Abstract
Decreases in body sizes of animals related to recent climate warming can affect population persistence and stability. However, direct observations of average sizes over time and their interrelationships with underlying density-dependent and density-independent processes remain poorly understood owing to the lack of appropriate long-term datasets. We measured body size of two species common to headwater streams in coastal and Cascades ecoregions of the Pacific Northwest of North America over multiple decades, comparing old-growth and managed forests. We found consistent decreases in median length of Coastal Cutthroat Trout Oncorhynchus clarkii clarkii, but a coexisting species, the Coastal Giant Salamander Dicamptodon tenebrosus, appears to be more resilient to size changes over time. Based on observed trends, adult trout have decreased in length by 6–13% over the last 30 years. Length decreased more in larger compared to smaller animals, suggesting that these effects reflect changes in growth trajectories. Results from a model-selection approach that included hydroclimatic and biological information as covariates in one of our study ecoregions demonstrated that stream temperature alone did not explain observed length reductions. Rather, a combination of density-dependent (animal abundances) and local density-independent factors (temperature, habitat, and streamflow) explained observed patterns of size. Continued decreases in size could lead to trophic cascades, biodiversity loss, or in extreme cases, species extirpation. However, the intricate links between density-independent and density-dependent factors in controlling population-level processes in streams need further attention.
Similar content being viewed by others
Introduction
Body size is one of the most obvious characteristics of organisms and its role in shaping the form and function of animals has attracted the attention of scientists for decades1,2,3. Smaller size at age confers intrinsic disadvantages3 including lower fitness and fecundity4 as well as shortcomings for competitive interactions with conspecifics5 and among species6. Shrinking size of organisms has been proposed as the third universal ecological response to climate change7,8,9, in addition to shifts in species distributions10 and phenology10,11,12. Conceptual models have also been developed to expand investigations of the effects of shrinking sizes from populations to ecosystems7,8,9, but empirical support to evaluate whether smaller sizes are consistent across taxa and ecoregions remains scarce8,9,13,14,15.
To date, the empirical information used to detect temporal changes in size in response to temperature relies on museum collections8,14, short-term laboratory experiments7,16,17, or harvested species7,15,18,19,20; but see reef fishes21. Size data from museum collections are biased by the lack of representativeness of populations22, and experimental studies oversimplify whole ecosystem processes that affect size. Size data from harvested or exploited species have inherent biases8,14,23 including the selective pressure of harvest on large individuals24,25,26,27, changes in capture effort, efficiencies, and shifts in management practices over time28,29,30,31. These additional factors can differentially affect size-specific life histories, especially for migratory species20,32,33. Collectively, these issues make it difficult to identify underlying mechanisms responsible for the observed shifts in size7,8,34. Hence, long-term data collected simultaneously from multiple species and their environment using consistent methods are crucial not only to test hypotheses about the coherence of responses across taxa and regions, but also to identify specific factors affecting size.
Body size provides a functional link between individual-level processes of behavior and physiology, including metabolism and growth, with higher-level population processes that can explain larger ecological patterns. For example, changes in temperature can lead to accelerated metabolism and growth rates earlier in life, resulting in smaller adults. Warmer environments can alter the distribution of food resources, which can negatively affect growth rates, or it could also reduce water availability leading to reduced habitat size and decrease the amount of resources per-capita, affecting size. A warming climate has been posited as the single factor explaining patterns of change in size in many cases7,8,18,35, but see reef fishes21. This proposition has been based, in part, on Bergmann’s ecogeographical rule36, which states that larger organisms tend to be found in colder environments whereas the opposite occurs in warmer regions. Bergmann's rule, however, does not seem to apply to ectotherms37, including freshwater fishes38 and amphibians39. Hence, a relationship between temperature and size may not be sufficient to explain the greater reductions in size observed in some freshwater species as compared to terrestrial animals7,14,17.
In ectotherms, including freshwater species, patterns of long-term changes in size due to warming are also affected by multiple biotic and abiotic factors that are often ignored or unexplored. Among them are the negative relationships between size and population density40,41, intra- and interspecific competition28,30, thermal dependence of growth and development modulated by site-specific conditions42, and combinations of these factors9. These biotic and abiotic factors operate at multiple scales43 with effects that can be cumulative. Recent evidence shows that additional factors other than climate can explain trends in size of salmonids in both freshwater (local environmental conditions15) and marine systems (competition44). Hence, overlooking the environmental complexity can potentially risk ignoring essential information needed to develop management actions for adapting to climate change.
Here, we evaluate whether sizes are shrinking under climate change using a long-term time series dataset of Coastal Cutthroat Trout (Oncorhynchus clarkii clarkii) in streams draining either old-growth or managed forests from two different ecoregions including the Oregon Coast (1962-2017) and Oregon Cascades (1987-2022), USA. We also test whether Coastal Giant Salamanders (Dicamptodon tenebrosus) display shrinking sizes in streams from one of the ecoregions over time (Oregon Cascades 1993–2022). In our study streams, trout have not been exploited by fishing, and the lifespan of both species is long enough to experience seasonal environmental stochasticity. We have long-term information about population abundances and local hydroclimate in one ecoregion, allowing us to develop and test multiple competing models that illustrate how biotic and abiotic factors can influence patterns of body size considering the local ecological context. These models underlie hypotheses based on the literature about potential influences of density-dependent and density-independent factors on size (Table 1). Our findings demonstrate that multi-decadal population studies can provide foundational information for answering complex questions that emerge from ongoing global environmental change.
Material and methods
Study sites
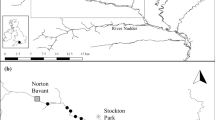
We used datasets collected from two sections of Mack Creek (old growth and second growth) and two tributaries of Drift Creek (Flynn Creek and Needle Branch) over the last 60 years (Fig. 1). Both Mack Creek and Drift Creek were originally studied as part of large multi-year efforts to assess the effects of forest harvest on freshwater ecosystems and are protected for research purposes64,65,66,67. Mack Creek is located in the Western Cascade Range of Oregon and Drift Creek lies in the Central Oregon Coast Range, approximately 150 km apart from each other. These drainages are not hydrologically connected. Drift Creek drains directly to the Pacific Ocean approximately 200 km south of the Columbia River estuary, whereas Mack Creek drains via Blue River to the McKenzie River to the Willamette River and ultimately to the Columbia River.
Map of our study sites including (a) Flynn Creek and Needle Branch, Coast Range, and (c) Mack Creek (old-growth and second-growth sections), Cascade Range. All sites are located in Oregon, USA. Individual body-size measurements of Coastal Cutthroat Trout and Coastal Giant Salamander were collected from our study sites over the last 60 years. [Figure developed Kelly Christiansen (USDA Forest Service, PNW Research Station); Created in ArcGIS PSMFC GIS, Airbus, USGS, NGA, NASA, CGIAR, NCEAS, NLS, OS, NMA, Geodatastyrelsen, GSA, GSI and the GIS User Community].
We considered datasets from the old-growth forest section at Mack Creek and Flynn Creek. These sites had no human-related disturbances of forest harvest, land-use changes, commercial, or recreational fishing during the study period and thus, the effects of climate change are isolated (Table 2). Dense old-growth forests including ancient Douglas-fir (Pseudotsuga menziesii), Western Redcedar (Thuja plicata), and Western Hemlock (Tsuga heterophylla) trees of more than 500 years old dominated the old-growth section of Mack Creek. At Flynn Creek, mature forests of approximately 75–155-year-old Douglas-fir and 75–115-year-old trees of Red Alder (Alnus rubra) were dominant vegetation in the basin.
