Abstract
The study explored the impact of pretreatment serum albumin-to-alkaline phosphatase ratio (AAPR) and changes in tumor blood supply on pathological complete response (pCR) in breast cancer (BC) patients following neoadjuvant chemotherapy (NACT). Additionally, a nomogram for predicting pCR was established and validated. The study included BC patients undergoing NACT at Yongchuan Hospital of Chongqing Medical University from January 2019 to October 2023. We analyzed the correlation between pCR and clinicopathological factors, as well as tumor ultrasound features, using chi-square or Fisher's exact test. We developed and validated a nomogram predicting pCR based on regression analysis results. The study included 176 BC patients. Logistic regression analysis identified AAPR [odds ratio (OR) 2.616, 95% confidence interval (CI) 1.140–5.998, P = 0.023], changes in tumor blood supply after two NACT cycles (OR 2.247, 95%CI 1.071–4.716, P = 0.032), tumor histological grade (OR 3.843, 95%CI 1.286–10.659, P = 0.010), and HER2 status (OR 2.776, 95%CI 1.057–7.240, P = 0.038) as independent predictors of pCR after NACT. The nomogram, based on AAPR, changes in tumor blood supply after two NACT cycles, tumor histological grade, and HER2 status, demonstrated a good predictive capability.
Similar content being viewed by others
Introduction
BC has emerged as the most prevalent form of cancer globally, posing a significant threat to women's health and quality of life1. Preoperative NACT has become an essential option in the comprehensive treatment of BC patients, and its survival benefit has long been shown to be equivalent to that of postoperative adjuvant chemotherapy2. In addition, NACT not only achieves tumor downstaging, increases the chance of surgery, and improves the breast-conservation rate, but also provides an opportunity to assess the sensitivity of chemotherapy. This aids clinicians in promptly adjusting the treatment regimen according to the tumor response, thereby enhancing patient survival rates3. Numerous studies indicate that patients receiving NACT and achieving pCR tend to have a more favorable clinical prognosis4,5. However, BC is a heterogeneous disease, and not all patients derive benefits from NACT6. Delaying surgery may even increase the risk of disease progression in chemotherapy-resistant tumors. Studies examining and characterizing biomarkers for predicting pCR have been undertaken. However, most of these studies have emphasized prognostic biomarkers rather than predictors identified before treatment initiation or at NACT onset7,8,9. Consequently, there is a need to identify factors influencing pCR in BC patients after NACT and develop predictive models to identify patients most likely to benefit from NACT. Previous studies indicate that peripheral inflammation indicators, such as neutrophil-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR), and platelet-to-lymphocyte ratio (PLR), are linked to the prognosis of BC patients. Using these metrics, the rate of PCR in NACT patients can be predicted, though the findings are controversial10,11,12,13. Liver-synthesized albumin possesses immunomodulatory functions and can be considered a surrogate marker for systemic inflammation. Low albumin levels are additionally linked to an insufficient organismal anti-cancer response14,15. Alkaline phosphatase (ALP) is considered to be linked to systemic inflammation and tumor advancement16,17. The ALB-to-ALP ratio (AAPR), derived by dividing the ALB level by the ALP level, is a composite index associated with systemic inflammation and is a superior predictor of tumor prognosis compared to ALB or ALP individually18. AAPR is a novel marker initially identified as a prognostic factor in hepatocellular carcinoma19. In recent years, it has been linked to the prognosis of hepatocellular carcinoma, nasopharyngeal carcinoma, small cell lung cancer, renal cancer, and various other malignant tumors20,21,22,23,24. Although a retrospective study has shown that low AAPR values are associated with a poorer survival prognosis in BC, there is a lack of high-quality evidence to support this, and the value of AAPR values in predicting prognosis in BC is currently fraught with controversy25.
