Abstract
Relationship between depressive disorder and autonomic nervous system has been already discussed. Reduced emotional regulation is supposed to be associated with prefrontal hypofunction and subcortical hyperactivity. The aim of this study was to determine the effect of vortioxetine on heart rate variability (HRV), a parameter of cardiac autonomic regulation, in depressed hospitalized paediatric patients and assess the clinical effectiveness of the drug in this population. We performed repeated polysomnography analyses at admission and after a short treatment in hospital (15.2 days on average) and measured various HRV parameters (RRi, pNN50, RMSSD, LF-HRV, HF-HRV) during wakefulness, N3 and REM sleep stages. Out of 27 study subjects, 67% have improved depression symptoms as well as anxiety and subjective sleep quality after short vortioxetine treatment. We have found a significant decrease in parasympathetic parameters pNN50, RMSSD and HF-HRV during N3 sleep phase, though not exclusively among vortioxetine responders. The anticipated increase in cardiovagal regulation after vortioxetine treatment was not demonstrated in this pilot study, possibly due to the drug’s multimodal mechanism and impact on the nucleus tractus solitarii, particularly its antagonism on 5HT-3 receptors. Application of selective drugs could further explain the effect of vortioxetine on HRV in depressed patients.
Similar content being viewed by others
Introduction
Mastering the capacity to adapt one’s emotional responses to match the specific context, referred to as emotion regulation, stands as a fundamental developmental milestone during early childhood and adolescence. These processes are underpinned by the autonomic nervous system (ANS), a central physiological network responsible for dynamically harmonizing real-time sensory inputs with internal motivational and goal-oriented1. The circuitry between ANS and central nervous system (CNS), shaped early in the course of the life, forms the basis for the adaptive neurovisceral regulation throughout one’s lifetime. Therefore, adolescence contributes significantly to mental well-being rendering this age group notably susceptible to mental disorders. Indeed, it is estimated that globally 14% of 10–19 aged children and adolescents experience a mental disorder. Major depressive disorder (MDD) is believed to affect approximately 1.1% of individuals in the 10–14-year-old group and 2.8% of those aged 15–192.
The autonomic imbalance is associated with a lack of physiological adaptability, dynamic flexibility, and health. There are two main theories that support the idea that cardiac vagal regulation indexed by heart rate variability (HRV) is associated with autonomic and emotional regulation. First, polyvagal theory3,4 specifies that the cardiac-linked vagal efferent modulation from the nucleus ambiguus is connected with cranial nerves involved in the emotional expression and vocalization important for emotional and affective regulation in people. Thus, vagally-mediated HRV index (high frequency, HF-HRV) could be considered as an index of emotional regulation. Further, Thayer and Lane5 reported the significance of cardiac autonomic regulation in the context of neurovisceral integration model (NIM). It is based on the existence of the central autonomic network (CAN) as functional cortico-subcortical neural circuits linking the heart to the prefrontal cortex (PFC). More specifically, the CAN described by Benarroch6 includes several brain structures such as PFC, amygdala, specific medullary areas, which are important for the complex heart rate (HR) control characterizing a healthy and adaptive regulatory system5. PFC is an executive brain region responsible for emotion regulation, decision-making, and social behaviour. It exerts an inhibitory influence on subcortical centres including the amygdala that allows the organism to adjust emotional and behavioural responses. Amygdala, important part of the limbic system, controls predominantly emotional regulation and basic autonomic arousal processes7. More specifically, amygdala, activated during threat/uncertainty, is under tonic inhibitory control of the PFC. Thus, in case of threat or novelty associated with emotional regulation, the PFC becomes hypoactive. This hypoactive state is related to inhibition of cardiovagal regulatory mechanisms accompanied by an increase in HR and decrease of vagally mediated HRV8,9,10. Therefore, the PFC and its abnormalities (e.g. prefrontal hypoactivity) could play a critical role in vagally-mediated dysregulation associated with MDD because of the PFC disruption to inhibit the amygdala as a region that mediates cardiovascular and autonomic responses11.
The relationship between autonomic dysfunction and emotion dysregulation (ED) has been described previously in the literature: ADHD in childhood was associated with abnormal parasympathetic mechanisms12, borderline personality disorder (BPD) symptom severity was significantly related to reduced resting-state HRV and increased HR13, loss of HRV complexity (a novel feature to detect cardiac abnormalities) has been associated with psychopathologies such as schizophrenia, bipolar disorder, and MDD10,11.
Latest meta-analysis on MDD and HRV that included 21 studies found lower HRV-measures in MDD compared to healthy controls14. Specifically, patients with MDD had lower HF-HRV, lower percentage of successive R-R intervals that differ by more than 50 ms (pNN50), increased low-frequency (LF)/HF ratio, which suggests lower parasympathetic effect, thus impaired adaptability to the stimuli. The effect of antidepressants on HRV was observed in smaller number of studies and showed a great variability based on the treatment choice, duration of intervention, and HRV measurement characteristics. Usually, treatment with tricyclic antidepressants showed reduction in HRV, which could be explained by their anticholinergic properties15,16 while studies with selective serotonin reuptake inhibitors (SSRIs) were more variable—with no or negative effect on HRV15,16,17. In adolescents, one study found that at 8-week follow-up of SSRI antidepressants, HRV parameters increased in depressed patients, which correlated with the improved MDD symptoms18. HRV parameters were used also to predict treatment response in MDD19. Interestingly, psychotherapy was effective for improving reduced HRV in MDD patients17.