We also considered datasets from streams with an adjacent second-growth forest in Mack and Needle Branch creeks (Table 2). The second-growth forest section of Mack Creek was clearcut in 1964. However, the collection of aquatic vertebrate data started 25 years after timber harvest. Evidence suggests that physical legacy effects of timber harvest should be minimal after 20 years68,69,70,71. Needle Branch had a clearcut (82% of the basin) in 1966 leaving no riparian buffer65 and a second clearcut in 2009 that included approximately 15 m wide riparian buffer on each side of the stream in the upper portion of the study reach (40%; 37 ha) with replanting within 2 years after harvest72. These second-growth forest stream sections illustrate a combined effect of climate change and legacies from past forest management.
Target species
Coastal Cutthroat Trout (Oncorhynchus clarkii clarkii) are distributed from Alaska to California73,74, whereas Coastal Giant Salamanders (Dicamptodon tenebrosus) are present from the coast of southern British Columbia to California75,76. These species are tertiary consumers that dominate headwaters in the Pacific Northwest of North America77. In its stream-living form, the lifespan of Coastal Cutthroat Trout is 4–5 years (and up to 7–8 years in some cases); individuals are sexually mature around age 1–2, and their home ranges are generally restricted to within 200 m of their birthplace78. During seasonal low flow, Coastal Cutthroat Trout prefer deeper pools79 with cover availability54.
The average Coastal Giant Salamander lifespan is unknown, but animals may live up to 25 years80. This species can reproduce as freshwater larvae (neotenes) or as transformed terrestrial adults, with size at maturity between 85 and 115 mm snout-to-vent length76. Coastal Giant Salamanders have restricted home ranges (< 30 m) as larvae81,82 and adults83. Salamander abundances are often negatively associated with wider and deeper streams in western North America55,84. Salamanders prefer small pools with slow water velocities55,56.
Animal collection and body size
We evaluated whether there is evidence of shrinking sizes under climate change using individual body length information for Coastal Cutthroat Trout (Oncorhynchus clarkii clarkii) from four stream sections in both ecoregions, and Coastal Giant Salamanders (Dicamptodon tenebrosus) from two stream sections in one ecoregion (Table 2). All experimental protocols and methods were conducted and approved in accordance with relevant guidelines and regulations from the ethics committee of the Office of Research Integrity at Oregon State University (Institutional Animal Care permits 3720, 4076, 4379, 4796, and 4816).
For Mack Creek, we included trout fork length (FL) and salamander snout-to-vent length (SVL) obtained using standard electrofishing procedures (details in Supporting Information). Trout datasets were available for years 1987–2022, whereas datasets for salamanders were available for years 1993–202285. We distinguished adult trout (Age 1+; FL > 70 mm) from trout young-of-year (YOY; FL ≤ 70 mm) using a visual evaluation of breaks between length classes on length-frequency histograms (Figs. S1, S2). For salamanders, however, we considered all size data owing to the difficulty of determining age based on length (Fig. S2).
Only datasets of trout fork length were available for Flynn Creek and Needle Branch (Fig. 1; Table 2). These datasets were obtained using standard electrofishing procedures during the periods 1962–1974, 1988–1997, and 2006–2017 (details in Supporting Information). Only adult trout (Age 1+) were initially targeted during sampling as questions about climate change and potential shifts in size distributions were not part of the original Alsea Watershed Study. Some inconsistencies in sampling effort resulted in only a few YOY captured for some years. Thus, we aggregated trout YOY data for the entire study period to visually inspect size distributions and separate them from adult trout (FL > 75; Fig. S1).
Statistical analysis
Trends in body size of trout and salamanders
We evaluated temporal trends in length of adult trout and salamanders using the non-parametric Mann–Kendall test for monotonic time series86 and the associated Sen’s slope estimator87 to estimate the magnitude of trends over time (i.e., mm decade−1). This rank-based test is robust for non-normal data, such as time series with outliers and non-linear trends88. To perform this analysis, we used the package ‘modifiedmk’89 implemented in R ver. 4.2.3. This R package extends the traditional Mann–Kendall test by incorporating modifications to account for serial correlation and offers various functions for trend detection and trend magnitude estimation. We adopted a block bootstrapped90 and bias corrected prewhitening91 procedure to account for potential serial correlation effects. We evaluated trends using multiple percentiles (i.e., 5th, 10th ⋯ 95th) estimated annually rather than rely on a single central-tendency metric per year (e.g., mean). This approach can correct size-related sampling biases and better describe the typical asymmetry of length distributions (e.g., fewer larger and presumable older animals versus more abundant smaller and presumable younger animals; Figs. S1, S2). The R script used to perform our trend analyses is provided in Supporting Information.
Model selection approach to explore factors affecting size at Mack Creek
Due to the absence of additional information at Flynn Creek and Needle Branch Creek, we performed the model section analysis only for Mack Creek where long-term time series of density-dependent (i.e., proportion of YOY trout and abundance of freshwater vertebrates85) and density-independent factors (i.e., temperature, discharge, and habitat size92,93) were available (Table 2).
We used a multi-model selection procedure as a robust information-theoretic approach for testing multiple hypotheses94,95. This analysis allowed us to evaluate the relative roles of density-dependent and density-independent factors affecting size of trout and salamanders (Table 1; Supporting Information). We did not include density-dependent factors related to biomass because time series of body mass were discontinuous. For density-independent factors, we used annual/seasonal metrics of temperature45 and discharge46 that describe the hydrologic regimes influenced by both snow and rain typical of our study region (Supporting Information). In addition, we added local habitat-size85 metrics including the maximum depth of cascades, pools, and side channels within each section of Mack Creek. We performed an additional trend analysis (see “Trends in body size of trout and salamander”) of these covariate factors to explore which of them might change over time. Before performing the model selection, we centered and standardized all density-dependent and density-independent factors (f) to make model coefficients comparable. We tested for potential multicollinearity and removed factors with strong correlation with others within each category (i.e., density-dependent or density-independent) using |r| > 0.7 as recommended threshold96,97.
We used generalized linear models (glm) with Gaussian error distributions focusing on main effects and, for simplicity, we did not consider pairwise interactions among factors. We implemented one set of models to predict the annual median length of trout and another set to predict the annual median length of salamanders. In both cases, we considered site (i.e., old-growth and second-growth) as a factor. We fitted all possible models (2f) on each case using the package ‘glmulti’98 implemented in R ver. 4.2.3. This R package is a tool for automated model selection and multi-model inference. It employs a genetic algorithm to search through the space of possible models and identifies the most appropriate one based on user-defined criteria. In our case, we separated and ranked all models for trout and salamanders and averaged top-supported models for each case using the Akaike’s Information Criterion corrected for small sample size (AICc). We selected the averaged model (delta AICc < 2) as the best-supported model and used the statistically significant standardized coefficients from conditional models to evaluate size effect among covariates for each case. The full average model assigned zero as the regression coefficient for factors that were not included in the model, whereas conditional averaged model only included the averages of each included factor in the model. The R script used to perform our trend analyses is provided in Supporting Information.