Despite the clinical ease of obtaining AAPR, there is a scarcity of studies utilizing AAPR to predict pCR rates in BC patients undergoing NACT. Clinical acquisition of ultrasound images of tumors is also straightforward. Patients' tumors are routinely assessed clinically before and during treatment, with breast ultrasound being the primary choice for evaluating tumors in BC patients due to its cost-effectiveness, non-invasiveness, and accuracy.Nevertheless, few studies have established predictive models for NACT efficacy based on ultrasound parameters and clinical indicators26. By integrating breast ultrasound with relevant clinicopathological indicators, we innovatively devised and validated a nomogram. This nomogram is based on AAPR values and changes in patients' tumor blood supply, aiming to predict the pCR in BC patients undergoing NACT.
Methods
Population
BC patients undergoing NACT at Yongchuan Hospital of Chongqing Medical University between January 2019 and October 2023 were included based on inclusion–exclusion criteria. Inclusion criteria were: (1) BC diagnosis confirmed by puncture pathology; (2) Completion of at least four cycles of NACT; (3) Subsequent surgery after NACT; (4) Availability of comprehensive clinicopathological and postoperative pathological data; (5) Assessment of the primary tumor using color doppler ultrasound before and after two cycles of NACT. Exclusion criteria were: (1) Antitumor therapy prior to NACT;(2) Locally advanced or distant metastases; (3) Lack of post-NACT surgery; (4) Inadequate clinicopathological and postoperative pathological data; (5) Non-assessment of the primary tumor by color doppler ultrasound before NACT or after two cycles of NACT. The study adhered to the principles of the Declaration of Helsinki and received approval from the Medical Research Ethics Committee of Yongchuan Hospital of Chongqing Medical University (No. 2023–003). The retrospective study did not involve patient privacy. Therefore, the waiver of informed consent was approved by the Ethics Committee of Yongchuan Hospital of Chongqing Medical University. The authors were not provided with information that could identify individual participants during or after data collection.
Clinicopathologic analysis
Prior to receiving NACT, patients underwent an assessment encompassing current and past disease history, age, body mass index (BMI), menstrual status, chemotherapy cycle, tumor size, lymph node status, ALB, ALP, AAPR, NLR, LMR in peripheral blood, hormone receptor (HR) status, human epidermal growth factor receptor 2 (HER2) status, Ki-67 index, NACT regimens, and other relevant information. Receiver operating characteristic (ROC) curves were utilized to calculate the optimal cut-off values for continuous variables. Subsequently, continuous variables were transformed into binary variables using the determined cut-off values. Clinical evaluation of breast tumors, encompassing tumor size and preoperative lymph node status, relied on breast ultrasound. Estrogen receptor (ER), progesterone receptor (PgR), HER2, and Ki-67 statuses were determined through immunohistochemistry (IHC) analysis of pre-treatment core biopsy specimens. HER2 status was classified as 0, 1, 2, or 3. HER2 was considered positive with a staining intensity score of3 + and considered negative with a score of 0 or 1 + . Furthermore, FISH analysis was used to determine HER2 status in tumors with a score of 2 + . Cancers with 1–100% of cells positive for ER/PgR expression were considered ER-positive/PgR-positive. The Ki-67 index was defined as the percentage of the total number of tumor cells (at least 1000) with nuclear staining over 10 high powered fields (× 40).
PCR was defined as no residual invasive cancer in the breast post-NACT and the absence of lymph node metastases during axillary surgery (ypT0ypN0) with no evidence of disease.
Ultrasound examination
The included patients underwent two-dimensional grey-scale ultrasound and color Doppler examinations before and after two cycles of NACT. Two breast ultrasound specialists independently reviewed all ultrasound images. Standard ultrasound characteristics of tumors in patients before and after two cycles of NACT were documented. These features encompass tumor size, morphology, borders, internal echogenicity, posterior echogenicity, Alder grading of blood flow signals, calcification, and changes in these ultrasound features.
