Abstract
The incidence of ulcerative colitis (UC) is on the rise globally. Shen-Zhu-Lian-Bai decoction (SZLBD) can relieve the clinical symptoms of UC. This study aimed to investigate the underlying molecular mechanism of SZLBD in the treatment of UC. The key treatment targets of SZLBD for UC were obtained based on the online database, and combined with the STRING database and Cytoscape 3.7.2 software, PPI network was constructed and visualized. The GEO database was utilized to validate the expression levels of core targets in UC. Metascape database GO functional annotation and KEGG pathway enrichment analysis. Molecular docking technology was used to verify the docking of core compounds with key targets. RT-qPCR and Western Blot were used to detect the expression of key targets in HCoEpiC cells for verification. After screening, 67 targets shared by SZLBD and UC were obtained. It is predicted that IL-6, IL-1B, and AKT1 might be the key targets of SZLBD in the treatment of UC. Quercetin was the main active ingredient. GEO results showed that the expression levels of IL-6, IL-1B and AKT1 were higher in the UC group compared to the control group. GO and KEGG analyses showed that these targets were related to apoptosis and inflammation. The results of molecular docking demonstrated that the AKT1 gene, a key target of quercetin, had the highest affinity of -9.2 kcal/mol. Cell experiments found that quercetin could affect the expression of IL-6, IL-1B, and AKT1. This study preliminarily explored and verified the mechanism of action of SZLBD in the treatment of UC, which provides a theoretical basis for subsequent in vivo mechanism studies.
Similar content being viewed by others
Introduction
Ulcerative colitis (UC) is a chronic inflammatory disease of the colon that typically presents with abdominal pain, diarrhea, and blood in the stool1. It is characterized by recurrence and remission of mucosal inflammation that begins in the rectum and extends near the colon. Endoscopic biopsy is the only way to diagnose UC2. Currently, the incidence of UC is on the rise globally2. Although the exact pathogenesis of UC is unknown, several factors, such as defects in the colonic epithelium, the mucus barrier, and the epithelial barrier3 have been identified to affect UC development. Medications for UC include 5-aminosalicylic acid drugs (5-ASA), steroids, and immunosuppressant. Although Western medicine can achieve certain an effect, the effect is not ideal4.
Many herbs have shown significant advantages in treating UC5. The commonly used traditional Chinese medicines (TCM) formulas for the treatment of UC include Ge-Gen-Qin-Lian decoction (GGQLD)6 and Shen-Ling-Bai-Zhu-San (SLBZS)7. Based on these two formulas, we combined clinical conditions to develop the TCM formula Shen-Zhu-Lian-Bai decoction (SZLBD). SZLBD is composed of Coptis chinensis Franch. (Huanglian), Phellodendron chinense Schneid. (Huangbai), Atractylodes macrocephala Koidz. (Baizhu), Portulacae oleracea L. (Machixian), Citrus reticulata Blanco (Chenpi), Codonopsis pilosula (Franch.) Nannf. (Dangshen), Paeonia lactiflora Pall. (Baishao). Huanglian and its active ingredient berberine have been proved to treat UC8. The ingredient of Huangbai can treat UC9. Baizhu Shaoyao San (BSS) composed of Baizhu, Baishao and Chenpi, so on, which can be used to treat UC10. Dangshen combined with astragalus polysaccharide (APS) can improve colitis in mice11. The above research indicates that the components of SZLBD play a role in treating UC. Therefore, we believe that SZLBD may have a good therapeutic effect on UC, but its related mechanism of action is still unclear.
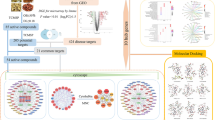
In this study, TCM formula SZLBD was used as the research object, and network pharmacology and molecular docking methods were applied to predict the effective compounds, key targets and signaling pathways of SZLBD in the treatment of UC, which were verified by cell experiments. The specific flow chart is shown in Fig. 1.