Results
Trends in body size
We found consistent trends toward decreases in median size over time (between 1.9 and 5 mm per decade) for adult trout at all study sites (Fig. 2; Tables S1–S4), but trends were less consistent for salamanders at Mack Creek (Fig. 3). At the Coast Range sites (Flynn Creek and Needle Branch), Sen’s slope values illustrated that shrinking size rates have occurred almost across the entire length distribution of adult trout (>10th percentile at Flynn Creek; >50th percentile at Needle Branch), yet large and presumably older trout seemed most affected (Fig. 4; Tables S1–S4). Similar patterns of shrinking size rates occurred for adult trout at both sections of Mack Creek (> 45th percentile in the old-growth section; >20th percentile in the second-growth section). In contrast, statistically significant shrinking size rates only occurred for large salamanders (80–90th percentiles) in the second-growth section of Mack Creek (Fig. 4; Tables S5, S6). Our findings were based on annual sampling efforts that resulted on an average of 204 individuals per site and taxon, for a total of 27,244 trout and 12,362 salamander individual observations during the entire study period (Tables S7, S8).
Median fork length (mm) of adult Coastal Cutthroat Trout across sites over time. Bias corrected prewhitened (BCP) Sen’s slope values (mm decade−1) represent the rate of change in median size over time (i.e., rate of shrinking size if negative). Shaded area marks 25th–75th percentile band. For detailed statistics, see Tables S1–S4 in Supporting Information.
Median snout-vent length (mm) of Coastal Giant Salamander in Mack Creek over time. Bias corrected prewhitened (BCP) Sen’s slope values (mm decade−1) represent the rate of change in median size over time (i.e., rate of shrinking size if negative). Shaded area indicates 25th–75th percentile band. For detailed statistics, see Tables S1–S4 in Supporting Information.
Bias corrected prewhitened (BCP) Sen’s slope values (mm decade−1) representing the rate of change in size (i.e., rate of shrinking size if negative) of adult Coastal Cutthroat Trout and Coastal Giant Salamander over time across length percentiles and sites. Filled symbols denote statistically significant trends (Mann–Kendall test p < 0.05). For detailed statistics, see Tables S1–S6 in Supporting Information.
Our temporal examination of covariate factors that potentially affect length of aquatic vertebrates in Mack Creek revealed that only the proportion of cold days (≤12 °C) and the variability of summer discharge have consistently decreased over time (Fig. 5; Table S9). Density fluctuated annually with no apparent long-term trends (Fig. 5a), except for the abundance of salamanders that increased between 1993 and 2008, then gradually decreased between 2008 and 2022 in the old-growth section. Overall, trout (YOY or adults) and salamander abundances were relatively comparable between stream sections, but adult trout seemed slightly more abundant in the second-growth section. Further, density-independent factors related to the size of stream habitats did not change over time, with pools as the deepest habitat units followed by cascades and side channels. Yet, it appeared that cascades were marginally deeper in the second-growth compared to the old-growth stream section. Additional metrics that describe the seasonality of temperature and discharge also fluctuated annually with no apparent long-term trends (Fig. 5b; Table S9). Yet, the warmest winter and summer seasons within our study period coincided with an extreme drought year in 2015.
Density-dependent (i.e., abundance of freshwater vertebrates) and density-independent factors (i.e., habitat size, temperature, and discharge) in Mack Creek over time. (a) Covariate factors measured during annual sampling events at the old-growth and second-growth sections of Mack Creek. (b) Hydroclimatic covariate factors were estimated based on daily time series obtained from a long-term stream gage station located in Mack Creek. See detailed description of these covariate factors in Table 1. *Statistically significant negative trends over time. For detailed trend analyses and statistics, see Table S9 in Supporting Information.
Model selection and hypotheses at Mack Creek
Overall, top-supported models (delta AICc < 2) that predicted the median size of adult trout and salamander included a combination of both density-dependent and density-independent factors (Tables 3, 4; Fig. 6). The proportion of cold days (≤12 °C) and proportion of days between 12 and 15 °C (optimal conditions for trout growth) were factors removed from the model selection procedure owing to collinearity (|r| > 0.92) with mean winter and summer temperature. The best-supported model (i.e., conditional average) of adult trout size included the abundance of both trout YOY and adult trout, as well as summer temperature and habitat size (Table 3; Fig. 6; Table S10). As hypothesized (Table 1), warmer summers and more abundant adult trout significantly reduced adult trout size. Also, both deeper side channels and more abundant trout YOY significantly increased the size of adult trout. The predicted effect of temperature was the greatest in the model, followed by adult trout abundance, depth of side-channel habitats, and abundance of trout YOY. Further, the best-supported model of salamander size included, as hypothesized (Table 1), the abundance of conspecifics and depth of cascade habitats, with both negatively affecting salamander size (Table 4; Fig. 6; Table S11). Conversely, the variability of summer stream discharge had a positive effect on salamander size. The median size of salamanders was consistently larger at the second-growth compared to the old-growth stream section of Mack Creek. Lastly, the greatest size effect in this model included site followed by the variability of summer stream discharge and salamander abundance.
Effect size (standardized values ± 95% CI) of covariate factors from the best-supported model (Tables 3, 4; Tables S10, S11) explaining median size of adult Coastal Cutthroat Trout and Coastal Giant Salamanders in Mack Creek. The best-supported model included both density-dependent (abundance of animals) and density-independent (stream discharge, temperature, habitat size) factors. These biotic and abiotic factors are fully described in Table 1.
Discussion
We show empirical support for consistent declines in trout length across ecoregions based on data that span decades, highlighting the importance of long-term ecological research in detecting this gradual shrinking of sizes over time. Based on observed trends, adult trout size reductions over the last 30 years are estimated to be between 6 and 13%. In Mack Creek, trends in length for salamanders are less consistent, suggesting complex responses between coexisting freshwater vertebrates to climate change. Our model-selection approach in Mack Creek offers additional evidence pointing to the possibility that reductions in size might not solely be related to climate warming, but also include a combination of density-dependent and density-independent factors, emphasizing the relevance of local ecological contexts. Our findings provide valuable insights into the responses of freshwater taxa to climate change that would not be possible without the careful maintenance and attention given to the consistent collection of these data as part of long-term research programs.
Consistent shrinking sizes of trout across sites, but not in stream salamanders
The consistent decline in sizes of trout from relatively pristine systems provides empirical support to the idea of shrinking size as an ecological response to climate change. To date, only a few examples from natural settings for large freshwater predators7,15,17 have been examined to test the hypothesis of smaller size of animals related to climate change in freshwaters; our study helps fill this information gap. Yet, there is conflicting evidence about whether a decline in size has occurred in salamanders with decreases63,99, increases99, and no apparent trends in size over time, except for larger animals (this study). Also, increases and decreases in size of salmonids and other exploited fishes illustrate complex interactions among size-selected harvest, environmental conditions, and other human influences such as hatchery-origin stocks15,30,44,100. Human-related influences on sampling and exploited animals can obscure trends in size.
Reductions in size of trout appear to reflect changes in growth trajectories, with greater magnitudes occurring for larger and likely older animals. In our study region, the increase in the frequency and intensity of droughts over time101 can differentially affect annual growth rates of larger trout, as shown after the occurrence of such extreme events50. Although the magnitude of decreases in size of adult trout is relatively small (mm per decade), trends are remarkably consistent and comparable in magnitude to other freshwater organisms17. In addition, the reduction in size we observed for larger salamanders (80th–90th size percentiles) in the second-growth section of Mack Creek is almost twice the magnitude reported for terrestrial salamanders63. More research is needed to understand the differential adaptation between coexisting species21 across ecoregions and to disentangle the role that local contexts play in modifying rates of shrinking sizes over time.