Statistical analyses
Statistical analyses were conducted utilizing R software (version 4.0.2) and SPSS (version 26.0). The maximum Youden index was employed to ascertain the optimal cut-off value, converting continuous variables into binary variables based on this determined threshold. Differences between the two groups were analyzed using the chi-square test or Fisher's exact test. Univariate and multivariate logistic regression analyses were employed to identify factors associated with pCR after NACT. Subsequently, based on the multivariate logistic regression analysis results, a nomogram prediction model for pCR after NACT was developed. The discriminatory performance of the nomogram was quantified through the measurement of Harrell's C-index. The model's discriminatory abilities were evaluated by measuring the area under the receiver operating characteristic (ROC) curve. A calibration curve was drawn to evaluate its calibration effect. Additionally, decision curve analysis was conducted to assess the net benefit of the nomogram. A significance level of P < 0.05 was considered statistically significant.
Ethical approval and consent to participate
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Yongchuan Hospital of Chongqing Medical University (No. 2023–003). The retrospective study did not involve patient privacy. Therefore, the waiver of informed consent was approved by the Ethics Committee of Yongchuan Hospital of Chongqing Medical University. The authors were not provided with information that could identify individual participants during or after data collection.
Result
Clinicopathological and ultrasound characteristics of BC patients received NACT
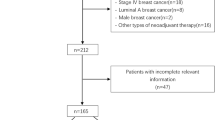
Our study finally included 176 patients, with a mean age of 49.56 ± 8.65 years. (Fig. 1)The optimal cut-off value for the AAPR was 0.881. All patients underwent ultrasound assessment of the primary tumor both before and after two cycles of NACT, followed by surgical intervention post-NACT. Postoperative pathology verified that 68 patients (38.64%) achieved pCR. Tables 1, 2 present the patients' clinicopathological characteristics and the tumors' ultrasonographic features. The chi-square test showed that serum albumin concentration, histological grading of the tumor, HER2 status and changes in tumor blood supply after two therapy cycles were significantly associated with the pcr rate in bc patients after NACT.
Logistic regression analysis for detecting the factors related to pCR after NACT
Univariate logistic regression analyses were conducted to identify factors potentially influencing pCR in BC patients after NACT. The results showed that serum albumin concentration, histological grading of the tumor, HER2 status, and tumor blood supply changes after two NACT cycles were significantly associated with the PCR rate in BC patients after NACT. Subsequently, the factors (P < 0.1) were included in the multivariate analysis. Our findings revealed that AAPR (P = 0.023), tumor histological grade (P = 0.010), HER2 status (P = 0.038), and changes in tumor blood supply (P = 0.032) independently predicted pCR in BC patients after NACT. (Table 3). Among them, AAPR and tumor histological grading were positively correlated with PCR; HER2 positivity and decreased tumor blood supply after two cycles of NACT were associated with a higher PCR.
Establishment and evaluation of the nomogram model
Using risk factors identified through multifactorial logistic regression analysis, we developed a nomogram for predicting pCR after NACT. The ROC curve was generated based on this model, yielding an area under the curve (AUC) of 0.803 (95% CI 0.723–0.884) (Fig. 2). The predictive model's concordance index (c-index) is 0.803, signifying its good discriminatory power. The calibration plot demonstrated good concordance between the predictions and actual observations (Fig. 3). The decision curve analysis (DCA) also indicated that the nomogram's pCR prediction results exhibited high accuracy for predicting pCR in NACT patients (Fig. 4). These findings imply that the nomogram is effective in predicting the pCR rate in BC patients receiving NACT (Fig. 5).
Discussion
NACT is currently widely employed in BC treatment, with robust evidence indicating that achieving pCR after NACT is indicative of a favorable prognosis27,28. Our study assessed the predictive significance of the AAPR and changes in tumor blood flow for pCR in BC patients undergoing NACT. We also investigated other factors that could impact pCR. Ultimately, a nomogram prediction model based on AAPR and changes in tumor blood supply was developed using logistic regression analysis results.
