot diagnoses (for GLMMs, we used the ‘DHARMa’ R package68). We used a mixed-effect Cox proportional hazards model to examine how the starting time of trial and the interaction between the type of the first-approached scent source and pesticide treatment affected the duration taken to approach the first scent source (‘coxme’ R package69). Due to the violation of the proportional hazard assumption for the predictor ‘scent source type’ when the model was fitted to the complete dataset, we ran the model separately on the ‘blend’ and ‘mineral oil’ datasets with starting time of trial and pesticide treatment as potential predictors. We included ‘Colony’ nested within ‘Day’ in the latter four mixed-effect models as a random term. To estimate the significance of potential predictors in the fitted models, we applied type III Wald χ2-tests using the Anova function of the ‘car’ R package70. Marginal means and post hoc comparisons with FDR adjustment were performed using the ‘emmeans’ R package71. All tests were two-tailed with α set to 0.05.
Ethical note
No permits or approvals were required for the experiment on commercially available buff-tailed bumblebees in Hungary. After completing the experiment, we euthanised the animals by freezing them to avoid mixing with the natural population.
Data availability
Data files supporting the results are archived and available at Figshare (https://figshare.com/s/0d7f3e158c0ccdff8d0b).
References
FAO. Pesticides use and trade, 1990–2021. FAOSTAT Analytical Briefs Series No. 70., Rome; https://doi.org/10.4060/cc6958en (2023)
Carriger, J. F. et al. Pesticides of potential ecological concern in sediment from South Florida canals: An ecological risk prioritization for aquatic arthropods. Soil Sedim. Contam. 15, 21–45 (2006).
Pimentel, D. & Burgess, M. Small amounts of pesticides reaching target insects. Environ. Dev. Sustain. 14, 1–2 (2012).
Smagghe, G. et al. Neonicotinoids and their substitutes in sustainable pest control. EASAC policy report 45. Schaefer Druck und Verlag GmbH (2023).
Rumschlag, S. L. et al. Consistent effects of pesticides on community structure and ecosystem function in freshwater systems. Nat. Commun. 11, 6333. https://doi.org/10.1038/s41467-020-20192-2 (2020).
Sánchez-Bayo, F. & Wyckhuys, K. A. Worldwide decline of the entomofauna: A review of its drivers. Biol. Conserv. 232, 8–27 (2019).
Vijver, M. G. et al. Postregistration monitoring of pesticides is urgently required to protect ecosystems. Environ. Toxicol. Chem. 36, 860–865 (2017).
Varga-Szilay, Z. & Pozsgai, G. Plant growers’ environmental consciousness may not be enough to mitigate pollinator declines: A questionnaire-based case study in Hungary. Pest Manag. Sci. 79, 1284–1294 (2023).
EC. European Commission implementing regulation (EU) 2018/113. OJEU 20, 7–10 (2018).
EFSA. Peer review of the pesticide risk assessment of the active substance acetamiprid. EFSA J. 14, e04610. https://doi.org/10.2903/j.efsa.2016.4610 (2016).
Decourtye, A. & Devillers, J. (2010). Ecotoxicity of neonicotinoid insecticides to bees in Insect nicotinic acetylcholine receptors, advances in experimental medicine and biology (ed Thany, S. H.), 683, 85–95 (Springer, 2010).
Shi, J. et al. Sublethal acetamiprid doses negatively affect the lifespans and foraging behaviors of honey bee (Apis mellifera L.) workers. Sci. Total Environ. 738, 139924. https://doi.org/10.1016/j.scitotenv.2020.139924 (2020).
Siviter, H., Richman, S. K. & Muth, F. Field-realistic neonicotinoid exposure has sub-lethal effects on non-Apis bees: A meta-analysis. Ecol. Lett. 24, 2586–2597 (2021).
Řezáč, M., Řezáčová, V. & Heneberg, P. Contact application of neonicotinoids suppresses the predation rate in different densities of prey and induces paralysis of common farmland spiders. Sci. Rep. 9, 5724. https://doi.org/10.1038/s41598-019-42258-y (2019).
Svoboda, J., Pech, P. & Heneberg, P. Low concentrations of acetamiprid, deltamethrin, and sulfoxaflor, three commonly used insecticides, adversely affect ant queen survival and egg laying. Sci. Rep. 13, 14893. https://doi.org/10.1038/s41598-023-42129-7 (2023).