Density-dependent and density-independent processes differentially affect size of trout and stream salamanders in Mack Creek
Findings from our model selection for Mack Creek demonstrate that the sizes of trout and stream salamanders are simultaneously influenced by density-dependent and density-independent processes. Therefore, reductions in size cannot be attributed to climate warming alone and are consistent with recent findings for salmonids in freshwater15,102 and marine systems44. Body size of an amphibian population varies with abundance and not climate103, emphasizing the importance of density-dependent factors. Considering how density-dependent and density-independent factors might influence the size of these freshwater vertebrates, our best-supported models highlight the importance of considering local ecological contexts to explain observed declines in size.
Density-dependent factors in the best-supported model predicting size of adult trout include positive associations with the abundance of trout YOY, and negative associations with the abundance of adult trout. For salamander size, the best-supported model includes negative associations with the abundance of salamanders. Both Coastal Cutthroat Trout and Coastal Giant Salamanders are generalist opportunistic feeders48,78,104. Headwater streams in the Pacific Northwest have low levels of primary and secondary productivity105. Thus, trout YOY represent a potential food source for large trout during summer47. Growth in salmonids is predominantly regulated by density-dependent factors, even at low population densities50,51,106 owing to competition for food and space107. Both trout and salamanders52 also exhibit territorial behavior107. Hence, density-dependence can decrease food availability per capita resulting in decreases in size of these freshwater vertebrates.
Density-independent factors are also incorporated in the best-supported models that predict size in adult trout and salamanders. Specifically, warmer summers negatively affect trout size. In Mack Creek, maximum stream temperatures are far below the critical thermal tolerances reported for Coastal Cutthroat Trout62, but warmer summers can increase metabolic costs61 with negative effects on growth50. In addition, our model shows that deeper side channels positively affect trout size. This is consistent with trout preferring relatively lentic and deeper habitats57 where there is a greater provision of in-stream cover54. Further, density-independent factors that describe seasonal stream discharge and local habitat size are also included in the best-supported model that predicts size of salamanders. Specifically, the higher variability of summer stream discharge exerts a positive influence, whereas deeper cascade habitats negatively influence salamander size. Higher variability of summer stream discharge would offer greater food opportunities owing to increases in total invertebrate-drift load during higher-flow events58. The negative association between size and depth of cascades aligns with stream salamander abundances as being inversely related to wider stream channels in western North America55,84 and their preference for smaller pool habitats with slower water velocities55,56.
Trends in size in the context of local forest management and climate change
Size reductions in adult trout differ in magnitude across our study sites, suggesting that the combination of local factors and climate change can modulate rates of size decrease over time. For example, median trout size decreases slightly more in sites with a legacy of forest harvest than in sites dominated by old-growth forests, evidence that relatively pristine ecosystems could buffer against the effects of climate change. The buffering capacity against the effects of climate change of relatively pristine systems108 such as old-growth forests109 has been overlooked in freshwater ecosystems110. It is plausible that late-successional forests reduce the consequences of thermal and hydrological stresses of climate change on the physiology and population dynamics of aquatic organisms. In Mack Creek, we show decreasing trends in the proportion of cold days (stream temperature ≤12 °C) consistent with steadily warming streams in winter over time45, potentially increasing temperature-size responses of aquatic organisms17. Forest management practices can be nuanced within and across streams68,69,70. In Needle Branch, forest management activities were much more detrimental to the stream channel and water quality in 1966 (clearcut with no buffer65,66) compared to 2009 (clearcut with buffer64,72). Potential increases in primary production and temperature (up to 30 °C)66 immediately after the clearcut in 1966 likely decreased when the second-growth canopy covered the stream. However, compared to pre-harvest conditions at Needle Branch, trout densities range from lower111 to relatively similar values64,112 over time. Collectively, our results illustrate the importance of considering legacy effects from past disturbance events in the face of climate change and their context-dependent associations to understand the importance of density-dependent and density-independent factors that influence body size of organisms.
Further research to understand indirect climate effects on body size
The role of climate warming affecting food, habitat availability, and emigration, and the effect of their interacting factors on shrinking sizes of trout and salamanders is complex, but merits future consideration. To our knowledge, comprehensive time series of food or cover availability and patterns of emigration in headwater streams over time do not exist for trout or salamanders. It is plausible that stream-living trout and salamanders are not limited by food owing to the predicted global increase in secondary production113 and insects114 due to climate warming. Alternatively, large individuals might emigrate downstream to larger habitats when their metabolic demands and competition with conspecifics54 make downstream areas more conducive to growth and survival. Further, the increase in frequency and magnitude of droughts in a warming climate115 can negatively affect habitat size, diversity, and connectivity116,117 including warmer waters and low dissolved oxygen concentrations116. These events can also affect seasonal food availability118,119 and species interactions117,120 resulting in lower annual growth50 and thus, indirectly affect body size.
The ecological implications of smaller sizes in trout and stream salamanders owing to climate warming and their potential changes in life histories of species are difficult to predict given the multiple independent factors and mechanisms that could be involved. Smaller size can result in lower fitness and fecundity4, diminished swimming speed or power121, and competitive disadvantages within conspecifics5 and among species6. Climate warming can have both short-term (e.g., energy expenditures as YOY) and long-term (e.g., how energy allocations affect life-history expression later in life) effects that will be difficult to separate, and further experiment under controlled conditions are warranted. For example, investment in gonadal development, especially eggs in females, can affect realized growth as metabolic costs likely increase with warmer environments122. Prolonged exposure to warming stress during early life stages of trout can negatively impact reproduction, but offsprings of next generation receiving similar thermal stress have shown higher growth and survival, suggesting a potential rapid adaptation to climate warming123. These complex developmental and evolutionary aspects are modulated by both individual condition and the environment124. Here, we focused on single effects without interactions, but our findings are sufficient to illustrate the potential role of multiple factors regulating size. Nevertheless, changes in body size resulting from climate warming will likely have far-reaching effects from individuals to ecosystems7. More long-term efforts across ecosystems are urgently needed to generalize predictions about the ecological responses of observed declines in size. Ultimately, expected changes in ecosystems related to climate warming are complex and occur at multiple scales involving biological and physical processes related to species and their life histories.
Conclusions
Our long-term data reveal compelling decreases in size of Coastal Cutthroat Trout, but not in Coastal Giant Salamanders. The associated mechanisms that explain these trends appear to be specific to local ecological and environmental contexts. Factors related to climate change, such as water temperature and flow variability, are related to the changes in size over time. However, size reductions also seem to be influenced by additional drivers unrelated to climate, such as density dependence. Detailed knowledge of the range of factors affecting size and the understanding of the complexity of species and ecosystems is crucial to effectively inform management and policy makers as they seek to adapt to climate change. Discoveries using these long-term datasets were not anticipated when these studies were designed, however, data on these species offer important insights allowing for the detection of environmental change, demonstrating the value of long-term ecological research.
Data availability
All data are available and archived at the following sites: https://doi.org/10.6073/pasta/7c78d662e847cdbe33584add8f809165, https://doi.org/10.6073/pasta/9437d1603044f5b92189110dd8343763, https://doi.org/10.6073/pasta/0066d6b04e736af5f234d95d97ee84f3.
References
Baker, J., Meade, A., Pagel, M. & Venditti, C. Adaptive evolution toward larger size in mammals. Proc. Natl. Acad. Sci. USA 112(16), 5093–5098. https://doi.org/10.1073/pnas.1419823112 (2015).
Calder, W. A. Size, Function, and Life History (Courier Corporation, 1996).
Peters, R. H. & Peters, R. H. The Ecological Implications of Body Size Vol. 2 (Cambridge University Press, 1986).
Barneche, D. R., Robertson, D. R., White, C. R. & Marshall, D. J. Fish reproductive-energy output increases disproportionately with body size. Science 360(6389), 642–645. https://doi.org/10.1126/science.aao6868 (2018).