Vortioxetine is one of the newest antidepressants that, in comparison to SSRIs, not only block serotonin reuptake but also directly modulates various serotonin (5-HT) receptors: it is a 5-HT3, 5-HT1D, and 5-HT7 antagonist, a 5-HT1A agonist, and a 5-HT1B partial agonist20. It has been approved for the treatment of MDD in adults but has been studied also in paediatric population21,22,23.
Measurement of HRV during daytime can be influenced by many confounding factors such as level of stress, emotions, exercise, body posture, alcohol of caffeine intake, and others. Therefore, measurement in resting states such as during sleep minimizes the confounders24. However, HRV can vary also between different sleep stages given the changes in ANS activity. Deep non-REM sleep (stage N3) is a state with the most profound vagal influence on HR. Subsequently, transition to REM sleep causes a decrease in vagal contribution to HR control and results in a relative prevalence of low-frequency HRV25.
The primary goal of this study was to establish the influence of vortioxetine on HRV during individual sleep stages using polysomnography (PSG) analysis in hospitalized children and adolescents with the diagnosis of MDD. The objective is to correlate a determined effect of vortioxetine with previous literature findings on SSRIs based on polyvagal theory and NIM. Simultaneously, we aimed to describe the effectiveness of antidepressant treatment with vortioxetine in these patients.
Results
Out of 30 patients, the data from both PSG assessments were successfully obtained only from 27 patients as shown in Fig. 1. Their basic demographic data are as following: average age 15.1 ± 1.5 years old, 20 (74%) were girls and median vortioxetine daily dose was 10 mg. Psychiatric rating scale scores at admission (Time 1) and discharge from hospital (Time 2) are displayed in Table 1 and divided into group of responders and non-responders. In all measured rating scales, there is a significant improvement in responder group.
We have found a significant decrease in parameters pNN50 (p = 0.0169), RMSSD (p = 0.0084), and HF-HRV (p = 0.0178) during deep sleep (N3 sleep stage) after short vortioxetine treatment. The effect size of these results was moderate.
Comparing separately the data only in the responder group (N = 18), there were no significant differences in HRV parameters before and after short vortioxetine therapy. However, we have observed a significant decrease of the parameter RMSSD (p = 0.0369, d = 0.42) during N3 sleep stage and interestingly, a significant decrease of HF (p = 0.0443, d = 0.45) during REM sleep in the non-responder group (N = 9). All results and values of HRV parameters in various sleep stages and wakefulness in the study subjects in Time 1 and Time 2 are displayed in Table 2.
When we compared the HRV parameters between responders and non-responders in both periods, the only parameter pNN50 was significantly higher at admission in non-responders at wake phase (p = 0.0371, d = 0.98).
We have not found any significant correlation between HRV parameters at Time 1 and scores of any rating scales (MADRS, HAM-A, AIS, CDI), nor of age.
Discussion
The present study brings new and original insights on autonomic regulation/dysregulation assessed by HRV during sleep in depressed paediatric patients before and after vortioxetine treatment in hospital. To the best of our knowledge, it is a first study describing effects of this antidepressant on HRV. The major findings of this study are following: (1) two thirds of depressed adolescent patients improved their depressive symptoms as well as symptoms of anxiety and insomnia after a short vortioxetine treatment during hospitalization; (2) short vortioxetine treatment significantly decreased parameters pNN50, RMSSD, and HF-HRV during N3 sleep stage in entire group but not in responder group separately. These major findings and possible mechanisms are discussed below.
Applying psychiatric rating scales showed that adolescent patients in our study suffered on average from moderate depression, mild to moderate anxiety and insomnia symptoms such as difficulties with falling asleep, night awakenings, shorter total sleep time, and worsened sleep quality. Vortioxetine proved efficacy, significantly improving the clinical status of depressed paediatric patients within a mean hospital treatment duration of 15.2 days, as evidenced by a 50% decrease in MADRS and CDI scores in two-thirds of the subjects (67%). Currently, pharmacological options for the treatment of depression and anxiety disorders in childhood are limited to fluoxetine in Slovakia, while in the United States, escitalopram is also utilised. Limited studies on the use of vortioxetine for paediatric depression have characterised its pharmacokinetic properties and confirmed its safety but have not shown statistically significant efficacy over placebo21,22,23. Improvement in CDI scores was significant in responder group in all five subcategories (negative mood, interpersonal problems, ineffectiveness, anhedonia, and negative self-esteem) while in no subcategory in non-responder group.