Our investigation revealed that pre-chemotherapy AAPR is an independent predictor of the response to NACT in BC patients, with AAPR values demonstrating a positive correlation with pCR rates. AAPR is a novel, convenient, cost-effective, and non-invasive indicator calculated from ALB and ALP levels, providing insights into systemic inflammation and nutritional status. ALB plays a role in maintaining the equilibrium of cell proliferation and metabolism, and reduced ALB levels might be linked to compromised immune function, impacting the effectiveness of antitumor therapy21,29.
Prior research has demonstrated that pretreatment ALB is a prognostic factor in diverse cancers such as lung, pancreatic, gastric, and colorectal30. In the univariate analysis of our study, ALB exhibited a positive correlation with pCR rates (P = 0.049). While our study did not reveal a significant correlation between ALP and pCR, pretreatment serum ALP levels were identified as an independent prognostic factor for disease-free survival and overall survival in triple-negative breast cancer (TNBC) patients in Chen et al.'s study31. Initially recognized as a prognostic factor for hepatocellular carcinoma, the AAPR, calculated from ALB and ALP, has been identified as a prognostic predictor for various cancers. Nevertheless, further exploration is required to fully understand the predictive value of the AAPR in BC prognosis. Long et al. conducted a retrospective study analyzing overall survival (OS) in 746 non-metastatic BC patients, revealing that a lower albumin-to-alkaline phosphatase ratio (AAPR) was linked to a shorter OS25. QU et al. explored the predictive value of the AAPR for pCR in BC patients undergoing NACT. Their findings revealed that the AAPR was an independent predictor positively associated with the pCR rate. This study corroborated our findings, and the concordance index (C-index) of the prediction model was comparable in both studies: 0.803 (95% CI 0.723–0.884) in our study vs. 0.792 (95% CI 0.737–0.848) in QU et al.'s study32. The results of this study provide additional support for the prognostic predictive value of the AAPR in BC.
Our study also found that changes in tumor blood supply observed by ultrasound after two chemotherapy cycles were independent predictors of response to NACT treatment in BC patients. Patients with decreased tumor blood supply after two cycles of chemotherapy were more likely to achieve PCR. Information regarding the patient's tumor blood flow could be readily obtained through color Doppler ultrasound before and during NACT. Research indicates that ultrasound can dynamically monitor tumor changes during NACT and evaluate the treatment's efficacy, enabling timely adjustments to the treatment plan if necessary33. Changes in ultrasound parameters during treatment provide more accurate predictions of the extent of pathological remission in NACT compared to ultrasound parameters assessed before treatment in the patient's tumor. Chen et al.'s study demonstrated a significant correlation between a decrease in tumor blood supply, evaluated through ultrasound during chemotherapy, and a higher rate of pCR, consistent with our findings26. However, the distinction lies in the fact that Chen et al. evaluated changes in tumor blood supply before and after the entire NACT cycle (4–8 cycles), while our study specifically assessed changes after two cycles of chemotherapy. In contrast, our study anticipated the neoadjuvant efficacy of patients earlier, facilitating earlier adjustments to the treatment plan for more significant patient benefit. Stevens et al. employed enhanced MRI to explore the predictive value of changes in tumor blood flow after one NACT cycle for pathological response. Regrettably, the study did not find a predictive association between changes in breast tumor blood flow after one cycle and pathological response34. Moreover, enhanced magnetic resonance imaging is costly and necessitates contrast injection, while ultrasound is non-invasive, cost-effective, convenient, and rapid. Ultrasound is commonly selected for follow-up assessments of the primary tumor in patients undergoing NACT. Thus, the changes in tumor blood flow are easily accessible parameters in clinical practice. This approach avoids additional economic burdens on BC patients undergoing NACT while evaluating and predicting treatment outcomes.
Apart from AAPR and changes in tumor blood supply after two cycles, multifactorial analysis revealed HER2 status and tumor histological grading as independent predictors. In line with earlier observations, patients with positive HER2 status and high tumor histological grading exhibited elevated rates of pCR4,35. Utilizing the readily available clinical indicators mentioned above, we constructed a nomogram to predict pCR in BC patients undergoing NACT. To our knowledge, this is the first model that combines AAPR with changes in tumor blood supply to predict pCR in BC patients receiving NACT.