Materials and methods
Screening of active compounds in SZLBD
The active compounds of 7 TCM in SZLBD were searched in the Traditional Chinese Medicine Systems Pharmacology (TCMSP) database (http://tcmspw.com/), and each compounds was screened according to the criteria of by the oral bioavailability (OB) ≥ 30% and drug likeness (DL) ≥ 0.18.
Screening of common targets of SZLBD and UC
The active compounds of SZLBD and their target information were retrieved from the TCMSP database, and the targets were converted into the corresponding human gene names using the STRING database (https://string-db.org/). UC-associated targets were obtained from the GeneCards database (https://www.genecards.org/) and the NCBI database by entering the keyword “ulcerative colitis”. All targets were limited to Homo sapiens. Next, the common targets of GeneCards and NCBI databases were retained as UC-related targets. Finally, the intersection targets of SZLBD-related targets and UC-related targets were considered as potential therapeutic target for SZLBD in the treatment of UC.
Protein–protein interaction (PPI) networks construction
The common targets of SZLBD and UC were entered into the STRING database (https://string-db.org/) for PPI network construction. The nodes and edges of the network represent proteins and protein–protein associations, respectively. PPI networks were visualized using Cytoscape 3.7.2 software, and topological features were analyzed to screen core targets.
GEO verification
We validated the expression levels of these core targets in UC using an external dataset from the Gene Expression Omnibus (GEO). We utilized the "limma" package in R language to obtain the expression levels of core genes in both the normal and UC groups. We then generated box plots using the Sangerbox online platform (http://sangerbox.com/home.html).
GO functional annotation and KEGG signaling pathway enrichment analysis
In order to understand the biological functions and related signaling pathways involved in the common targets of SZLBD and UC, GO functional annotation (including biological process (BP), cell ingredient (CC) and molecular function (MF)) and KEGG signaling pathway enrichment analysis were performed for the common targets using Metascape database (https://metascape.org/). In the end, there are only p-values < 0.01, count > 3, enrichment factor (enrichment factor is the ratio between observed counts and counts expected by chance) > 1.5 is considered a significant enrichment result12,13.
Molecular docking
The molecular structure of the core active compounds were downloaded from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/). The protein crystal structure of the key targets were obtained from the RCSB PDB database (PDB, https://www.rcsb.org/). The structure of compounds and proteins were processed by AutoDockTools (version 1.5.6). Optimal combinations of docking score were visualized by PyMOL.
Experimental verification
Cell culture and quercetin administration
Human colonic epithelial cells (HCoEpiC) were purchased from Saibaikang Company (Shanghai, China). HCoEpiC cells were cultured at 37 °C under 5% CO2-humidified air in DMEM supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin and 100 μg/mL streptomycin14.
Screening of LPS concentration
HCoEpiC was made into 5 × 104 cells/mL suspension, inoculated in culture plates or culture flasks, and cultured in a 37 °C incubator with 5% CO2 for 24 h. Cells were treated with different concentrations of LPS (5, 10, 15 μg/mL). After 24 h, cell viability was assessed using the CCK-8 assay by measuring absorbance in each well to calculate cell survival rates.
Quercetin cytotoxicity test
Quercetin was purchased from MCE Company. The cells were randomly divided into 7 groups, i.e., the normal group, the model group, and the quercetin-added group (0, 20, 40, 60, and 80 μM). Except the normal group, all groups were given LPS with a final concentration of 10 μg/mL to create an inflammatory injury model. In addition to the normal group and the model group, each group was added with the corresponding test drug and incubated at 37 °C with 5% CO2 for 24 h. Cell viability was assessed using the CCK-8 assay by measuring absorbance in each well to calculate cell survival rates.
Real-time quantitative PCR (RT-qPCR)
Total cellular RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Subsequently, cDNA was synthesized using a PrimeScript RT reagent kit (Takara Biotechnology, Dalian, China). Real-time PCR was then performed using an ABI 7500 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). The primer sequences are shown in Table 1.
