Abstract
Rose flowers (Rosa hybrida L.) are highly perishable and have a limited vase life. This study evaluated the effects of preharvest foliar applications of γ-aminobutyric acid (GABA) and calcium chloride (CaCl2), individually and combined, on antioxidant responses and vase life of cut Jumilia rose flowers. Treatments included foliar sprays of GABA at 0, 20, 40, and 60 mM and CaCl2 at 0, 0.75%, and 1.5%, applied in a factorial design within a completely randomized setup before harvest. Results showed GABA and CaCl2 interaction (especially, 60 mM GABA and 1.5% CaCl2) significantly increased enzymatic antioxidants including superoxide dismutase, catalase, and peroxidase, as well as non-enzymatic antioxidants such as flavonoids, carotenoids, phenolics, and antioxidant activity in petals compared to control. SOD activity in roses, treated with CaCl2 (1.5%) and GABA (60 mM), peaked at 7.86 units. mg−1 protein min−1, showing a nearly 2.93-fold increase over the control (2.68 units. mg−1 protein min−1). A parallel trend was observed for CAT activity. These treatments also reduced petal malondialdehyde content and polyphenol oxidase activity. Protein content and vase life duration increased in all treatments. Plants treated with a combination of GABA (20 mM) and CaCl2 (0.75%), GABA (60 mM) and CaCl2 (1.5%), or GABA (40 mM) individually exhibited the longest vase life duration. The co-application of GABA and CaCl2 improved the antioxidant activity and postharvest quality of cut roses by reducing PPO activity and MDA contents, increasing protein content and prolonging vase life. This treatment is a potential postharvest strategy to improve antioxidant capacity and delay senescence in cut roses.
Similar content being viewed by others
Introduction
Roses (Rosa hybrida L.) are considered the most diverse and widespread commercial flower in the global ornamental plant industry1. In terms of production and economic importance, they rank first due to their growth pattern, wide range of flower colors, and variety of flower shapes2. When it comes to the marketing appeal of roses, a number of characteristics play a crucial role in meeting consumer demands and enhancing the value and significance of roses in the marketplace. These include factors such as flower color, stem diameter/length, flower bud height/diameter, and, most importantly, vase life3. This vital parameter, i.e., vase life, depends on a range of factors, e.g., variety, optimal growing conditions, a/biotic stresses, harvesting processes, post-harvest handling procedures, etc.4. Fertilization management and some plant growth bioregulators application during plant growth improve the quality and vase life of cut flowers.
γ-aminobutyric acid (GABA) is a four-carbon, non-proteinogenic amino acid. This naturally occurring bioactive compound is crucial in various plant physiological processes, such as growth, signal transmission, and stress responses. Research has demonstrated that applying GABA before and after harvest can prolong the vase life of numerous cut flowers. These include rose5, gerbera6,7, carnation8, protea9, anthurium10,11, daffodil12, and tuberose13. The mechanisms behind this extension of flower vase life are complex. GABA appears to regulate the expression of genes involved in hormone biosynthesis, transcriptional regulation, reactive oxygen species (ROS) generation, polyamine metabolism, and both enzymatic and non-enzymatic antioxidant reactions14. This compound plays a critical role in modulating the antioxidant system during plant growth by affecting the transcription of genes responsible for encoding antioxidant enzymes15. GABA application also has a significant impact on respiratory metabolism, leading to changes in the activity of numerous enzymes within the tricarboxylic acid (TCA) cycle16.
Calcium (Ca) is an essential macronutrient that plays a critical role in maintaining the structural integrity of cell walls, preserving membrane stability, and orchestrating cellular signaling processes in plants17. Its presence enhances the strength of cell walls by forming bonds with pectins, thus increasing rigidity and firmness, which provides essential mechanical support to plants18,19. In addition, the presence of Ca inhibits the activity of polygalacturonase enzymes, facilitating the maintenance of the middle lamella20. Furthermore, this essential macronutrient can modulate the activities of ACC synthase and ACC oxidase, leading to a reduction in endogenous ethylene production by the plant, thus slowing the process of senescence. A study by Islam, et al.21 showed an increase in antioxidant responses in tomato plants after the application of exogenous Ca. Due to these beneficial properties, the application of Ca salts, such as calcium chloride, calcium phosphate, calcium citrate, calcium oxide, and calcium lactate, has been reported to significantly prolong the vase life of cut flowers in various species, including Heliconia spp22, Gerbera23, and Gladiolus24. Ca has also been shown to delay senescence of cut roses by protecting both membrane phospholipids and membrane proteins from degradation while reducing ethylene production25.