The nomogram is a simple and effective tool for predicting prognosis. The C-index of the nomogram was 0.803 (95% CI 0.723–0.884), and the AUC value of the ROC curve was 0.803, which indicated that the nomogram prediction model had good accuracy in the prediction of pCR. In addition, the calibration plot and DCA curve also indicated that the prediction model had good predictive ability. Therefore, this study provides a novel, simple, and feasible prediction model for BC patients with pCR after NACT.
However, there are limitations in this study. Firstly, BC is a heterogeneous disease, and the relatively small sample size hinders the execution of subgroup analyses, particularly for independent investigations of hormone receptor-positive BC with low pCR rates. Secondly, the study population was drawn from a single center and solely validated through internal validation. Additional external validation from diverse centers is essential to assess the model's accuracy. Finally, this study was retrospective, posing challenges in obtaining more comprehensive clinical information. The analysis focused only on common clinicopathological factors, laboratory indicators, and ultrasound parameters. Thus, enlarging the sample size and incorporating more indicators are imperative for enhancing the prediction model in subsequent research.
Conclusion
This study developed a simple, innovative, and easily applicable nomogram prediction model utilizing four independent predictors: AAPR, changes in tumor blood flow, HER2 status, and tumor histological grading. The model demonstrates effective predictive capabilities for pCR after NACT, integrating changes in ultrasound image parameters with clinicopathological indicators. Further exploration of its potential is warranted in future research.
Data availability
Any request for data and materials should be made in writing to the corresponding author, and these will be considered.
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71(3), 209–249. https://doi.org/10.3322/caac.21660 (2021).
Rastogi, P. et al. Preoperative chemotherapy: updates of national surgical adjuvant breast and bowel project protocols B-18 and B-27. J. Clin. Oncol. 26(5), 778–785. https://doi.org/10.1200/jco.2007.15.0235 (2008).
Tang, L., Shu, X. & Tu, G. Exploring the influencing factors of the pathologic complete response in estrogen receptor-positive, HER2-negative breast cancer after neoadjuvant chemotherapy: a retrospective study. World J. Surg. Oncol. 20(1), 27. https://doi.org/10.1186/s12957-022-02492-7 (2022).
Spring, L. M. et al. Pathologic complete response after neoadjuvant chemotherapy and impact on breast cancer recurrence and survival: a comprehensive meta-analysis. Clin. Cancer Res. 26(12), 2838–2848. https://doi.org/10.1158/1078-0432.Ccr-19-3492 (2020).
Feng, K. et al. A review of studies on omitting surgery after neoadjuvant chemotherapy in breast cancer. Am. J. Cancer Res. 12(8), 3512–3531 (2022).
von Minckwitz, G. et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J. Clin. Oncol. 30(15), 1796–1804. https://doi.org/10.1200/jco.2011.38.8595 (2012).
Fournier, M. V. et al. A predictor of pathological complete response to neoadjuvant chemotherapy stratifies triple negative breast cancer patients with high risk of recurrence. Sci. Rep. 9(1), 14863. https://doi.org/10.1038/s41598-019-51335-1 (2019).
Hatzis, C. et al. A genomic predictor of response and survival following taxane-anthracycline chemotherapy for invasive breast cancer. Jama 305(18), 1873–1881. https://doi.org/10.1001/jama.2011.593 (2011).
Chung, W. S. et al. An integrative clinical model for the prediction of pathological complete response in patients with operable stage II and stage III triple-negative breast cancer receiving neoadjuvant chemotherapy. Cancers 14(17), 4170. https://doi.org/10.3390/cancers14174170 (2022).
Marín Hernández, C. et al. Usefulness of lymphocyte-to-monocyte, neutrophil-to-monocyte and neutrophil-to-lymphocyte ratios as prognostic markers in breast cancer patients treated with neoadjuvant chemotherapy. Clin. Transl. Oncol. 20(4), 476–483. https://doi.org/10.1007/s12094-017-1732-0 (2018).