Western blot analysis
The cells were lysed using RIPA lysis buffer after treatment. After centrifugation at 4 °C and 3000 rpm for 10 min, the supernatant was collected. Subsequently, protein lysates were separated and electrophoresed on PVDF membranes using 10% SDS-PAGE. The PVDF membranes were then incubated in 5% skim milk TBST solution for 2 h, followed by washing with TBST solution without skim milk three times for 10 min each. Afterwards, the membranes were incubated overnight at 4 °C with IL-6, IL-1B, and AKT1 rabbit polyclonal antibodies (sourced respectively from ZEN BIO, proteintech, and ZEN BIO). The membranes were moistened with TBST solution three times for 10 min each, followed by incubation at room temperature for 1.5 h in HRP-labeled goat anti-rabbit IgG solution (dilution ratio: 1/20,000). Finally, the membranes were washed with TBST solution three times for 10 min each, and then tested using a Tanon chemiluminescence imager. The images were quantitatively analyzed using ImageJ software.
Results
Screening results of active compounds in SZLBD
A total of 90 active compounds of SZLBD were obtained based on the TCMSP database and the screening criteria of OB ≥ 30% and DL ≥ 0.18 (Table 2). Combined with the TCMSP database, 185 SZLBD related targets were obtained, and the ingredients—compounds -target network was shown in Fig. 2. As a result, the main active compounds of SZLBD included quercetin and kaempferol, among others, each of which regulated multiple targets.
Common targets of SZLBD and UC
According to GeneCards and NCBI databases, 547 UC-related genes were obtained. A total of 67 common targets of SZLBD-UC were obtained by intersecting SZLBD-related targets and UC-related targets. The UC-herb-ingredient-target network is displayed in Fig. 3.
Common targets of SZLBD and UC. The red represents UC (ulcerative colitis), the teal represents traditional Chinese medicine (HL, Huanglian; CP, Chenpi; BZ, Baizhu; HB, Huangbai; MCX, Machixian; BS, Baishao; DS, Dangshen), the pink represents active ingredients, and the blue represents the targets of SZLBD acting on UC.
PPI network of SZLBD-UC
A PPI network with 67 nodes and 912 edges was constructed by using the STRING database and visualized through Cytoscape software (Fig. 4). Based on the topological analysis results of PPI network, we found that IL-6, IL-1B and AKT1 had high degree values, so we speculated that the above targets may be the key targets for SZLBD treatment of UC.
GEO verification
Based on the GSE75214 dataset (which includes 11 control samples and 97 UC patient samples), we analyzed the expression levels of IL-6, IL-1B, and AKT1 between the control and UC groups. The results showed that compared to the control group, the expression levels of IL-6, IL-1B, and AKT1 were higher in the UC group (Fig. 5).
GO functional annotation and KEGG signaling pathway enrichment analysis
A total of 1186 items were obtained from GO functional annotation analysis, and the significant enrichment results are shown in Fig. 6. Among them, there were 1072 BP entries (Fig. 6A), mainly involving inflammatory response, positive regulation of cell death and regulation of inflammatory response, etc. There were 34 CC entries (Fig. 6B), mainly involving membrane raft, transcription regulator complex and extracellular matrix, etc. There were 80 MF entries (Fig. 6C), involving cytokine activity, RNA polymerase II-specific DNA-binding transcription factor binding and protein homodimerization activity, etc.
A total of 138 pathways were obtained from KEGG signaling pathway enrichment analysis, and the significant results are shown in Fig. 5D. Signaling pathways associated with UC therapy mainly include IL-17 signaling pathway, PI3K-Akt signaling pathway and inflammatory bowel disease, etc.