Additionally, our treatment of acute MDD episode significantly improved sleep measured by AIS and anxiety symptomatology measured by HAM-A. In the context of sleep disorders, Du et al. reported a case, in which a three-month trial of vortioxetine at 10 mg led to an improvement in REM sleep behaviour disorder (RBD)26. We have also previously described a case report of depressed adolescent that took vortioxetine and improved symptoms of RBD27. Insomnia is one of the most common symptoms occurring in depressed patients. The presence of depressive symptoms often results in disruptions to sleep, suggesting that the enhancement in subjective sleep quality scores may stem from a reduction in depressive symptomatology. However, it is also important to mention that the hospitalization of a patient is associated with a regular regimen, which may also contribute to improvement of subjective sleep assessment scores. In adults, vortioxetine at doses 2.5–10 mg once daily did not show significant improvements in HAM-A scores in patients with primary generalized anxiety disorder28. We demonstrated a significant reduction of the described anxiety symptomatology in the paediatric patients. Thus, this is the first study describing the clinical effect of vortioxetine on anxiety symptomatology in a population of children and adolescents.
The improvement in the clinical condition of the patients that we demonstrated in this work using scaling methods (MADRS, CDI, HAM-A) can also be interpreted as an improvement of ED, which in the context of polyvagal theory should lead to an increase in vagal activity and HRV. Based on the NIM, we expected an increase in parasympathetic (PNS) activity indicating increased prefrontal activity resulting in inhibition of subcortical regions associated with a reduction in depressive symptomatology.
We have firstly looked at the entire sample group but considering the clinical improvement (50% decrease on MADRS), we further analysed separately the sample of vortioxetine responders and non-responders. The results in the whole group showed a reduction in the vagal parameters pNN50, RMSSD and HF-HRV during N3 sleep stage after vortioxetine treatment compared to the baseline values. However, we have not found any significant effect of vortioxetine only in responder group, even though there was a trend, and the effect size was considered moderate. The reason can be a small sample size and a short duration of the treatment. Since the effect of vortioxetine on HRV has not yet been investigated, it can be discussed in the context of other work on SSRI treatment. The results of these studies, however, have also been inconclusive, showing a non-significant or negative effect of SSRI treatment in adult patients. Specifically, Kemp et al.15 did not demonstrate an effect of SSRIs on HRV, whereas Licht et al. demonstrated a reduction in HRV during SSRI use. Patients who started taking SSRIs experienced a reduction in HRV whereas patients who discontinued SSRIs experienced an increase in HRV. The results of Licht et al.29 are thus consistent with our results.
In the population of children and adolescents, there have been published only four studies examining the effect of antidepressants on HRV. In two of them, the patients were taking tricyclic antidepressants whose effect on HRV due to their anticholinergic effects cannot be compared with results in our research30,31. Vloet et al. in their cross-sectional study on 23 depressed patients did not observe significant effect of antidepressant treatment with SSRIs and mirtazapine on HRV32. Koenig et al. demonstrated an increase in HRV associated with an increase in cortical thickness in frontal region after 8 weeks of SSRIs (fluoxetine or sertraline) use, which subsequently may influence HRV due to predicted increase in vagal activity33. We hypothesize that one of the reasons for different results may be a difference in the drug chosen and duration of the treatment. It can be assumed that the antidepressant effect associated with the inhibitory effect of the frontal region on subcortical structures correlates with the length of antidepressant treatment (a sufficient period is needed for adaptation of physiological mechanisms regulating autonomic activity).
Interestingly, in the non-responder group, we have observed a significant decrease in parameter RMSSD during N3 sleep stage and in parameter HF during REM sleep. Transition from non-REM to REM sleep stage is characterised by decrease of vagal contribution to HR control, resulting in a relative prevalence of low-frequency HRV, which can be interpreted as a condition of sympathetic (SNS) dominance in the autonomic balance (complex character of LF-HRV: mainly PNS at rest, but reflects SNS dominance in the periods of demand)25. Thus, we found the more profound effect of this parasympathetic to sympathetic transition in non-responder MDD patients. However, the variability of this result was very high, which together with the small sample size do not enable us to draw any clear conclusion of this finding and the bigger studies in future should confirm this.
Moreover, HRV measurement can be influenced by several factors that can bias the results. According to Herzig et al. the appropriate measurement time is during deep sleep, which minimalizes confounding factors34. Consistently, our study demonstrated that changes in vagal regulation can be captured during N3 stage while they were not detectable during wakefulness and REM phase (apart from the previously mentioned result with high variability). There is the most pronounced (PNS) modulation of cardiac activity during the N3 phase35, which probably explains the highest sensitivity of this sleep stage to study changes in PNS activity. Another mechanism may involve the effect of vortioxetine on the nucleus tractus solitarii (NTS). Inhibition of serotonin reuptake as well as direct agonism and antagonism of vortioxetine at serotonin receptors interact with cardiovascular regulation at many levels from the PFC to the cardiac receptors36. If we assume that clinical improvement in patients correlates with an increase in prefrontal activity, higher inhibitory effect and a subsequent reduction in the activity of subcortical structures, then demonstrated reduction in vagal regulation of the heart may result from a possible effect of vortioxetine on the regulatory centres responsible for cardiovascular integrity. A possible explanation for the reduced cardiovagal activity evaluated by HRV could be precisely the antagonism on 5HT-3 localized on the NTS. Activation of 5HT-3 receptors on the NTS leads to vagal bradycardia whereas their inhibition attenuates the activity of the cardioinhibitory centre and thus leads to tachycardia as a consequence of reduced vagal influences36. Therefore, it is not excluded that an increase in prefrontal activity with subsequent subcortical inhibition represents only a secondary effect on vagal-mediated effect on HRV, which is masked by a dominant effect at the NTS level. Considering these assumptions, it is important to make further research on autonomous nervous system and antidepressant therapies using highly selective drugs and longer observation period.