Zhang, F. et al. A nomogram to predict the pathologic complete response of neoadjuvant chemotherapy in triple-negative breast cancer based on simple laboratory indicators. Ann. Surg. Oncol. 26(12), 3912–3919. https://doi.org/10.1245/s10434-019-07655-7 (2019).
Graziano, V. et al. Combination of peripheral neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio is predictive of pathological complete response after neoadjuvant chemotherapy in breast cancer patients. Breast 44, 33–38. https://doi.org/10.1016/j.breast.2018.12.014 (2019).
Acikgoz, O. et al. Pretreatment platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio as a predictor of pathological complete response to neoadjuvant chemotherapy in patients with breast cancer: single center experience from Turkey. Anticancer Drugs 33(10), 1150–1155. https://doi.org/10.1097/cad.0000000000001389 (2022).
Bernardi, M. et al. Albumin in decompensated cirrhosis: new concepts and perspectives. Gut 69(6), 1127–1138. https://doi.org/10.1136/gutjnl-2019-318843 (2020).
Garcia-Martinez, R. et al. Immunomodulatory and antioxidant function of albumin stabilises the endothelium and improves survival in a rodent model of chronic liver failure. J. Hepatol. 62(4), 799–806. https://doi.org/10.1016/j.jhep.2014.10.031 (2015).
Xiao, Y. et al. Dynamic serum alkaline phosphatase is an indicator of overall survival in pancreatic cancer. BMC Cancer 19(1), 785. https://doi.org/10.1186/s12885-019-6004-7 (2019).
Karhade, A. V. et al. Prognostic value of serum alkaline phosphatase in spinal metastatic disease. Br. J. Cancer 120(6), 640–646. https://doi.org/10.1038/s41416-019-0407-8 (2019).
Li, B. et al. Prognostic value of a nomogram based on the dynamic albumin-to-alkaline phosphatase ratio for patients with extensive-stage small-cell lung cancer. Onco. Targets Ther. 13, 9043–9057. https://doi.org/10.2147/ott.S262084 (2020).
Chan, A. W. et al. Albumin-to-alkaline phosphatase ratio: a novel prognostic index for hepatocellular carcinoma. Dis. Markers 2015, 564057. https://doi.org/10.1155/2015/564057 (2015).
Zhang, F. et al. Albumin-to-alkaline phosphatase ratio as a predictor of tumor recurrence and prognosis in patients with early-stage hepatocellular carcinoma undergoing radiofrequency ablation as initial therapy. Int. J. Hyperthermia 38(1), 1–10. https://doi.org/10.1080/02656736.2020.1850885 (2021).
Kim, J. S. et al. The prognostic value of albumin-to-alkaline phosphatase ratio before radical radiotherapy in patients with non-metastatic nasopharyngeal carcinoma: a propensity score matching analysis. Cancer Res. Treat. 51(4), 1313–1323. https://doi.org/10.4143/crt.2018.503 (2019).
Zhou, S., Wang, H., Jiang, W., Yu, Q. & Zeng, A. Prognostic value of pretreatment albumin-to-alkaline phosphatase ratio in extensive-disease small-cell lung cancer: a retrospective cohort study. Cancer Manag. Res. 12, 2015–2024. https://doi.org/10.2147/cmar.S247967 (2020).
Won, I., Shim, S. R., Kim, S. I., Kim, S. J. & Cho, D. S. Albumin-to-alkaline phosphatase ratio as a novel prognostic factor in patients undergoing nephrectomy for non-metastatic renal cell carcinoma: propensity score matching analysis. Clin. Genitourin. Cancer 20(3), e253–e262. https://doi.org/10.1016/j.clgc.2022.01.012 (2022).