Molecular docking
In this study, the main active ingredient quercetin was selected as the ligand molecule and the key targets IL-6, IL-1B and AKT1 were selected as receptor molecules for molecular docking, and the results showed that quercetin had a strong affinity with IL-6, IL-1B and AKT1 (Fig. 7). Quercetin and IL-6 formed stable complexes by interacting with amino acid GLU127, ARG141, GLU137 and GLN130 (Fig. 7A). Quercetin mainly interacted with amino acid residues ASN92, LYS148, CLU149, PRO176 and TYR175 of IL-1B (Fig. 7B). In addition, quercetin mainly interacted with AKT1 residues LEU52, GLU49, ARG48, CLY327, ARG328, TYR326, ALA329 and GLY393 (Fig. 7C). The molecular docking scores of quercetin with IL-6, IL-1B and AKT1 proteins were − 7.2 kcal/mol, − 7.0 kcal/mol and − 9.2 kcal/mol, respectively. Based on molecular docking, it was preliminarily verified that quercetin had good binding ability to IL-6, IL-1B and AKT1, which further proved the reliability of the prediction results of the network pharmacology.
Experimental verification
In order to verify that quercetin can interact with IL-6, IL-1B and AKT1 to participate in the treatment of UC by SZLBD, cellular validation was performed in this study. Firstly, an inflammatory cell model was constructed by treating with DMEM containing different concentrations of LPS (5, 10, 15 μg/mL) for 24 h. Cell viability was determined, and the optimal concentration was determined to be 10 μg/mL (Fig. 8A). After treating with LPS (10 μg/mL) for 24 h, cells were treated with different concentrations of quercetin (0, 20, 40, 60, and 80 μM) for 24 h, and cell viability was measured to determine the optimal concentration, which was found to be 40 μg/mL (Fig. 8B). We examined the effect of quercetin on inflammatory factors in HCoEpiC cells. Compared with the control group, the mRNA and protein expression levels of IL-6, IL-1B, and AKT1 were significantly increased in the model group. Compared with the model group, the mRNA and protein expression levels of IL-6, IL-1B, and AKT1were significantly decreased in the quercetin-treated group (Fig. 8C–I).
Quercetin improves LPS-induced inflammatory damage of HCoEpiC cells. (A) The choice of LPS safety and effective concentration; (B) The choice of quercetin safety and effective concentration; (C) Levels of mRNA expression of IL-6; (D) Levels of mRNA expression of IL-1B; (E) Levels of mRNA expression of AKT1; (F–I) Expression of IL-6, IL-1B and AKT1 proteins. *p < 0.05,**p < 0.01,***p < 0.001.
Discussion
Literature has reported that Huanglian significantly inhibits the level of proinflammatory cytokines (IL-17) in the treatment of UC15. It has been found that Huangbai can improve chronic inflammatory injury caused by endothelial dysfunction by regulating the PI3K-Akt-mTOR signaling pathway16. Baizhu and Chenpi are the main ingredients of Baizhu Shaoyao San, another Chinese medicine for the treatment of UC, which can regulate inflammatory factors and intestinal flora17. Machixian has anti-ulcer and anti-inflammatory effects. Studies have shown that Machixian can reduce the symptoms of colitis18. Dangshen and Baishao are herbs from TCM formulas Zhen-Wu-Bu-Qi Decoction for the treatment of UC19, which exhibit anti-inflammatory properties by modulating the PI3K-AKT, MAPK signaling pathway, and NF-κB signaling pathway. In our study, 90 active compounds and 185 targets of SZLBD were identified from the TCMSP database. The compounds-targets network diagram shows the potential synergies between multiple ingredients and their targets. Quercetin, the main active ingredient, comes from Huanglian, Huangbai and Machixian. In conclusion, these three herbs of SZLBD can treat and improve UC by acting on the IL-17 and PI3K-Akt signaling pathways, which is consistent with and more complete than previous studies.