Furthermore, our correlation analysis did not confirm the relationships between the results of the scales assessing depressive symptomatology and HRV parameters during any of the sleep stages. Meta-analysis including healthy adolescent subjects did not find a significant correlation between depressive symptomatology and HRV33. There is a greater number of studies addressing this topic in adults in contrast to the paediatric population. Kemp et al. found a correlation between depressive symptomatology and HRV reduction in adult patients in their meta-analysis15. According to the latest meta-analysis in children and adolescents, there was no significant association in HRV parameters and depression symptoms, however, gender was a significant moderator for this relationship, revealing a more pronounced negative association among a group with the greater percentage of females37. This correlates with our results and percentage of girls in the present study. We have neither demonstrated a significant correlation between HRV and anxiety symptomatology nor sleep disturbances.
This study has several limitations. Hospitalization itself can influence the HRV parameters in both ways—it can represent a stressful uncomfortable situation but at the same time a regular regimen can have a positive effect on sleep and other symptoms. The study included a small sample size, and a relatively short vortioxetine treatment period was analysed, although the clinical improvement was observed. Moreover, there was a gender disparity (more female than male patients) and a lack of a control group. Considering no control nor placebo group in this study, we cannot draw any clear conclusion on the effect of vortioxetine on the clinical psychiatric symptoms in this group of adolescent patients. Future studies with a proper design (randomized placebo-controlled) need to confirm the present pilot findings.
To sum up, in this study we have observed a clinical efficacy of short vortioxetine treatment in two thirds of the hospitalized children and adolescents with depressive disorder. Moreover, improvement of sleep and anxiety symptoms was also noted. This short treatment led to decrease of pNN50, RMSSD and HF-HRV during N3 sleep phase in the entire group but this effect was not confirmed in the responder group separately. We have confirmed that appropriate HRV measurement is during deep sleep minimizing confounding factors. Decreased HRV parameters in this study could be explained by the antagonist effect of vortioxetine on 5HT-3 receptors localized on the NTS but further studies using selective substances and longer observation period are warranted.
Methods
Participants
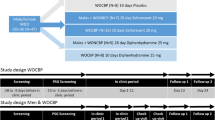
This study included 30 adolescent patients (12–18 years old, 9 boys) with the diagnosis of MDD without psychotic symptoms according to DSM-538 admitted to Psychiatric Clinic of Jessenius Faculty of Medicine and University Hospital Martin in Slovakia. Exclusion criteria were as following: bipolar affective disorder type I and II, autism, substance use disorder in last 6 months, mental anorexia and/or bulimia, intellectual disability (IQ ≤ 70), neurologic disorder, currently on medication significantly affecting sleep architecture, pregnancy, obesity or malnutrition, cardiovascular, endocrine, metabolic, and other diseases affecting the ANS, and alcohol or caffeine intake. Patients were antidepressant-free at least for a month before the inclusion in our study. Regarding their history, 11 individuals had previous experience with antidepressants (fluoxetine, fluvoxamine, or sertraline). The process of final inclusion of the participants is showed in Fig. 1.
The study was approved by the Ethics Committee of Jessenius Faculty of Medicine in Martin (EK87/2019) and the study subjects as well as their parents/legal guardians were informed about the study details and gave written consents to participate.
Study protocol
At hospital admission, patients’ condition and symptoms were thoroughly assessed by the paediatric psychiatrists and psychiatric rating scales such as Montgomery-Asberg Depression Rating Scale (MADRS), Hamilton Anxiety Rating Scale (HAM-A), Athens Insomnia scale (AIS), and Child Depression Inventory (CDI). Subsequently at night, the first PSG assessment was performed and the next day vortioxetine was administered for the first time. The initial dose was 5 mg a day and was titrated according to the clinical symptoms and response up to maximum dose of 20 mg once daily in the morning (naturalistic setting). The follow-up PSG assessment was made in the end of acute symptom hospitalization (15.2 days on average) and patients were divided into responders (clinical improvement of at least 50% in MADRS; hospital discharge) and non-responders (sent to long-term psychiatric care facilities).
Clinical assessment
The MADRS is a clinician-rated measure of depressive severity and comprises 10 items that enable to detect depression changes. The items are evaluated on a seven-point Likert scale (0–6), thus the maximum overall score is 60 points. Scores 0–6 represent the absence of symptoms, 7–19 mild depression, 20–34 moderate depression and 35–60 indicate the greatest depression severity39,40. Although MADRS is preferably used in adults, its reliability was confirmed also in children41. We have used this instrument together with the CDI, a self-report scale, which measures depression symptoms specifically in children and adolescents. It includes 27 items rated 0–2 with a total score up to 54 points. The cut-off score that differentiates between the depressive and general population is 1942,43.