Zhao, M. et al. Prognostic value of preoperative albumin-to-alkaline phosphatase ratio in patients with muscle-invasive bladder cancer after radical cystectomy. Onco. Targets Ther. 13, 13265–13274. https://doi.org/10.2147/ott.S285098 (2020).
Long, Z. Q. et al. Prognostic impact of the pretreatment albumin to alkaline phosphatase ratio for nonmetastatic breast cancer patients. Cancer Manag. Res. 11, 4809–4814. https://doi.org/10.2147/cmar.S200759 (2019).
Chen, H. et al. The role of nomogram based on the combination of ultrasound parameters and clinical indicators in the degree of pathological remission of breast cancer. J. Oncol. 2023, 3077180. https://doi.org/10.1155/2023/3077180 (2023).
Xia, L. Y., Hu, Q. L., Zhang, J., Xu, W. Y. & Li, X. S. Survival outcomes of neoadjuvant versus adjuvant chemotherapy in triple-negative breast cancer: a meta-analysis of 36,480 cases. World J. Surg. Oncol. 18(1), 129. https://doi.org/10.1186/s12957-020-01907-7 (2020).
van Mackelenbergh, M. T. et al. Outcome after neoadjuvant chemotherapy in estrogen receptor-positive and progesterone receptor-negative breast cancer patients: a pooled analysis of individual patient data from ten prospectively randomized controlled neoadjuvant trials. Breast Cancer Res. Treat. 167(1), 59–71. https://doi.org/10.1007/s10549-017-4480-5 (2018).
Nojiri, S. & Joh, T. Albumin suppresses human hepatocellular carcinoma proliferation and the cell cycle. Int. J. Mol. Sci. 15(3), 5163–5174. https://doi.org/10.3390/ijms15035163 (2014).
Gupta, D. & Lis, C. G. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr. J. 9, 69. https://doi.org/10.1186/1475-2891-9-69 (2010).
Chen, B. et al. Pre-treatment serum alkaline phosphatase and lactate dehydrogenase as prognostic factors in triple negative breast cancer. J. Cancer. 7(15), 2309–2316. https://doi.org/10.7150/jca.16622 (2016).
Qu, F. et al. Construction and validation of a serum albumin-to-alkaline phosphatase ratio-based nomogram for predicting pathological complete response in breast cancer. Front Oncol. 11, 681905. https://doi.org/10.3389/fonc.2021.681905 (2021).
Song, L. et al. Diagnostic evaluations of ultrasound and magnetic resonance imaging in mammary duct ectasia and breast cancer. Oncol. Lett. 15(2), 1698–1706. https://doi.org/10.3892/ol.2017.7514 (2018).
Stevens, W. et al. Breast tumour volume and blood flow measured by MRI after one cycle of epirubicin and cyclophosphamide-based neoadjuvant chemotherapy as predictors of pathological response. Br. J. Radiol. 94(1123), 20201396. https://doi.org/10.1259/bjr.20201396 (2021).
Ma, R. et al. A nomogram based on platelet-to-lymphocyte ratio for predicting pathological complete response of breast cancer after neoadjuvant chemotherapy. BMC Cancer 23(1), 245. https://doi.org/10.1186/s12885-023-10703-x (2023).
Acknowledgements
We thank all the investigators who participated in this study.
Funding
This work was supported by the Life Oasis Public Service Center, Qujiang District, Quzhou City, China, under Grant number [KM-ZLGJ-018].
Author information
Authors and Affiliations
Contributions
Tingjian Zhang and Yuyao Liu contributed equally to this study. All authors contributed to the study's conception and design. Tingjian Zhang, Yuyao Liu, and TianTian performed material preparation, data collection, and analysis. Tingjian Zhang wrote the first draft of the manuscript, and all authors commented on previous versions. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, T., Liu, Y. & Tian, T. Predicting pathological complete response after neoadjuvant chemotherapy in breast cancer by clinicopathological indicators and ultrasound parameters using a nomogram. Sci Rep 14, 16348 (2024). https://doi.org/10.1038/s41598-024-64766-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-64766-2
- Springer Nature Limited