Arican, Y. E., Karaman, E. F. & Özden, S. The sub-chronic effects of acetamiprid on the global DNA methylation levels in Sprague-Dawley rat brain and liver. Istanbul J. Pharm. 49, 167–172 (2019).
Gupta, S., Gajbhiye, V. T. & Gupta, R. K. Effect of light on the degradation of two neonicotinoids viz acetamiprid and thiacloprid in soil. Bull. Environ. Contam. Toxicol. 81, 185–189 (2008).
Potts, J., Jones, D. L., Macdonald, A., Ma, Q. & Cross, P. Acetamiprid fate in a sandy loam with contrasting soil organic matter contents: A comparison of the degradation, sorption and leaching of commercial neonicotinoid formulations. Sci. Total Environ. 842, 156711. https://doi.org/10.1016/j.scitotenv.2022.156711 (2022).
Pohorecka, K. et al. Residues of neonicotinoid insecticides in bee collected plant materials from oilseed rape crops and their effect on bee colonies. J. Apic. Sci. 56, 115–134 (2012).
Stejskalová, M., Konradyová, V., Suchanová, M. & Kazda, J. Is pollinator visitation of Helianthus annuus (sunflower) influenced by cultivar or pesticide treatment?. Crop Prot. 114, 83–89 (2018).
Capela, N. et al. Exposure and risk assessment of acetamiprid in honey bee colonies under a real exposure scenario in Eucalyptus sp. landscapes. Sci. Total Environ. 840, 156485. https://doi.org/10.1016/j.scitotenv.2022.156485 (2022).
Chen, D. et al. Nationwide biomonitoring of neonicotinoid insecticides in breast milk and health risk assessment to nursing infants in the Chinese population. J. Agric. Food Chem. 68, 13906–13915 (2020).
Andrione, M., Vallortigara, G., Antolini, R. & Haase, A. Neonicotinoid-induced impairment of odour coding in the honeybee. Sci. Rep. 6, 38110. https://doi.org/10.1038/srep38110 (2016).
Ke, L., Chen, X., Dai, P. & Liu, Y. J. Chronic larval exposure to thiacloprid impairs honeybee antennal selectivity, learning and memory performances. Front. Physiol. 14, 1114488. https://doi.org/10.3389/fphys.2023.1114488 (2023).
Straub, F., Orih, I. J., Kimmich, J. & Ayasse, M. Negative effects of the neonicotinoid clothianidin on foraging behavior and antennal sensitivity in two common pollinator species, Osmia bicornis and Bombus terrestris. Front. Ecol. Evol. 9, 697355. https://doi.org/10.3389/fevo.2021.697355 (2021).
Tatarko, A. R., Leonard, A. S. & Mathew, D. A neonicotinoid pesticide alters Drosophila olfactory processing. Sci. Rep. 13, 10606. https://doi.org/10.1038/s41598-023-37589-w (2023).
Muth, F. & Leonard, A. S. A neonicotinoid pesticide impairs foraging, but not learning, in free-flying bumblebees. Sci. Rep. 9, 4764. https://doi.org/10.1038/s41598-019-39701-5 (2019).
Moffat, C. et al. Neonicotinoids target distinct nicotinic acetylcholine receptors and neurons, leading to differential risks to bumblebees. Sci. Rep. 6, 24764. https://doi.org/10.1038/srep24764 (2016).
Baines, D., Wilton, E., Pawluk, A., de Gorter, M. & Chomistek, N. Neonicotinoids act like endocrine disrupting chemicals in newly-emerged bees and winter bees. Sci. Rep. 7, 10979. https://doi.org/10.1038/s41598-017-10489-6 (2017).
Hutchinson, L. A. et al. Using ecological and field survey data to establish a national list of the wild bee pollinators of crops. Agric. Ecosyst. Environ. 315, 107447. https://doi.org/10.1016/j.agee.2021.107447 (2021).
Johnson, C. A., Dutt, P. & Levine, J. M. Competition for pollinators destabilizes plant coexistence. Nature 607, 721–725 (2022).
Bommarco, R., Marini, L. & Vaissière, B. E. Insect pollination enhances seed yield, quality, and market value in oilseed rape. Oecologia 169, 1025–1032 (2012).
Osborne, J. L. et al. Bumblebee flight distances in relation to the forage landscape. J. Anim. Ecol. 77, 406–415 (2008).
Baron, G. L., Jansen, V. A., Brown, M. J. & Raine, N. E. Pesticide reduces bumblebee colony initiation and increases probability of population extinction. Nat. Ecol. Evol. 1, 1308–1316 (2017).