Quercetin (3, 5, 7, 3’, 4’-pentahydroxyflavonoid) is one of the main representatives of flavonoids. In plants, it exists mainly in the form of glycosides. In addition, quercetin has a wide range of biological effects, including antioxidant, anticancer, anti-inflammatory, antidiabetic and antibacterial effects20. It exerts its anti-inflammatory effect mainly by inhibiting the release of cytokines, reducing the production of COX and LOX, and maintaining the stability of mast cells21. A study of pulpitis has reported that quercetin reduces the production of pro-inflammatory cytokines IL-6 and IL-1722. Besides, quercetin-induced miR-369-3p modulates inflammatory cascades in chronic inflammatory responses and increases IL-6 production23. Quercetin can also inhibit inflammation by regulating the PI3K-AKT signaling pathway, thus reducing oxidative stress damage in GES-1 cells24. In mice with DSS-induced colitis, quercetin can significantly improve moderate DSS- induced colitis and reduce intestinal inflammation25. Additionally, in the study of Huai Hua San for treating UC, quercetin has also been found to play an important role in exerting anti-inflammatory effects26. In our study, both the prediction of active compounds and cell verification experiments indicated that quercetin was a key ingredient of SZLBD and played a vital role in the treatment of UC by regulating inflammation-related processes.
It is well known that IL-1B and IL-6 are pro-inflammatory factors. In a past study, administration of a water-soluble artemisinin analog (SM934) was found to improve UC by reducing DSS-induced mRNA and protein levels of IL-1 B and IL-627. Protein kinase B (AKT) is a member of the human serine-threonine kinase and the AGC family of protein kinases. Samples of human patients with UC showed severe down regulation of AKT1 function, accompanied by decreased AKT1 activity and increased inflammation28. More drugs for the treatment of UC work through the pathway where AKT1 is located. Dihydroartemisinin can alleviate DSS-induced colitis via the PI3K-AKT signaling pathway29. Huangqin decoction ameliorates DSS-induced colitis by inhibiting the Ras-PI3K-Akt-HIF-1α pathway30. Quercetin can alleviate inflammation by reducing the levels of TNF-α, IL-1β, IL-6, and IL-10, thus treating DSS-induced colitis in mice31. The results of network pharmacology analysis and cell experiments in this study showed that quercetin, the main ingredient of SZLBD, targeted IL-6, IL-1B and AKT1 in the treatment of UC. We speculated that quercetin might improve UC by reducing the expression of pro-inflammatory factors and the PI3K-Akt signaling pathway.
Overall, this study suggested that quercetin might be the main active ingredient in SZLBD. IL-6, IL-1B, and AKT1 were potential therapeutic targets of SZLBD in the treatment of UC. In addition, the role of SZLBD in UC might be achieved by modulating the balance of cytokines in the immune system and inflammation-related pathways, such as the IL-17 and PI3K-AKT pathways. These findings confirm that network pharmacology is an important method for predicting active compounds and targets. This study provides a theoretical basis for SZLBD in the treatment of UC and a comprehensive approach for finding more Chinese medicines to treat UC.
However, our study still has some limitations. First, most targets in the databases are verified, and unverified or undocumented ones may be overlooked. Second, quercetin, although identified as the main active ingredient of SZLBD, cannot completely replace SZLBD. Third, network pharmacology analysis ignores the absorption pathways, effective sites, active compounds, and metabolic forms of bioactive substances. Additionally, we have not yet explored the roles of other key ingredients within the decoction. Finally, we have not yet conducted animal studies to validate the efficacy of the SZLBD. Therefore, we need more in vitro and in vivo research to further explore the underlying molecular mechanisms by which SZLBD treats UC.
Conclusion
In this study, we combined network pharmacology, molecular docking, and in vitro experiments to explore and verify the mechanism by which SZLBD targets and their main bioactive constituents effectively exert anti-UC effects. We found that SZLBD acted on multiple targets of multiple signaling pathways, and intervened in the PI3K-Akt and IL-17 pathways to delay the progression in UC. In the follow-up study, we plan to design in vivo and in vitro animal pharmacological experiments to deeply investigate the mechanism of action of SZLBD in the treatment of UC, so as to provide more references for its clinical application and development.