There is limited existing literature on the combined use of GABA and Ca, especially regarding their influence on the antioxidant responses of rose flowers. Therefore, the main objective of this study was to evaluate the effects of GABA and Ca treatments, both individually and in combination, on the antioxidant responses of Jumilia rose cut flowers, with the ultimate goal of prolonging their vase life.
Materials and methods
Plant materials and experimental setup
The experiment was carried out in a factorial design within a completely randomized setup with three replications and five plants per replication, at the hydroponic greenhouse facilities of Sida Rose Company (Yasuj, Kohgiluyeh and Boyer-Ahmad Province, Iran, 2022).
Rosa hybrid cv. Jumilia grafted on Natal Briar rootstock was selected for the study, which were purchased from a local commercial producer that is approved by the Iranian medicinal plants association. These grafted plants were planted in 100 cm × 40 cm plastic pots, filled with a 1:1 ratio cocopeat/perlite (v/v). To ensure optimal growth conditions, the plants were fertigated with a carefully formulated nutritional solution, administered via an open drip irrigation system. The nutrient solution was delivered to the plants five times daily at 2 h intervals. Throughout the growth period, standard horticultural practices were employed, including pruning, pest and disease management, and branch bending. The greenhouse maintained average day/night temperatures of 24 ± 4/15 ± 2 °C and 40–60% relative humidity. Additional information about the water quality and nutrient solution composition can be found in Tables 1 and 2, respectively.
Treatments
The experiment involved two factors: the application of γ-amino butyric acid (GABA) at four different concentrations (0, 20, 40, and 60 mM), calcium chloride (CaCl2) at three levels (0, 0.75, and 1.5%): To apply these treatments, a hand-sprayer was used to evenly spray GABA and CaCl2 solutions to the plants, ensuring that the flowers were thoroughly wet to the point of runoff. Control flowers were sprayed solely with distilled water. This procedure was repeated three times, each spaced 7 days apart, leading up to the harvest of the flowers. Upon reaching the commercial harvest stage, the flowers were carefully cut and placed in containers filled with water. These containers were stored in a room where the temperature remained at an average of 24 ± 4/15 ± 2 °C during the day and night, while the relative humidity was maintained within the range of 40–60%. In this experiment, 15 rose branches were considered for each treatment, 10 branches for the flower vase life and 5 branches for other tests (biochemical and enzymatic).
Enzyme extraction
First, 0.5 g of petals were frozen and subsequently ground into a fine powder using liquid nitrogen and a mortar and pestle. The resulting powder was then homogenized in a 2 mL extraction buffer consisting of 50 mM potassium phosphate buffer at pH 8.0. To this buffer, 10% (w/v) polyvinylpyrrolidone (PVP), 0.1 mM ethylenediaminetetraacetic acid (EDTA), and 1 mM dithiothreitol (DTT) were added. The homogenate was centrifuged at 10,000 × g for 30 min, maintaining a temperature of 4 °C. Following centrifugation, the resulting supernatants were carefully collected.
Superoxide dismutase activity (SOD)
To determine the activity of superoxide dismutase (SOD, EC 1.15.1.1), the following procedure was followed: 0.1 mL of the enzymatic extract was added to a tube containing a mixture of 13 mM L-methionine, 25 mM nitroblue tetrazolium chloride (NBT), 0.1 mM EDTA, 50 mM sodium carbonate, and 2 mM riboflavin, all dissolved in a 50 mM phosphate buffer at pH 7.8, as described by Dhindsa, et al.26. The tube was then placed under two 15 W fluorescent lamps for a duration of 15 min. A control sample, without the enzyme, was also prepared to determine the maximal color change. To stop the reaction, the lights were switched off, and the tubes were kept in the dark. Additionally, a non-irradiated complete reaction mixture served as a blank. The absorbance was measured at 560 nm spectrophotometrically, and one unit of enzyme activity was defined as the amount of enzyme required to reduce the absorbance reading to 50% compared to the tubes lacking the enzyme. The SOD activity was expressed as units per milligram of protein per minute.
Catalase activity
Catalase (CAT, EC 1.11.1.6) activity was assessed spectrophotometrically following the method outlined by Chance and Maehly27. This involved monitoring the decrease in absorbance at 240 nm attributed to the consumption of H2O2. A reaction mixture of 1 mL was prepared, comprising 50 mM potassium phosphate buffer at pH 7.0 and 15 mM H2O2. The reaction was initiated by introducing 50 μL of the crude extract into this solution. The CAT activity was quantified and expressed as units, μmol of H2O2 consumed per minute, per milligram of protein.