The HAM-A is a 14-item rating tool administered by clinicians to measure the severity of anxiety symptoms. Each item is scored from 0 (not present) to 4 (severe). Scores of 17 and less indicate mild symptoms, 18–24 mild to moderate anxiety symptoms and 25–30 moderate to severe anxiety severity44. Assessment in adolescent population has been also validated45.
The AIS is a valid instrument for insomnia assessment and screening in adolescents. It contains 8 items rated from 0 (no symptoms) to 3 (serious problem). The original cut-off point was 646 while the newer study suggested it to be 747.
These rating scales were administered at admission and at the day of PSG follow-up.
Polysomnography
PSG analyses were conducted in a specialized room designed to minimize any potential sources of stress. This room was equipped with a camera for monitoring, and the actual assessment took place in an adjacent room. To ensure patients were comfortable and familiar with the surroundings, they were given ample time to acquaint themselves with the room before the analysis began. For ECG monitoring, 7 ECG channels (3 physical and 4 derived) were used as part of the polysomnograph. Three physical electrodes were placed just below the right clavicle (collar bone) and to the left of the midclavicular line (slightly lower than right) and the fifth intercostal space48. The sampling rate for ECG monitoring was 500 Hz. ECG was continuously recorded using Sleepware G3 software49. Once the electrodes were in place, patients were allowed to rest in the room and naturally fall asleep while being monitored. During the monitoring process, we carefully identified the different sleep stages and recorded the corresponding time periods of electrocardiogram (ECG) activity during both non-rapid eye movement (NREM) and rapid eye movement (REM) sleep phases.
Heart rate variability assessment
HRV parameters were evaluated from ECG recordings during wakefulness and individual sleep stages (REM and N3 stage of NREM) measured during the regular sleeping time of patients of duration at least 7 h by Alice 6 device (Philips Respironics, Canada) and programme Sleepware G3 before and after short antidepressant therapy during hospitalization (on average 15.2 days). After recording, ECG were manually controlled, and artifacts removed. Then the R-R intervals of 300 time-series long were exported from ECG recordings to csv files and used in the ECG analysis for evaluated time and spectral HRV parameters. Artifacts were detected and removed or replaced automatically according to HRVAS IBI50. More specifically, artifacts were detected according to a time-varying threshold (TV-TH) estimated from the time-varying distribution of R-R intervals. First, the quartile deviation of 90 surrounding beats was counted for each beat. Next, the results were multiplied by a factor 5.2. Then, ectopic beats in the form positive–negative-positive or negative–positive-negative, short beats form negative–positive and long beats form positive–negative patterns into the R-R time series were included as artifacts. Subsequently, extra or missed beats were detected by comparing R-R values with median of the surrounding 10 R-R intervals (medianRR). Additionally, an extra beat was detected if the R-R time series condition has been met:
And the missed beat was detected according the condition as follows:
where RR(i)—representing the R-R interval, RR(i + 1)—characterizing the following R-R interval after RR(i), medianRR – was the median of the surrounding 10 R-R intervals, and TV-TH was a time-varying threshold. Consequently, ectopic beats were corrected by replacing artifact R-R intervals (too long, or too short beats) by interpolation, extra beats were corrected by removing the extra R-oscillation and recalculating the R-R interval series, and missed beats were corrected by adding new R-oscillation occurrence time50.
In time-domain analysis, mean R-R intervals as the average of beat-to-beat deviations in the duration of consecutive R-R intervals and the root square of consecutive differences (rMSSD, ms) were assessed independently during the stages of long sleep (N3 and REM) and wakefulness before sleep51,52. In spectral power analysis, time series of R-R intervals were resampled using cubic spline interpolation with a frequency of 4 Hz. Subsequently, detrending was performed using the smoothing parameter Λ = 50050. Then, spectral powers in the low-frequency band (LF-HRV; 0.04–0.15 Hz) and high frequency band (HF-HRV; 0.15–0.40 Hz) of HRV were analysed using fast Fourier transform with Welch periodogram52 during monitoring sleep phases.
Time- and frequency-domain methods of the HRV analysis are the most frequently used for personalised antidepressant treatment16. More specifically, time-domain parameters RMSSD and pNN50 represent measures mediated mainly by parasympathetic efferent nerves53. Further, frequency (spectral) analysis assesses the underlying variance (power) in the HR pattern at different frequencies. High frequency (HF) is a marker of cardiovagal and emotional modulation, and low frequency (LF) is more complex—it may be produced by both the PNS and SNS, and blood pressure regulation via baroreceptors51. The evaluated parameters with their characteristics are displayed in Table 3.