Newman, M. A. Social behavior and interspecific competition in two trout species. Physiol. Zool. 29(1), 64–81 (1956).
Persson, L. Asymmetrical competition: Are larger animals competitively superior?. Am. Naturalist 126(2), 261–266 (1985).
Daufresne, M., Lengfellner, K. & Sommer, U. Global warming benefits the small in aquatic ecosystems. Proc. Natl. Acad. Sci. USA 106(31), 12788–12793. https://doi.org/10.1073/pnas.0902080106 (2009).
Gardner, J. L., Peters, A., Kearney, M. R., Joseph, L. & Heinsohn, R. Declining body size: A third universal response to warming?. Trends Ecol. Evolut. 26(6), 285–291. https://doi.org/10.1016/j.tree.2011.03.005 (2011).
Ohlberger, J. Climate warming and ectotherm body size—From individual physiology to community ecology. Funct. Ecol. 27(4), 991–1001. https://doi.org/10.1111/1365-2435.12098 (2013).
Parmesan, C. & Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37. https://doi.org/10.1038/nature01286 (2003).
Cohen, J. M., Lajeunesse, M. J. & Rohr, J. R. A global synthesis of animal phenological responses to climate change. Nat. Clim. Change 8(3), 224–228. https://doi.org/10.1038/s41558-018-0067-3 (2018).
Thackeray, S. J. et al. Phenological sensitivity to climate across taxa and trophic levels. Nature. 535, 241. https://doi.org/10.1038/nature18608 (2016).
Connette, G. M., Crawford, J. A. & Peterman, W. E. Climate change and shrinking salamanders: Alternative mechanisms for changes in plethodontid salamander body size. Glob. Change Biol. 21(8), 2834–2843. https://doi.org/10.1111/gcb.12883 (2015).
Naya, D. E., Naya, H. & Cook, J. Climate change and body size trends in aquatic and terrestrial endotherms: Does habitat matter?. PloS ONE 12(8), e0183051. https://doi.org/10.1371/journal.pone.0183051 (2017).
Solokas, M. A. et al. Shrinking body size and climate warming: Many freshwater salmonids do not follow the rule. Glob. Change Biol. 29(9), 2478–2492. https://doi.org/10.1111/gcb.1662 (2023).
Doan, N. X. et al. Extreme temperature impairs growth and productivity in a common tropical marine copepod. Sci. Rep. 9(1), 4550. https://doi.org/10.1038/s41598-019-40996-7 (2019).
Forster, J., Hirst, A. G. & Atkinson, D. Warming-induced reductions in body size are greater in aquatic than terrestrial species. Proc. Natl. Acad. Sci. USA 109(47), 19310–19314. https://doi.org/10.1073/pnas.1210460109 (2012).
Baudron, A. R., Needle, C. L., Rijnsdorp, A. D. & Marshall, C. T. Warming temperatures and smaller body sizes: Synchronous changes in growth of North Sea fishes. Glob. Change Biol. 20(4), 1023–1031. https://doi.org/10.1111/gcb.12514 (2014).
Hoy, S. R., Peterson, R. O. & Vucetich, J. A. Climate warming is associated with smaller body size and shorter lifespans in moose near their southern range limit. Glob. Change Biol. 24(6), 2488–2497. https://doi.org/10.1111/gcb.14015 (2018).
Oke, K. B. et al. Recent declines in salmon body size impact ecosystems and fisheries. Nat. Commun. 11(1), 4155. https://doi.org/10.1038/s41467-020-17726-z (2020).
Asta, A. et al. Fish body sizes change with temperature but not all species shrink with warming. Nat. Ecol. Evolut. 4(6), 809–814. https://doi.org/10.1038/s41559-020-1171- (2020).
Campbell Grant, E. H. Please don’t misuse the museum: ‘Declines’ may be statistical. Glob. Change Biol. 21(3), 1018–1024. https://doi.org/10.1111/gcb.12702 (2015).
Boutin, S. & Lane, J. E. Climate change and mammals: Evolutionary versus plastic responses. Evolut. Appl. 7(1), 29–41. https://doi.org/10.1111/eva.12121 (2014).
Fagen, R. Long-term trends in maximum size of sport-caught Chinook salmon (Oncorhynchus tshawytscha): A data-analytic approach to weights of first-prize fish in four southeastern Alaska salmon derbies. Fisheries Res. 6(2), 125–134. https://doi.org/10.1016/0165-7836(88)90032-X (1988).
Jennings, S., Greenstreet, S. P. R. & Reynolds, J. D. Structural change in an exploited fish community: A consequence of differential fishing effects on species with contrasting life histories. J. Animal Ecol. 68(3), 617–627. https://doi.org/10.1046/j.1365-2656.1999.00312.x (1999).
Mkumbo, O. C. & Marshall, B. E. The Nile perch fishery of Lake Victoria: Current status and management challenges. Fisheries Manag. Ecol. 22(1), 56–63. https://doi.org/10.1111/fme.12084 (2015).
Ngor, P. B. et al. Evidence of indiscriminate fishing effects in one of the world’s largest inland fisheries. Sci. Rep. 8(1), 8947. https://doi.org/10.1038/s41598-018-27340-1 (2018).
Bigler, B. S., Welch, D. W. & Helle, J. H. A review of size trends among North Pacific salmon (Oncorhynchus spp). Can. J. Fisheries Aquat. Sci. 53(2), 455–465. https://doi.org/10.1139/f95-181 (1996).
Gwinn, D. C. et al. Rethinking length-based fisheries regulations: The value of protecting old and large fish with harvest slots. Fish Fisheries 16(2), 259–281. https://doi.org/10.1111/faf.12053 (2015).
Jeffrey, K. M., Côté, I. M., Irvine, J. R. & Reynolds, J. D. Changes in body size of Canadian Pacific salmon over six decades. Can. J. Fisheries Aquatic Sci. 74(2), 191–201. https://doi.org/10.1139/cjfas-2015-0600 (2016).
Rypel, A. L., Lyons, J., Griffin, J. D. T. & Simonson, T. D. Seventy-year retrospective on size-structure changes in the recreational fisheries of Wisconsin. Fisheries 41(5), 230–243. https://doi.org/10.1080/03632415.2016.1160894 (2016).
Flitcroft, R. L., Arismendi, I. & Santelmann, M. V. A review of habitat connectivity research for Pacific salmon in marine, estuary, and freshwater environments. J. Am. Water Resources Assoc. 55(2), 430–441. https://doi.org/10.1111/1752-1688.12708 (2019).
Haig, S. M., Murphy, S. P., Matthews, J. H., Arismendi, I. & Safeeq, M. Climate-altered wetlands challenge waterbird use and migratory connectivity in arid landscapes. Sci. Rep. 9(1), 4666. https://doi.org/10.1038/s41598-019-41135-y (2019).
Gérard, M. et al. Shift in size of bumblebee queens over the last century. Glob. Change Biol. 26(3), 1185–1195. https://doi.org/10.1111/gcb.14890 (2020).
Gardner, J. L. et al. Dynamic size responses to climate change: Prevailing effects of rising temperature drive long-term body size increases in a semi-arid passerine. Glob. Change Biol. 20(7), 2062–2075 (2014).
Bergmann, C. Uber die Verhaltnisse der warmeokonomie der Thiere zu uber Grosso. Gottinger Studien 3, 595–708 (1847).
Angilletta, M. J. & Dunham, A. E. The temperature-size rule in ectotherms: Simple evolutionary explanations may not be general. Am. Naturalist 162(3), 332–342. https://doi.org/10.1086/377187 (2003).