Data availability
All data generated or analysed during this study are included in this article. Further inquiries can be directed to the corresponding authors.
Abbreviations
- UC:
-
Ulcerative colitis
- SZLBD:
-
Shen-Zhu-Lian-Bai decoction
- TCM:
-
Traditional Chinese medicines
- TCMSP:
-
Traditional Chinese Medicine Systems Pharmacology
- PPI:
-
Protein–protein interaction
- BP:
-
Biological process
- CC:
-
Cellular ingredient
- MF:
-
Molecular function
References
Adams, S. M. & Bornemann, P. H. Ulcerative colitis. Am. Fam. Physician 87, 699–705 (2013).
Ungaro, R., Mehandru, S., Allen, P. B., Peyrin-Biroulet, L. & Colombel, J. F. Ulcerative colitis. Lancet 389, 1756–1770 (2017).
Kobayashi, T. et al. Ulcerative colitis. Nat. Rev. Dis. Primers 6, 74 (2020).
Buurman, D. J. et al. Ulcerative colitis patients with an inflammatory response upon mesalazine cannot be desensitized: A randomized study. Scand. J. Gastroenterol. 50, 399–405 (2015).
Langhorst, J. et al. Systematic review of complementary and alternative medicine treatments in inflammatory bowel diseases. J. Crohns Colitis 9, 86–106 (2015).
Xu L, Zhang J, Wang Y, Zhang Z, Wang F, Tang X: Uncovering the mechanism of Ge-Gen-Qin-Lian decoction for treating ulcerative colitis based on network pharmacology and molecular docking verification. Biosci. Rep. 41(2), (2021).
Chao, L. et al. Shen-Ling-Bai-Zhu-San improves dextran sodium sulfate-induced colitis by inhibiting caspase-1/Caspase-11-mediated pyroptosis. Front. Pharmacol. 11, 814 (2020).
Li, T., Wu, M., Wang, L., Song, X. & Feng, P. Huang Lian for ulcerative colitis: A protocol of systematic review and meta-analysis of randomized clinical trials. Medicine (Baltimore) 99, e22457 (2020).
Su, S. et al. Phellodendrine promotes autophagy by regulating the AMPK/mTOR pathway and treats ulcerative colitis. J. Cell Mol. Med. 25, 5707–5720 (2021).
Cai, H. et al. Investigation on spectrum-effect correlation between constituents absorbed into blood and bioactivities of Baizhu Shaoyao San before and after processing on ulcerative colitis rats by UHPLC/Q-TOF-MS/MS coupled with gray correlation analysis. Molecules 24, 940 (2019).
Tang, S. et al. Combination of polysaccharides from Astragalus membranaceus and Codonopsis pilosula ameliorated mice colitis and underlying mechanisms. J. Ethnopharmacol. 264, 113280 (2021).
Zhou, Q., Zhang, F., He, Z. & Zuo, M. Z. E2F2/5/8 serve as potential prognostic biomarkers and targets for human ovarian cancer. Front. Oncol. 9, 161 (2019).
Li, L., Lv, J., He, Y. & Wang, Z. Gene network in pulmonary tuberculosis based on bioinformatic analysis. BMC Infect. Dis. 20, 612 (2020).
Yan, Y. et al. Comparison of the effects of essential oil obtained from the crude and bran-processed atractylodes lancea on lipopolysaccharide-Induced Inflammatory injury of human colonic epithelial cells by downregulating the IKK/NF-κB signaling pathway. Evid. Based Complement Alternat. Med. 2021, 5219129 (2021).
Wang, Y. et al. Coptisine ameliorates DSS-induced ulcerative colitis via improving intestinal barrier dysfunction and suppressing inflammatory response. Eur. J. Pharmacol. 896, 173912 (2021).
Song, T. & Chen, W. D. Berberine inhibited carotid atherosclerosis through PI3K/AKTmTOR signaling pathway. Bioengineered 12, 8135–8146 (2021).