Mitrović, P. M. et al. White mustard (Sinapis alba L.) oil in biodiesel production: A review. Front. Plant Sci. 11, 299. https://doi.org/10.3389/fpls.2020.00299 (2020).
Saunier, A., Grof-Tisza, P. & Blande, J. D. Effect of ozone exposure on the foraging behaviour of Bombus terrestris. Environ. Pollut. 316, 120573. https://doi.org/10.1016/j.envpol.2022.120573 (2023).
De Bruyne, M. & Baker, T. C. Odor detection in insects: Volatile codes. J. Chem. Ecol. 34, 882–897 (2008).
Stanley, D. A. et al. Neonicotinoid pesticide exposure impairs crop pollination services provided by bumblebees. Nature 528, 548–550 (2015).
Stanley, D. A. & Raine, N. E. Chronic exposure to a neonicotinoid pesticide alters the interactions between bumblebees and wild plants. Funct. Ecol. 30, 1132–1139 (2016).
Lämsä, J., Kuusela, E., Tuomi, J., Juntunen, S. & Watts, P. C. Low dose of neonicotinoid insecticide reduces foraging motivation of bumblebees. Proc. R. Soc. B 285, 20180506. https://doi.org/10.1098/rspb.2018.0506 (2018).
Kenna, D. et al. Pesticide exposure affects flight dynamics and reduces flight endurance in bumblebees. Ecol. Evol. 9, 5637–5650 (2019).
Brown, L. A., Ihara, M., Buckingham, S. D., Matsuda, K. & Sattelle, D. B. Neonicotinoid insecticides display partial and super agonist actions on native insect nicotinic acetylcholine receptors. J. Neurochem. 99, 608–615 (2006).
Palmer, M. J. et al. Cholinergic pesticides cause mushroom body neuronal inactivation in honeybees. Nat. Commun. 4, 1634. https://doi.org/10.1038/ncomms2648 (2013).
Stanley, D. A., Smith, K. E. & Raine, N. E. Bumblebee learning and memory is impaired by chronic exposure to a neonicotinoid pesticide. Sci. Rep. 5, 16508. https://doi.org/10.1038/srep16508 (2015b).
Stanley, D. A., Russell, A. L., Morrison, S. J., Rogers, C. & Raine, N. E. Investigating the impacts of field-realistic exposure to a neonicotinoid pesticide on bumblebee foraging, homing ability and colony growth. J. Appl. Ecol. 53, 1440–1449 (2016).
Muth, F., Francis, J. S. & Leonard, A. S. Modality-specific impairment of learning by a neonicotinoid pesticide. Biol. Lett. 15, 20190359. https://doi.org/10.1098/rsbl.2019.0359 (2019).
Christen, V., Kunz, P. Y. & Fent, K. Endocrine disruption and chronic effects of plant protection products in bees: Can we better protect our pollinators?. Environ. Pollut. 243, 1588–1601 (2018).
Vandenberg, L. N. et al. Hormones and endocrine-disrupting chemicals: Low-dose effects and nonmonotonic dose responses. Endocr. Rev. 33, 378–455 (2012).
Chandler, A. J., Drummond, F. A., Collins, J. A., Lund, J. & Alnajjar, G. Exposure of the common Eastern bumble bee, Bombus impatiens (Cresson), to sub-lethal doses of acetamiprid and propiconazole in wild blueberry. J. Agric. Urban Entomol. 36, 1–23 (2020).
Elango, D. et al. Biodegradation of neonicotinoid insecticide acetamiprid by earthworm gut bacteria Brucella intermedium PDB13 and its ecotoxicity. Microbiol. Res. 268, 127278 (2023).
Guo, L. et al. Biodegradation of the neonicotinoid insecticide acetamiprid by actinomycetes Streptomyces canus CGMCC 13662 and characterization of the novel nitrile hydratase involved. J. Agric. Food Chem. 67, 5922–5931 (2019).
Ma, X., Li, H., Xiong, J., Mehler, W. T. & You, J. Developmental toxicity of a neonicotinoid insecticide, acetamiprid to zebrafish embryos. J. Agric. Food Chem. 67, 2429–2436 (2019).
Varga-Szilay, Z. & Tóth, Z. Is acetamiprid really not that harmful to bumblebees (Apidae: Bombus spp.). Apidologie 53, 2. https://doi.org/10.1007/s13592-022-00909-6 (2022).