Belk, M. C. & Houston, D. D. Bergmann’s Rule in ectotherms: A test using freshwater fishes. Am. Naturalist 160(6), 803–808. https://doi.org/10.1086/343880 (2002).
Adams, D. C. & Church, J. O. Amphibians do not follow Bergmann’s rule. Evolution 62(2), 413–420. https://doi.org/10.1111/j.1558-5646.2007.00297.x (2008).
Damuth, J. Of size and abundance. Nature 351(6324), 268–269. https://doi.org/10.1038/351268a0 (1991).
Schmid, P. E., Tokeshi, M. & Schmid-Araya, J. M. Relation between population density and body size in stream communities. Science 289(5484), 1557–1560. https://doi.org/10.1126/science.289.5484.1557 (2000).
Burton, T., Killen, S. S., Armstrong, J. D. & Metcalfe, N. B. What causes intraspecific variation in resting metabolic rate and what are its ecological consequences?. Proc. R. Soc. B Biol. Sci. 278(1724), 3465–3473. https://doi.org/10.1098/rspb.2011.1778 (2011).
Brown, J. H. & Nicoletto, P. F. Spatial scaling of species composition: Body masses of North American land mammals. Am. Naturalist 138(6), 1478–1512. https://doi.org/10.1086/285297 (1991).
Ohlberger, J., Cline, T. J., Schindler, D. E. & Lewis, B. Declines in body size of sockeye salmon associated with increased competition in the ocean. Proc. R. Soc. B. https://doi.org/10.1098/rspb.2022.2248 (2023).
Arismendi, I., Johnson, S. L., Dunham, J. B. & Haggerty, R. Descriptors of natural thermal regimes in streams and their responsiveness to change in the Pacific Northwest of North America. Freshw. Biol. 58(5), 880–894. https://doi.org/10.1111/fwb.12094 (2013).
Olden, J. D. & Poff, N. L. Redundancy and the choice of hydrologic indices for characterizing streamflow regimes. River Res. Appl. 19(2), 101–121. https://doi.org/10.1002/rra.700 (2003).
Baldwin, C. M., Beauchamp, D. A. & Van Tassell, J. J. Bioenergetic assessment of temporal food supply and consumption demand by salmonids in the Strawberry Reservoir food web. Trans. Am. Fisheries Society 129, 429–450 (2000).
Parker, M. S. Feeding ecology of stream-dwelling Pacific Giant Salamander larvae (Dicamptodon tenebrosus). Copeia 1994(3), 705–718. https://doi.org/10.2307/1447187 (1994).
Sheldon, K. A. & Richardson, J. S. Season-specific survival rates and densities of coastal cutthroat trout across stream sizes in southwestern British Columbia. Ecol. Freshw. Fish 31(1), 102–117 (2022).
Arismendi, I., Penaluna, B.E., Gregory, S.V. Trout under drought: A long-term study of annual growth and condition of stream-living coastal cutthroat trout (Oncorhynchus clarkii clarkii). in (Lobon-Cervia, J., Budy, P., Gresswell, R. eds.) Advances in the Ecology of Stream-Dwelling Salmonids. Fish & Fisheries Series, Vol. 44. (Springer, 2024). https://doi.org/10.1007/978-3-031-44389-3_16
Grossman, G. D. & Simon, T. N. Density-dependent effects on salmonid populations: A review. Ecol. Freshw. Fish 29, 400–418. https://doi.org/10.1111/eff.12523 (2020).
Nussbaum, R.A., E.D. Brodie, Jr., & Storm, R.M. (1983) Amphibians and reptiles of the Pacific Northwest. University of Idaho Press.
Jaeger, R. G. Density-dependent and density-independent causes of extinction of a salamander population. Evolution 34(4), 617–621. https://doi.org/10.2307/2408016 (1980).
Penaluna, B. E., Dunham, J. B. & Andersen, H. V. Nowhere to hide: The importance of instream cover for stream-living Coastal Cutthroat Trout during seasonal low flow. Ecol. Freshw. Fish 30, 256–269 (2021).
Roni, P. Habitat use by fishes and Pacific Giant Salamanders in small western Oregon and Washington streams. Trans. Am. Fisheries Society 131(4), 743–761. https://doi.org/10.1577/1548-8659(2002)131%3c0743:hubfap%3e2.0.co;2 (2002).
Welsh, H. H. & Lind, A. J. Multiscale habitat relationships of stream amphibians in the Klamath-Siskiyou region of California and Oregon. J. Wildlife Manag. 66(3), 581–602 (2002).
Glova, G. L. Interaction for food and space between experimental populations of juvenile coho salmon (Oncorhynchus kisutch) and coastal cutthroat trout (Salmo clarki) in a laboratory stream. Hydrobiologia 131, 155–168 (1986).
Wooster, D., Miller, S. W. & DeBano, S. J. Impact of season-long water abstraction on invertebrate drift composition and concentration. Hydrobiologia 772, 15–30 (2016).
Dodds, W. K. et al. Surprises and insights from long-term aquatic data sets and 559 experiments. BioScience 62(8), 709–721. https://doi.org/10.1525/bio.2012.62.8.4 (2012).
Millidine, K. J., Armstrong, J. D. & Metcalfe, N. B. Presence of shelter reduces maintenance metabolism of juvenile salmon. Funct. Ecol. 20(5), 839–845. https://doi.org/10.1111/j.1365-2435.2006.01166.x (2006).
Dwyer, W. P. & Kramer, R. H. The influence of temperature on scope for activity in Cutthroat Trout, Salmo clarki. Trans. Am. Fisheries Society 104(3), 552–554. https://doi.org/10.1577/1548-8659(1975)104%3c552:tiotos%3e2.0.co;2 (1975).
Beitinger, T. L., Bennett, W. A. & McCauley, R. W. Temperature tolerances of North American freshwater fishes exposed to dynamic changes in temperature. Environ. Biol. Fishes 58(3), 237–275. https://doi.org/10.1023/A:1007676325825 (2000).
Caruso, N. M., Sears, M. W., Adams, D. C. & Lips, K. R. Widespread rapid reductions in body size of adult salamanders in response to climate change. Glob. Change Biol. 20(6), 1751–1759. https://doi.org/10.1111/gcb.12550 (2014).
Bateman, D. S. et al. Fish response to contemporary timber harvest practices in a second-growth forest from the central Coast Range of Oregon. Forest Ecol. Manag. 411, 142–157. https://doi.org/10.1016/j.foreco.2018.01.030 (2018).
Moring, J. R. (1975). The Alsea Watershed Study: Effects of logging on the aquatic resources of three headwater streams of the Alsea River, Oregon. Part I–Biological Studies. Oregon State University. https://ir.library.oregonstate.edu/concern/technical_reports/765372384
Stednick, J. D. (ed.) Hydrological and Biological Responses to Forest Practices (Springer, 2008).
Swanson, F. J., Gregory, S. V., Sedell, J. R., & Campbell, A. G. (1982). Land-water interactions: the riparian zone. in (Edmonds, R. L. Ed.) Analysis of Coniferous Forest Ecosystems in the Western United States. (pp 267–291). US/IBP Synthesis Series 14. Hutchinson Ross Publishing Co.
Jones, J. A. & Post, D. A. Seasonal and successional streamflow response to forest cutting and regrowth in the northwest and eastern United States. Water Resources Res. https://doi.org/10.1029/2003wr002952 (2004).