Peroxidase activity
The guaiacol peroxidase (POD, EC 1.11. 1.7) activity was determined as follows: A 50 μL portion of the crude enzyme preparation was added to a 2 mL solution containing 50 mM potassium phosphate buffer (pH 7.0), 13 mM guaiacol, and 5 mM H2O2. The increase in absorbance, resulting from the oxidation of guaiacol (with an extinction coefficient of 26.6 mM-−1 cm−1), was monitored at 470 nm for one minute. The peroxidase activity was quantified and expressed in units, μmol guaiacol oxidized per minute, per milligrams of protein28.
Polyphenol oxidase and ACC synthase activity
Polyphenol oxidase (PPO, EC1.10.3.1) activity was determined following the protocol established by Kumar and Khan29. The assay mixture for PPO consisted of 2 mL of 0.1 M phosphate buffer at pH 6.0, 1 mL of 0.1 M catechol, and 0.5 mL of the enzyme extract. This mixture was incubated at 25 °C for 5 min, and the reaction was halted by adding 1 mL of 2.5 N H2SO4. The absorbance of the resulting purpurogallin was measured at 495 nm. PPO activity is expressed in units (μmol catechol oxidized per minute) per mg protein.
The ACC synthase activity in petals was measured according to the method of Jiang et al.30 with some modification. After preparation the samples, the ACC was converted to ethylene. The ethylene in the headspace was then measured using a Shimadzu GC 9A gas chromatograph. A unit of ACC synthase activity was defined as the amount of enzyme that catalyzed the formation of 1 nmol of ACC per hour under the stated assay conditions.
Malondialdehyde content
Malondialdehyde (MDA) content was assessed following the thiobarbituric acid (TBA) reaction, as originally outlined by Ali, et al.31, with minor adjustments. Initially, 200 mg of petal samples were homogenized in 2 mL of 0.1% trichloroacetic acid and subjected to centrifugation at 10,000 × g for 15 min. Next, 1 mL of the resulting supernatant was combined with 2.5 mL of 0.5% thiobarbituric acid in 20% trichloroacetic acid and subjected to incubation in hot water at 95 °C for 30 min. The reaction was promptly terminated by cooling the mixture on ice, followed by centrifugation at 10,000 × g for 30 min. The absorbance was measured at 532 and 600 nm. The concentration of MDA was determined by subtracting the nonspecific absorption at 600 nm from the absorption at 532 nm, using an absorbance coefficient of extinction (155 mM−1 cm−1).
Vase life
Sharp secateurs were used to cut flowers. The selection criteria included choosing flowers at the tight bud stage, characterized by fully developed coloration while the petals remained tightly closed, yet to begin unfolding. For the assessment of vase life, a total of 15 flower stems were harvested from each treatment group. These stems were then recut to a standardized length of 50 cm and placed individually in containers filled with water. The containers were maintained at room temperature, consistently set at 25 °C, with relative humidity levels ranging between 85 and 90%. In accordance with the criteria established by Jowkar, et al.32: the vase life was carefully monitored and evaluated on a daily basis. The end of the vase life was determined when either the neck of the flower was bent or the five outermost petals showed visible signs of wilting.
Total flavonoid content
To determine the total flavonoid content in the methanolic extract solution, the aluminum chloride assay, as described by Tohidi et al.34, was employed. An aliquot of 125 μl from the extract solution was mixed with 75 μl of 5% NaNO2 solution. The mixture was then allowed to stand for 5 min before the addition of 150 μl of aluminum chloride (10%) solution. Following this, 750 μl of NaOH solution (1 M) was incorporated, and the final volume of the mixture was adjusted to 2500 μl using deionized water. After incubating the mixture for 15 min, the absorbance was measured at 510 nm using a spectrophotometer. The total flavonoid content was quantified and expressed as milligrams of quercetin equivalent per gram of fresh weight.
Total phenolic content
Initially, 1.25 g of petal samples were combined with 25 mL of 80% methanol in an orbital shaker set at 150 rpm and maintained at a temperature of 25 °C. The mixture was allowed to shake for a duration of 24 h. The total phenolic compounds in the resulting methanolic extract solution was determined using the Folin Ciocalteu method, following the protocol outlined by Tohidi, et al.34, with minor adjustments. Specifically, 0.5 mL of the filtered methanolic extract was added to a test tube containing a mixture of 2.5 mL of the Folin Ciocalteu reagent (diluted tenfold) and 2 mL of 7.5% sodium carbonate. The contents of the test tube were thoroughly mixed. After heating this mixture at 45 °C for 15 min, the absorbance was measured at 765 nm using a spectrophotometer. Gallic acid was utilized as the standard for quantifying the total phenolic content. The resulting data were expressed as milligrams of gallic acid equivalent per gram of fresh weight.