El Hassani, A. K. et al. Effects of sublethal doses of acetamiprid and thiamethoxam on the behavior of the honeybee (Apis mellifera). Arch. Environ. Contam. Toxicol. 54, 653–661 (2008).
Tóth, Z. & Kovács, Z. Chronic acetamiprid exposure moderately affects the foraging behaviour of buff-tailed bumblebees (Bombus terrestris). Ethology 130, e13425. https://doi.org/10.1111/eth.13425 (2024).
Camp, A. A. et al. Effects of the neonicotinoid acetamiprid in pollen on Bombus impatiens microcolony development. Environ. Toxicol. Chem. 39, 2560–2569 (2020).
Camp, A. A., Williams, W. C., Eitzer, B. D., Koethe, R. W. & Lehmann, D. M. Effects of the neonicotinoid acetamiprid in syrup on Bombus impatiens (Hymenoptera: Apidae) microcolony development. PLoS ONE 15, e0241111. https://doi.org/10.1371/journal.pone.0241111 (2020).
Straub, F. et al. Land-use-associated stressors interact to reduce bumblebee health at the individual and colony level. Proc. R. Soc. B 290, 20231322. https://doi.org/10.1098/rspb.2023.1322 (2023).
Zioga, E., White, B. & Stout, J. C. Honey bees and bumble bees may be exposed to pesticides differently when foraging on agricultural areas. Sci. Total Environ. 896, 166214. https://doi.org/10.1016/j.scitotenv.2023.166214 (2023).
Siviter, H. & Muth, F. Do novel insecticides pose a threat to beneficial insects ?. Proc. R. Soc. B 287, 20201265. https://doi.org/10.1098/rspb.2020.1265 (2020).
Molnár, B. P., Tóth, Z. & Kárpáti, Z. Synthetic blend of larval frass volatiles repel oviposition in the invasive box tree moth, Cydalima perspectalis. J. Pest Sci. 90, 873–885 (2017).
Roelofs, W.L. Electroantennogram assays: Rapid and convenient screening procedures for pheromones in Techniques in Pheromone Research (eds Hummel, H.E. & Miller, T.A.) 131–159 (Springer, 1984)
Ephrussi, B. & Beadle, G. W. A technique of transplantation for Drosophila. Am. Nat. 70, 218–225 (1936).
Friard, O. & Gamba, M. BORIS: A free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol. Evol. 7, 1325–1330 (2016).
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/ (2022).
Pinheiro, J., Bates, D., DebRoy, S., Sarkar, D. & R Core Team nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1-157. https://CRAN.R-project.org/package=nlme (2021).
Brooks, M. E. et al. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 9, 378–400 (2017).
Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. R package version 0.4.6. https://CRAN.R-project.org/package=DHARMa (2022).
Therneau, T. M. Coxme: Mixed effects cox models. R Package Version 2.2–18.1. https://CRAN.R-project.org/package=coxme (2022a).
Fox, J. & Weisberg, S. An R companion to applied regression (3rd ed.) (Sage, 2019). https://socialsciences.mcmaster.ca/jfox/Books/Companion
Lenth, R. Emmeans: Estimated marginal means, aka least-squares means. R package version 1.8.6. https://CRAN.R-project.org/package=emmeans (2023).
Therneau, T. M. A Package for Survival Analysis in R. R package version 3.3–1. https://CRAN.R-project.org/package=survival (2022b).
Therneau, T. M., & Grambsch, P. M. Modeling survival Data: Extending the cox model. (Springer, 2000).
Acknowledgements
Z.T. was financially supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences (MTA, BO/00634/21/8). M.O.S. was financially supported by the New National Excellence Program of the Ministry for Innovation and Technology from the source of the National Research, Development and Innovation Fund (ITM, ÚNKP-23-4).
Funding
Open access funding provided by HUN-REN Centre for Agricultural Research.
Author information
Authors and Affiliations
Contributions
Z.K. and Z.T. conceived and designed the study, and performed the experiment. Z.T. performed the statistical analysis, wrote the initial manuscript, and revised and edited the subsequent versions. Z.K. and M.O.S. performed the residue analysis. Z.K., M.O.S. and Z.T. contributed substantially to the text and revisions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kárpáti, Z., Szelényi, M.O. & Tóth, Z. Exposure to an insecticide formulation alters chemosensory orientation, but not floral scent detection, in buff-tailed bumblebees (Bombus terrestris). Sci Rep 14, 14622 (2024). https://doi.org/10.1038/s41598-024-65388-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-65388-4
- Springer Nature Limited