Mellina, E. & Hinch, S. G. Influences of riparian logging and in-stream large wood removal on pool habitat and salmonid density and biomass: A meta-analysis. Can. J. Forest Res. 39(7), 1280–1301. https://doi.org/10.1139/X09-037 (2009).
Moore, R. D. & Wondzell, S. Physical hydrology and the effects of forest harvesting in the Pacific Northwest: A review. J. Am. Water Resources Assoc. 41(4), 763–784 (2005).
Penaluna, B. E. et al. Local variability Mediates vulnerability of trout populations to land use and climate change. PloS ONE 10(8), e0135334. https://doi.org/10.1371/journal.pone.0135334 (2015).
Hatten, J. A. et al. Effects of contemporary forest harvesting on suspended sediment in the Oregon Coast Range: Alsea Watershed Study revisited. Forest Ecol. Manag. 408, 238–248. https://doi.org/10.1016/j.foreco.2017.10.049 (2018).
Behnke, R. J. Native Trout of Western North America 6 (American Fisheries Society Monograph (USA), 1992).
Penaluna, B. E. et al. Conservation of native Pacific trout diversity in western North America. Fisheries 41(6), 286–300 (2016).
Good, D. A. Hybridization and cryptic species in Dicamptodon (Caudata: Dicamptodontidae). Evolution 43(4), 728–744. https://doi.org/10.1111/j.1558-5646.1989.tb05172.x (1989).
Nussbaum, R. A. (1976). Geographic variation and systematics of salamaders of the genus Dicamptodon Strauch (Ambystomatidae). Miscellaneous Publications No. 149. Museum of Zoology, University of Michigan. https://deepblue.lib.umich.edu/bitstream/handle/2027.42/56393/MP149.pdf?sequence=1
Hawkins, C. P., Murphy, M. L., Anderson, N. & Wilzbach, M. A. Density of fish and salamanders in relation to riparian canopy and physical habitat in streams of the northwestern United States. Can. J. Fisheries Aquat. Sci. 40(8), 1173–1185 (1983).
Trotter, P. C. Coastal Cutthroat Trout: A life history compendium. Trans. Am. Fisheries Society 118(5), 463–473. https://doi.org/10.1577/1548-8659(1989)118%3c0463:cctalh%3e2.3.co;2 (1989).
Heggenes, J., Northcote, T. & Peter, A. Seasonal habitat selection and preferences by cutthroat trout (Oncorhynchus clarki) in a small, coastal stream. Can. J. Fisheries Aquat. Sci. 48, 1364–1370. https://doi.org/10.1139/f91-163 (1991).
Duellman, W. E. & Trueb, L. Biology of Amphibians (Johns Hopkins University Press, 1994).
Chelgren, N. D. & Adams, M. J. Inference of timber harvest effects on survival of stream amphibians is complicated by movement. Copeia 105(4), 712–725 (2017).
Sagar, J. P., Olson, D. H. & Schmitz, R. A. Survival and growth of larval coastal giant salamanders (Dicamptodon tenebrosus) in streams in the Oregon Coast Range. Copeia 2007(1), 123–130 (2007).
Johnston, B. & Frid, L. Clearcut logging restricts the movements of terrestrial Pacific giant salamanders (Dicamptodon tenebrosus Good). Can. J. Zool. 80(12), 2170–2177. https://doi.org/10.1139/z02-213 (2002).
Adams, M. J. & Bury, R. B. The endemic headwater stream amphibians of the American Northwest: Associations with environmental gradients in a large forested preserve. Glob. Ecol. Biogeogr. 11(2), 169–178. https://doi.org/10.1046/j.1466-822X.2002.00272.x (2002).
Gregory, S. & Arismendi, I. Aquatic vertebrate population study in Mack Creek, Andrews Experimental Forest, 1987 to present. Long-Term Ecol. Res. https://doi.org/10.6073/pasta/7c78d662e847cdbe33584add8f809165 (2020).
Mann, H. B. Nonparametric tests against trend. Econometrica J. Econometr. Society. 13(3), 245–259 (1945).
Sen, P. K. Robustness of some nonparametric procedures in linear models. Ann. Math. Stat. 39(6), 1913–1922 (1968).
Esterby, S. R. Review of methods for the detection and estimation of trends with emphasis on water quality applications. Hydrol. Process. 10(2), 127–149 (1996).
Patakamuri, S. K., & O’Brien, N. (2022). Modifiedmk: Modified versions of Mann Kendall and Spearman’s Rho trend tests. R package version 1.6, https://CRAN.R-Project.org/package=modifiedmk
Yue, S. & Pilon, P. A comparison of the power of thettest, Mann-Kendall and bootstrap tests for trend detection/Une comparaison de la puissance des teststde Student, de Mann-Kendall et du bootstrap pour la détection de tendance. Hydrol. Sci. J. 49(1), 21–37. https://doi.org/10.1623/hysj.49.1.21.53996 (2004).
Hamed, K. H. Enhancing the effectiveness of prewhitening in trend analysis of hydrologic data. J. Hydrol. 368, 143–155 (2009).
Johnson, S., Wondzell, S. & Rothacher, J. Stream discharge in gaged watersheds at the HJ Andrews Experimental Forest, 1949 to present. Long-Term Ecol. Res. https://doi.org/10.6073/pasta/0066d6b04e736af5f234d95d97ee84f3 (2020).
Gregory, S. & Johnson, S. Stream and air temperature data from stream gages and stream confluences in the Andrews Experimental Forest, 1950 to present. Long-Term Ecol. Res. https://doi.org/10.6073/pasta/9437d1603044f5b92189110dd8343763 (2019).
Burnham, K. P., Anderson, D. R. & Huyvaert, K. P. AIC model selection and multimodel inference in behavioral ecology: some background, observations, and comparisons. Behav. Ecol. Sociobiol. 65(1), 23–35 (2011).
Barton, K. & Barton, M. K. Package ‘MuMIn’. Version 1, 18 (2018).
Dormann, C. F. et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 36(1), 27–46. https://doi.org/10.1111/j.1600-0587.2012.07348.x (2013).
Moriasi, D. N. et al. Model evaluation guidelines for systematic quantification of accuracy in watershed simulations. Trans. ASABE. 50(3), 885–900. https://doi.org/10.13031/2013.23153 (2007).
Calcagno, V. & de Mazancourt, C. Glmulti: An R package for easy automated model selection with (generalized) linear models. J. Stat. Softw. 34(12), 1–29. https://doi.org/10.18637/jss.v034.i12 (2010).
McCarthy, T., Masson, P., Thieme, A., Leimgruber, P. & Gratwicke, B. The relationship between climate and adult body size in redback salamanders (Plethodon cinereus). Geo Geogr. Environ. 4(1), e00031. https://doi.org/10.1002/geo2.31 (2017).
Helle, J. H., Martinson, E. C., Eggers, D. M. & Gritsenko, O. Influence of salmon abundance and ocean conditions on body size of Pacific salmon. North Pacific Anadromous Fish Commission Bull. 4, 289–298 (2007).
Iglesias, V., Travis, W. R. & Balch, J. K. Recent droughts in the United States are among the fastest-developing of the last seven decades. Weather Clim. Extremes 37, 100491 (2022).
Al-Chokhachy, R. et al. Stream size, temperature, and density explain body sizes of freshwater salmonids across a range of climate conditions. Can. J. Fisheries Aquat. Sci. 79(10), 1729–1744. https://doi.org/10.1139/cjfas-2021-0343 (2022).
Green, D. M. & Middleton, J. Body size varies with abundance, notclimate, in an amphibian population. Ecography 36, 947–955 (2013).