Total antioxidant activity
The total antioxidant activity was determined using the DPPH (2,2-diphenyl-picryl-hydrazyl) radical degradation method. Specifically, 10 μL of petal extract was combined with 4 mL of distilled water in test tubes, followed by the addition of 1 mL of a 250 μM DPPH solution. Subsequently, the test tubes were left undisturbed in darkness for a 30-min incubation period. Afterward, the absorbance was measured at 517 nm using a spectrophotometer. The antioxidant activity was then calculated as the percentage of inhibition relative to the control, employing the following formula35: .
where A blank is the absorbance of the control reaction, and A sample is the absorbance of the test compound in the sample.
Petal carotenoid content
Petals (0.5 g) were extracted in 5 mL of acetone (80%), then centrifuged (8000 × g) for 10 min. The supernatant was used to make a final volume of 100 mL of the petal extract. Extraction of petal tissue with the buffer continued three times. The absorbance of the extract was read at 470 nm with a spectrophotometer and 80% acetone was used as a blank. Carotenoid content of petal tissue was determined using the following formula36: .
It should be noted that all the protocols used in this study are in accordance with national, and international guidelines and legislation.
Statistical analysis
Data were analyzed by SAS (9.4M7), and means were compared using Duncan’s multiple range tests at the 5% probability level. When the interaction between treatments was significant, as determined by ANOVA, main effects were not presented. (The raw data is attached as a supplementary file).
Results
Vase life
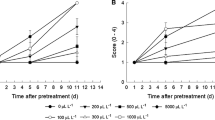
In all samples, an increase in vase life was observed, and this increase was significantly higher than that of the control group (Fig. 1). The longest vase life duration was noted in plants that were treated with combinations of GABA (20 mM) and CaCl2 (0.75%), GABA (60 mM) and CaCl2 (1.5%), or GABA (40 mM) applied individually.
Total protein content
In Fig. 2, the interaction effects of different concentrations of GABA (0, 20, 40, 60 mM) and CaCl2 (0, 0.75%, 1.5%) on the total protein content in rose petals are depicted. An increase in this parameter was observed across all treatments, with all treated samples exhibiting significantly higher protein content compared to the control group. The application of CaCl2 alone increased this parameter; however, the effect of GABA was more pronounced. In samples that received GABA treatments (alone), the protein content was higher than in those treated with CaCl2 alone. A synergistic effect was observed between these two compounds, as the combined effect was greater than the sum of their separate effects. Notably, in plants treated with the highest levels of GABA (60 mM) and CaCl2 (1.5%), the protein content was elevated to 9.17 mg. g−1 FW, representing an approximately 1.77-fold increase when compared to the control samples (5.18 mg. g−1 FW).
Flavonoid content, carotenoids and antioxidant activity
Table 3 demonstrates the interaction effect of different levels of GABA and CaCl2 on non-enzymatic antioxidants and total antioxidant activity in rose petals. An increase in flavonoid content in petals was observed with the elevation of both GABA and CaCl2 levels. The highest concentration of flavonoids (21.17 mg. g−1 FW) was obtained when roses were treated with a CaCl2 solution at a 1.5% concentration and GABA at 40 mM. A significant increase in petal carotenoid concentration was also noted in response to CaCl2 and/or GABA, whether applied individually or in combination. In all treated plants, this parameter was significantly higher compared to the control group, with samples treated with CaCl2 (1.5%) and GABA (60 mM) recording 29.53 mg. g−1 FW carotenoids, representing an increase of over 2.5 times in comparison to the control group. A dose-dependent increase in total phenolic compounds in the petals was observed with the application of GABA and CaCl2, reaching 26.55 mg. g−1 FW at the highest treatment levels. This concentration was 1.62 times higher than that of the control group, which displayed 16.36 mg. g−1 FW. Additionally, a significant enhancement in the antioxidant activity of the petals was observed with the application of CaCl2 and/or GABA, whether applied individually or in combination. The control group exhibited significantly lower antioxidant activity (26.61%) compared to all other treatments. Generally, the highest levels of carotenoids, phenolic content, and antioxidant activity were observed when using the highest combined concentrations of GABA and CaCl2.