Statistical analyses
The data were visualized and statistically analysed by the GraphPad 8.0.1 (GraphPad Prism, San Diego, CA, USA). Shapiro–Wilk test of normality was performed to assess parametric and non-parametric distribution of the data. The effect of short antidepressant treatment determined via psychiatric rating scale scores was analysed by paired t-test (parametric data) or Wilcoxon test (non-parametric data). Spearman tests were used to assess the correlations between HRV parameters and psychiatric rating scales scores, and age. Mixed ANOVA was used to compare HRV parameters in two times (Time 1, Time 2) and different stages (wakefulness, N3 stage, REM) in three groups (all subjects, responders, non-responders) and then in responders compared to non-responders. The p-value was corrected post-hoc by Benjamini, Krieger and Yekutieli two-stage step-up method to control false discovery rate. Such P-value ≤ 0.05 was defined as statistically significant. Effect size was calculated by post-hoc comparison of means and standard deviations of two groups using G*Power 3.1.9.4 (Dusseldorf University, Dusseldorf, Germany).
Ethical approval
The study was approved by the Ethics Committee of Jessenius Faculty of Medicine in Martin (EK87/2019) and all the methods were performed in accordance with the Declaration of Helsinki and other relevant guidelines and regulations.
Informed consent
To ensure ethical standards are upheld, all participants involved in this study and their parents/legal guardians have been provided with comprehensive information regarding the research objectives, procedures, potential risks, and benefits. Furthermore, each participant has voluntarily agreed to participate by providing explicit informed consent. Informed consent was obtained also from their parents/legal guardians.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on request.
References
Berry, D., Palmer, A. R., Distefano, R. & Masten, A. S. Autonomic complexity and emotion (dys-)regulation in early childhood across high- and low-risk contexts. Dev. Psychopathol. 31(3), 1173–1190. https://doi.org/10.1017/S0954579419000683 (2019).
WHO, ‘Mental health of adolescents’. Accessed 24 Oct 2023. https://www.who.int/news-room/fact-sheets/detail/adolescent-mental-health (2023).
Porges, S. W. Orienting in a defensive world: Mammalian modifications of our evolutionary heritage. A Polyvagal theory. Psychophysiology 32(4), 301–318. https://doi.org/10.1111/j.1469-8986.1995.tb01213.x (1995).
Porges, S. W. The polyvagal perspective. Biol. Psychol. 74(2), 116–143. https://doi.org/10.1016/j.biopsycho.2006.06.009 (2007).
Thayer, J. F. & Lane, R. D. A model of neurovisceral integration in emotion regulation and dysregulation. J. Affect. Disord. 61(3), 201–216. https://doi.org/10.1016/S0165-0327(00)00338-4 (2000).
Benarroch, E. E. The central autonomic network: Functional organization, dysfunction, and perspective. Mayo Clin. Proc. 68(10), 988–1001. https://doi.org/10.1016/s0025-6196(12)62272-1 (1993).
Yang, T. T. et al. Increased Amygdala activation is related to heart rate during emotion processing in adolescent subjects. Neurosci. Lett. 428(2–3), 109–114. https://doi.org/10.1016/j.neulet.2007.09.039 (2007).
Hänsel, A. & von Känel, R. The ventro-medial prefrontal cortex: A major link between the autonomic nervous system, regulation of emotion, and stress reactivity?. Biopsychosoc. Med. 2, 21. https://doi.org/10.1186/1751-0759-2-21 (2008).
Thayer, J. F. & Lane, R. D. Claude Bernard and the heart–brain connection: Further elaboration of a model of neurovisceral integration. Neurosci. Biobehav. Rev. 33(2), 81–88. https://doi.org/10.1016/j.neubiorev.2008.08.004 (2009).
Bujnakova, I. et al. Autism spectrum disorder is associated with autonomic underarousal. Physiol. Res. 65(Suppl 5), S673–S682. https://doi.org/10.33549/physiolres.933528 (2016).
Tonhajzerova, I. et al. Cardiac autonomic regulation is impaired in girls with major depression. Progr. Neuro-Psychopharmacol. Biol. Psychiatry 34(4), 613–618. https://doi.org/10.1016/j.pnpbp.2010.02.023 (2010).
Musser, E. D. et al. Emotion regulation via the autonomic nervous system in children with attention-deficit/hyperactivity disorder (ADHD). J. Abnorm. Child. Psychol. 39(6), 841–852. https://doi.org/10.1007/s10802-011-9499-1 (2011).
Weise, S. et al. Emotion dysregulation and resting-state autonomic function in adolescent borderline personality disorder-A multimodal assessment approach. Personal. Disord. 11(1), 46–53. https://doi.org/10.1037/per0000367 (2020).
Koch, C., Wilhelm, M., Salzmann, S., Rief, W. & Euteneuer, F. A meta-analysis of heart rate variability in major depression. Psychol. Med. 49(12), 1948–1957. https://doi.org/10.1017/S0033291719001351 (2019).
Kemp, A. H. et al. Impact of depression and antidepressant treatment on heart rate variability: A review and meta-analysis. Biol. Psychiatry 67(11), 1067–1074. https://doi.org/10.1016/j.biopsych.2009.12.012 (2010).
van Zyl, L. T., Hasegawa, T. & Nagata, K. Effects of antidepressant treatment on heart rate variability in major depression: A quantitative review. BioPsychoSocial Med. 2(1), 12. https://doi.org/10.1186/1751-0759-2-12 (2008).