Esselstyn, J. A. & Wildman, R. C. Observations of Juga in the diet of larval Pacific Giant Salamanders (Dicamptodon tenebrosus). Northwestern Naturalist 78(2), 70–73. https://doi.org/10.2307/3536849 (1997).
Minshall, G. W. et al. Interbiome comparison of stream ecosystem dynamics. Ecol. Monogr. 53(1), 1–25. https://doi.org/10.2307/1942585 (1983).
Lobón-Cerviá, J. Density-dependent growth in stream-living brown trout Salmo trutta L. Funct. Ecol. 21(1), 117–124 (2007).
Chapman, D. W. Food and space as regulators of salmonid populations in streams. Am. Nat. 100(913), 345–357 (1966).
Stralberg, D. et al. Climate-change refugia in boreal North America: What, where, and for how long?. Front. Ecol. Environ. 18(5), 261–270. https://doi.org/10.1002/fee.2188 (2020).
Betts, M. G., Phalan, B., Frey, S. J. K., Rousseau, J. S. & Yang, Z. Old-growth forests buffer climate-sensitive bird populations from warming. Diversity Distributions 24(4), 439–447. https://doi.org/10.1111/ddi.12688 (2018).
Ebersole, J. L., Quiñones, R. M., Clements, S. & Letcher, B. H. Managing climate refugia for freshwater fishes under an expanding human footprint. Front. Ecol. Environ. 18(5), 271–280. https://doi.org/10.1002/fee.2206 (2020).
Gregory, S. V., Schwartz, J. S., Hall, J. D., Wildman, R. C. & Bisson, P. A. Long-term trends in habitat and fish populations in the Alsea basin. In Hydrological and Biological Responses to Forest Practices (ed. Stednick, J. D.) 237–257 (Springer, 2008).
Bateman, D. S. et al. Fish response to successive clearcuts in a second-growth forest from the central Coast range of Oregon. Forest Ecol. Manag. 496, 119447 (2021).
Patrick, C. J. et al. Precipitation and temperature drive continental-scale patterns in stream invertebrate production. Sci. Adv. 5(4), eaav2348. https://doi.org/10.1126/sciadv.aav2348 (2019).
van Klink, R. et al. Meta-analysis reveals declines in terrestrial but increases in freshwater insect abundances. Science 368(6489), 417–420. https://doi.org/10.1126/science.aax9931 (2020).
Zhao, C., Brissette, F., Chen, J. & Martel, J. Frequency change of future extreme summer meteorological and hydrological droughts over North America. J. Hydrol. 584, 124316 (2020).
Lake, P. S. Ecological effects of perturbation by drought in flowing waters. Freshw. Biol. 48, 1161–1172. https://doi.org/10.1046/j.1365-2427.2003.01086.x (2003).
Lennox, R. J., Crook, D. A. & Moyle, P. B. Toward a better understanding of freshwater fish responses to an increasingly drought-stricken world. Rev. Fish Biol. Fisheries 29, 71–92. https://doi.org/10.1007/s11160-018-09545-9 (2019).
Bogan, M. T., Boersma, K. S. & Lytle, D. A. Resistance and resilience of invertebrate communities to seasonal and supraseasonal drought in arid-land headwater streams. Freshw. Biol. 60, 2547–2558. https://doi.org/10.1111/fwb.12522 (2015).
Piniewski, M. et al. Responses of fish and invertebrates to floods and droughts in Europe. Ecohydrology. 10, e1793. https://doi.org/10.1002/eco.1793 (2017).
Humphries, P. & Baldwin, D. S. Drought and aquatic ecosystems: An introduction. Freshw. Biol. 48, 1141–1146. https://doi.org/10.1046/j.1365-2427.2003.01092.x (2003).
Cano-Barbacil, C. et al. Key factors explaining critical swimming speed in freshwater fish: A review and statistical analysis for Iberian species. Sci. Rep. 10, 18947. https://doi.org/10.1038/s41598-020-75974-x (2020).
Simpson, A. Differences in body size and lipid reserves between maturing and nonmaturing Atlantic salmon parr, Salmo salar L. Can. J. Zool. 70, 1737–1742 (1992).
Butzge, A. J. et al. Early warming stress on rainbow trout juveniles impairs male reproduction but contrastingly elicits intergenerational thermotolerance. Sci. Rep. 11(1), 17053. https://doi.org/10.1038/s41598-021-96514 (2021).
McMillan, J. R. et al. Individual condition and stream temperature influence early maturation of rainbow and steelhead trout, Oncorhynchus mykiss. Environ. Biol. Fishes 93, 343–355. https://doi.org/10.1007/s10641-011-9921-0 (2012).
Acknowledgements
Jim Hall for the vision of stream research in the H.J. Andrews Experimental Forest. Randy Wildman and Linda Ashkenas assisted in the field for more than 30 years at the H.J. Andrews Experimental Forest. Gary Lamberti, Kelly Moore, Josh Williams, Justin Silbernagel, Sam Rabe, Nick Chambers, David Leer, Francisco Tinoco-Pickens, Alex Koeberle, Lauren Zatkos, Emilee Mowlds, Andres Olivos and many others helped in the field at the H.J. Andrews Experimental Forest. Data and facilities were provided by the HJ Andrews Experimental Forest and Long Term Ecological Research (LTER) program, administered cooperatively by the USDA Forest Service Pacific Northwest Research Station, Oregon State University, and the Willamette National Forest. This material is based upon work supported by the National Science Foundation under the LTER Grants: LTER1 DEB-8012162 (1980–1985), LTER2 DEB- 8514325 (1985–1990), LTER3 DEB-9011663 (1990–1996), LTER4 DEB- 9632921 (1996–2002), LTER5 DEB-0218088 (2002–2008), LTER6 DEB-0823380 (2008–2014), LTER7 DEB-1440409 (2014–2020), LTER8 DEB-2025755 (2020–2026). John Schwartz, Randy Wildman, Linda Ashkenas, and many others helped in the field at the AWS. Jason Dunham and Dave Hockman-Wert provided support to access the data for the AWS. The original AWS from 1957 to 1961 was funded by Oregon State Game Commission, Oregon State College, Oregon Natural Resources Committee, U. S. Geological Survey, Georgia-Pacific Corporation, and Oregon Cooperative Wildlife Research Unit. The Game Commission and Oregon natural Resources Committee remained the early major funders until 1974. The AWS sampling from 1988 to 1996 was supported by the COPE Program, which received funding from the state of Oregon and the forest industry. The Watershed Research Cooperative of Oregon State University provided logistical and institutional support for the AWS. The National Council for Air and Stream Improvement (NCASI) and Plum Creek Timber Company (now Weyerhaeuser Company) and the Oregon Forest and Industries Council (OFIC) provided funds to support the AWS. Research conducted under Oregon State University Institutional Animal Care permits 3720, 4076, 4379, 4796, and 4816. Kathryn Ronnenberg for copyediting and providing feedback on figures. Two anonymous reviewers provided insightful comments that improved our manuscript.
Author information
Authors and Affiliations
Contributions
I.A. had the original idea for the manuscript; I.A., S.V.G., and D.S.B collected biological data; I.A. analyzed the data and made the figures; I.A. and B.E.P. developed ideas and wrote the first draft of the manuscript; all co-authors substantially contributed to revisions of the original draft. All authors gave final approval for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arismendi, I., Gregory, S.V., Bateman, D.S. et al. Shrinking sizes of trout and salamanders are unexplained by climate warming alone. Sci Rep 14, 13614 (2024). https://doi.org/10.1038/s41598-024-64145-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-64145-x
- Springer Nature Limited