Ma, Q. et al. A review of pharmacological and clinical studies on the application of Shenling Baizhu San in treatment of ulcerative colitis. J. Ethnopharmacol. 244, 112105 (2019).
Wang, Z. et al. Protective effects of polysaccharide extracted from Portulacae oleracea L. on colitis induced by dextran sulfate sodium. J. Med. Food 23, 125–131 (2020).
Zhai, L. et al. Therapeutic effects and mechanisms of Zhen-Wu-Bu-Qi decoction on dextran sulfate sodium-induced chronic colitis in mice assessed by multi-omics approaches. Phytomedicine 99, 154001 (2022).
Li, Y. et al. Quercetin, inflammation and immunity. Nutrients 8, 167 (2016).
Tang, S. M. et al. Pharmacological basis and new insights of quercetin action in respect to its anti-cancer effects. Biomed. Pharmacother. 121, 109604 (2020).
Mahmoud Hashemi, A. et al. Quercetin decreases Th17 production by down-regulation of MAPK- TLR4 signaling pathway on T cells in dental pulpitis. J. Dent. (Shiraz) 19, 259–264 (2018).
Galleggiante, V. et al. Quercetin-Induced miR-369-3p suppresses chronic inflammatory response targeting C/EBP-β. Mol. Nutr. Food Res. 63, e1801390 (2019).
Yao, X. & Mei, Y. Quercetin improves mitochondrial function and inflammation in H(2)O(2)-induced oxidative stress damage in the gastric mucosal epithelial cell by regulating the PI3K/AKT signaling pathway. Evid. Based Complement Alternat. Med. 2021, 1386078 (2021).
Riemschneider, S. et al. Indol-3-carbinol and quercetin ameliorate chronic DSS-induced colitis in C57BL/6 mice by AhR-mediated anti-inflammatory mechanisms. Int. J. Environ. Res. Public Health 18, 2262 (2021).
Liu, J. et al. Network pharmacology prediction and molecular docking-based strategy to discover the potential pharmacological mechanism of Huai Hua San against ulcerative colitis. Drug Des. Dev. Ther. 15, 3255–3276 (2021).
Yan, Y. X. et al. Artemisinin analogue SM934 ameliorates DSS-induced mouse ulcerative colitis via suppressing neutrophils and macrophages. Acta Pharmacol. Sin. 39, 1633–1644 (2018).
Mustfa, S. A. et al. SUMOylation pathway alteration coupled with downregulation of SUMO E2 enzyme at mucosal epithelium modulates inflammation in inflammatory bowel disease. Open Biol 7, 170024 (2017).
Li, N. & Sun, W. Dihydroartemisinin protects against dextran sulfate sodium-induced colitis in mice through inhibiting the PI3K/AKT and NF-κB Signaling pathways. Biomed. Res. Int. 2019, 1415809 (2019).
Li, M. Y. et al. Anti-inflammatory effects of Huangqin decoction on dextran sulfate sodium-induced ulcerative colitis in mice through regulation of the gut microbiota and suppression of the Ras-PI3K-Akt-HIF-1α and NF-κB pathways. Front. Pharmacol. 10, 1552 (2019).
Wang, L. et al. pH sensitive quercetin nanoparticles ameliorate DSS-induced colitis in mice by colon-specific delivery. Mol. Nutr. Food Res. 68(2), e2300051 (2024).
Funding
This study was supported by 2022 Scientific Research Project of Guangdong Provincial Bureau of Traditional Chinese Medicine (20221394).
Author information
Authors and Affiliations
Contributions
Li Zhu was in charge of conducting experiments and writing the article. Jinghua Liang performed network pharmacology analysis, conceived and designed experiments.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhu, L., Liang, J. Network pharmacological prediction of the mechanism of action of Shen-Zhu-Lian-Bai Decoction in the treatment of ulcerative colitis. Sci Rep 14, 14183 (2024). https://doi.org/10.1038/s41598-024-64683-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-64683-4
- Springer Nature Limited