Chen, S., Wang, H., Yue, J., Guan, N. & Wang, X. Intervention methods for improving reduced heart rate variability in patients with major depressive disorder: A systematic review and meta-analysis. Compr. Psychiatry 119, 152347. https://doi.org/10.1016/j.comppsych.2022.152347 (2022).
Koenig, J. et al. Increases in orbitofrontal cortex thickness following antidepressant treatment are associated with changes in resting state autonomic function in adolescents with major depression—Preliminary findings from a pilot study. Psychiatry Res. Neuroimaging 281, 35–42. https://doi.org/10.1016/j.pscychresns.2018.08.013 (2018).
Choi, K. W. & Jeon, H. J. Heart rate variability for the prediction of treatment response in major depressive disorder. Front. Psychiatry https://doi.org/10.3389/fpsyt.2020.00607 (2020).
D’Agostino, A., English, C. D. & Rey, J. A. Vortioxetine (Brintellix): A new serotonergic antidepressant. P T 40(1), 36–40 (2015).
Findling, R. L. et al. Pharmacokinetics and safety of vortioxetine in pediatric patients. J. Child Adolesc. Psychopharmacol. 27(6), 526–534. https://doi.org/10.1089/cap.2016.0155 (2017).
Findling, R. L. et al. A 6-month open-label extension study of vortioxetine in pediatric patients with depressive or anxiety disorders. J. Child Adolesc. Psychopharmacol. 28(1), 47–54. https://doi.org/10.1089/cap.2017.0047 (2018).
Findling, R. L. et al. Vortioxetine for major depressive disorder in adolescents: 12-week randomized, placebo-controlled, fluoxetine-referenced, fixed-dose study. J. Am. Acad. Child Adolesc. Psychiatry 61(9), 1106-1118.e2. https://doi.org/10.1016/j.jaac.2022.01.004 (2022).
de Vries, H., Oldenhuis, H., van der Schans, C., Sanderman, R. & Kamphuis, W. Does wearable-measured heart rate variability during sleep predict perceived morning mental and physical fitness?. Appl. Psychophysiol. Biofeedback 48(2), 247–257. https://doi.org/10.1007/s10484-022-09578-8 (2023).
Vanoli, E. et al. Heart rate variability during specific sleep stages. Circulation 91(7), 1918–1922. https://doi.org/10.1161/01.CIR.91.7.1918 (1995).
Du, Y. et al. Vortioxetine improves rapid eye movement sleep behavior disorder: A case report. Medicine (Baltimore) 99(26), e21003. https://doi.org/10.1097/MD.0000000000021003 (2020).
Mlyncekova, Z. et al. Vortioxetine effects on sleep architecture in treatment of comorbid depression in adolescent patient with Narcolepsy and cataplexy: Case report. Neuro Endocrinol. Lett. 42(8), 503–507 (2021).
Mahableshwarkar, A. R., Jacobsen, P. L., Chen, Y. & Simon, J. S. A randomised, double-blind, placebo-controlled, duloxetine-referenced study of the efficacy and tolerability of vortioxetine in the acute treatment of adults with generalised anxiety disorder. Int. J. Clin. Pract. 68(1), 49–59. https://doi.org/10.1111/ijcp.12328 (2014).
Licht, C. M. M., de Geus, E. J. C., van Dyck, R. & Penninx, B. W. J. H. Longitudinal evidence for unfavorable effects of antidepressants on heart rate variability. Biol. Psychiatry 68(9), 861–868. https://doi.org/10.1016/j.biopsych.2010.06.032 (2010).
Mezzacappa, E., Steingard, R., Kindlon, D., Philip Saul, J. & Earls, F. Tricyclic antidepressants and cardiac autonomic control in children and adolescents. J. Am. Acad. Child Adolesc. Psychiatry 37(1), 52–59. https://doi.org/10.1097/00004583-199801000-00017 (1998).
Srinivasan, K., Ashok, M. V., Vaz, M. & Yeragani, V. K. Effect of imipramine on linear and nonlinear measures of heart rate variability in children. Pediatr. Cardiol. 25(1), 20–25. https://doi.org/10.1007/s00246-003-0468-5 (2004).
Vloet, T. D. et al. Mean heart rate and parameters of heart rate variability in depressive children and the effects of antidepressant medication. Zeitschrift für Kinder- und Jugendpsychiatrie und Psychotherapie 47(3), 253–260. https://doi.org/10.1024/1422-4917/a000672 (2019).
Koenig, J., Kemp, A. H., Beauchaine, T. P., Thayer, J. F. & Kaess, M. Depression and resting state heart rate variability in children and adolescents—a systematic review and meta-analysis. Clin. Psychol. Rev. 46, 136–150. https://doi.org/10.1016/j.cpr.2016.04.013 (2016).
Herzig, D. et al. Reproducibility of heart rate variability is parameter and sleep stage dependent. Front. Physiol. 8, 1100. https://doi.org/10.3389/fphys.2017.01100 (2017).
Chouchou, F. & Desseilles, M. Heart rate variability: A tool to explore the sleeping brain?. Front. Neurosci. 8, 402. https://doi.org/10.3389/fnins.2014.00402 (2014).
Ramage, A. G. & Villalón, C. M. 5-Hydroxytryptamine and cardiovascular regulation. Trends Pharmacol. Sci. 29(9), 472–481. https://doi.org/10.1016/j.tips.2008.06.009 (2008).
Baumeister-Lingens, L. et al. Vagally-mediated heart rate variability and depression in children and adolescents—A meta-analytic update. J. Affect. Disord. 339, 237–255. https://doi.org/10.1016/j.jad.2023.07.027 (2023).
APA, (ed.) Diagnostic and Statistical Manual of Mental Disorders: DSM-5, 5th edn, (American Psychiatric Association, 2013).
Montgomery, S. A. & Asberg, M. A new depression scale designed to be sensitive to change. Br. J. Psychiatry 134, 382–389. https://doi.org/10.1192/bjp.134.4.382 (1979).
Quilty, L. C. et al. The structure of the Montgomery-Åsberg depression rating scale over the course of treatment for depression. Int. J. Methods Psychiatr. Res. 22(3), 175–184. https://doi.org/10.1002/mpr.1388 (2013).
Shailesh, J. et al. Psychometric evaluation of the CDRS and MADRS in assessing depressive symptoms in children. J. Am. Acad. Child Adolesc. Psychiatry 46(9), 1201–1212 (2007).
Kovacs, M. & Beck, A. An empirical-clinical approach toward a definition of childhood depression. In Depression in Childhood Diagnosis, Treatment and Conceptual Models (eds Schulterbrandt, J. G. & Raskin, A.) 1–25 (Raven Press, New York, 1977).
Matthey, S. & Petroversuski, P. The children’s depression inventory: Error in cutoff scores for screening purposes. Psychol. Assess. 14(2), 146–149. https://doi.org/10.1037/1040-3590.14.2.146 (2002).
Thompson, E. Hamilton rating scale for anxiety (HAM-A). Occup. Med. 65(7), 601. https://doi.org/10.1093/occmed/kqv054 (2015).
Clark, D. B. & Donovan, J. E. Reliability and validity of the hamilton anxiety rating scale in an adolescent sample. J. Am. Acad. Child Adolesc. Psychiatry 33(3), 354–360. https://doi.org/10.1097/00004583-199403000-00009 (1994).
Soldatos, C. R., Dikeos, D. G. & Paparrigopoulos, T. J. The diagnostic validity of the athens insomnia scale. J. Psychosom. Res. 55(3), 263–267. https://doi.org/10.1016/s0022-3999(02)00604-9 (2003).
Chung, K. F., Kan, K. K. K. & Yeung, W. F. Assessing insomnia in adolescents: Comparison of insomnia severity index, athens insomnia scale and sleep quality index. Sleep Med. 12(5), 463–470. https://doi.org/10.1016/j.sleep.2010.09.019 (2011).
Pandi-Perumal, S. R., Spence, D. W. & BaHammam, A. S. Polysomnography: An overview. In Primary Care Sleep Medicine: A Practical Guide (eds Pagel, J. F. & Pandi-Perumal, S. R.) 29–42 (Springer, New York, 2014).
Berry, R. B. et al. AASM scoring manual updates for 2017 (Version 2.4). J. Clin. Sleep Med. 13(5), 665–666. https://doi.org/10.5664/jcsm.6576 (2017).
Tarvainen, M. P., Ranta-Aho, P. O. & Karjalainen, P. A. An advanced detrending method with application to HRV analysis. IEEE Trans. Biomed. Eng. 49(2), 172–175. https://doi.org/10.1109/10.979357 (2002).
Shaffer, F. & Ginsberg, J. P. An overview of heart rate variability metrics and norms. Front. Public. Health 5, 258. https://doi.org/10.3389/fpubh.2017.00258 (2017).
Malik, M. et al. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Eur. Heart J. 17(3), 354–381. https://doi.org/10.1093/oxfordjournals.eurheartj.a014868 (1996).
Kleiger, R. E., Stein, P. K., Bosner, M. S. & Rottman, J. N. Time domain measurements of heart rate variability. Cardiol. Clin. 10(3), 487–498 (1992).
Acknowledgements
This work was supported by Scientific Grant Agency of the Ministry of Education, Science, Research and Sport of the Slovak Republic under grant VEGA 1/0048/24.
Author information
Authors and Affiliations
Contributions
I.O., I.H. and I.T. designed the study, P.H., I.H., D.F., N.F., Z.M., V.K., A.M. and T.K. performed clinical assessments of patients and polysomnography analyses, Z.V. and M.K. performed statistical analyses, P.H., M.K. and I.T. performed the literature search and wrote the first draft of the paper, I.T., I.O. and J.M. supervised the revision of the paper. All authors contributed to and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Krivosova, M., Hutka, P., Ondrejka, I. et al. Vortioxetine’s impact on the autonomic nervous system in depressed children and adolescents: analysis of the heart rate variability. Sci Rep 14, 14442 (2024). https://doi.org/10.1038/s41598-024-65278-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-65278-9
- Springer Nature Limited