Enzymatic antioxidant and polyphenol oxidase activities
Table 4 shows the interaction effects of different levels of GABA and CaCl2 on enzymatic antioxidant and polyphenol oxidase activities in rose petals. SOD activity exhibited a significant rise in response to higher concentrations of both GABA and CaCl2. The SOD activity in roses treated with a combination of CaCl2 (1.5%) and GABA (60 mM) reached 7.86 Units. mg−1 protein min−1, marking an approximately 2.93-fold increase compared to the control group (2.68 Units. mg−1 protein min−1). Similar trend was observed for the CAT activity. When roses were treated with CaCl2 (1.5%) and GABA (60 mM), the CAT activity reached 12.1 Units. mg−1 protein min−1, nearly tripling the activity level observed in the control group. The POD activity also followed a similar pattern of increase with the rise in CaCl2 and GABA concentrations. Samples treated with the highest levels of both compounds displayed a POD activity of 4.88 Units. mg−1 protein min−1 which was higher than all other treatments. In contrast, PPO activity showed a reverse response to elevated CaCl2 and GABA levels. The combination of CaCl2 (1.5%) and GABA (60 mM) resulted in a PPO activity of 1.84 Units. mg−1 protein min−1, which was lower than all other treatments.
ACC synthase activity
In the present study the application of GABA and CaCl2 significantly (P < 0.01) increased the ACC synthase activity in the petals of the cut rose flowers (Table 4). The results showed that the foliar pre-harvest application of GABA and CaCl2 reduced the ACC synthase activity. The highest and lowest of ACC synthase activity was obtained in the rose plants treated with GABA at 40 mM and CaCl2 at 1.5 and 0.75% rates and the untreated flowers (3.57, 3.96, and 8.18 nmol g-1 FW, respectively). In fact, the combined application of GABA and CaCl2 decreased ACC synthase activity by 151% compared to the untreated plants (Table 4).
MDA
The petal MDA content exhibited a significant decrease in roses subjected to higher levels of GABA and CaCl2. Specifically, in samples treated with CaCl2 (1.5%) and GABA (60 mM), the MDA content in the petals decreased by 20% relative to the control group (Fig. 3).
Correlation between parameters
Vase life showed no significant correlation with the parameters studied. It displayed a non-significant negative correlation with ACC and PPO, as well as a non-significant positive relationship with other characteristics. ACC had a highly significant positive correlation with PPO and a significant negative correlation with Ca, total phenolic compounds, SOD, CAT, and total antioxidant activity. Total phenolic compounds exhibited a negative correlation with ACC and PPO, but showed a significant positive correlation with ACC, SOD, CAT, and total antioxidant activity. PPO had a highly significant positive correlation with ACC but significant negative correlations with Ca, total phenolic compounds, SOD, CAT, and total antioxidant activity. SOD showed a highly significant positive correlation with Ca, Total Phenol, CAT, and total antioxidant activity, while maintaining a significant negative correlation with ACC and PPO. CAT had a highly significant negative correlation with ACC and PPO, and a significant positive correlation with other parameters, excluding vase life. Total antioxidant activity also demonstrated a highly significant negative correlation with ACC and PPO, along with a significant positive correlation with Ca, total phenolic compounds, SOD, and CAT (Table 5 and Fig. 4).
Discussion
Our results demonstrated that exogenous application of both GABA and CaCl2 can synergistically enhance the enzymatic/non-enzymatic antioxidant response in rose flowers. The increased flavonoid, carotenoid, phenolic, and antioxidant activity levels as well as higher SOD, CAT, and POD activity observed with combined GABA and CaCl2 application align with previous investigations showing similar antioxidant-elevating properties of these compounds. A number of investigations have ascertained that GABA can induce the activation of the antioxidant defense system in response to abiotic stresses, thereby mitigating oxidative damage caused by the production of reactive oxygen species (ROS)37,38,39. Subsequent to the application of exogenous GABA, a significant rise in the activity of enzymatic antioxidants within leaves is observed. This is accompanied by a reduction in the accumulation of ROS, consequently enhancing the resilience of seedlings to adverse environmental conditions40,41,42. In an in vitro experiment, it was demonstrated that GABA and proline reduced ROS levels. GABA exhibits a superior capacity for the removal of superoxide anions (O2-) and hydrogen peroxide (H2O2) compared to proline43.
Ca, through its binding affinity with the phospholipid bilayer, exerts regulatory control over membrane architecture, signaling cascades, and membrane functionality. Consequently, this involvement facilitates the reinforcement and improvement of structural integrity within membrane organelles in plants mitigating adverse environmental changes44. Previous studies have documented the beneficial influence of exogenous Ca application in alleviating environmental changes in plants. These advantageous effects have been attributed to physiological mechanisms, which include osmotic adjustment, improved antioxidant (enzymatic/non-enzymatic) responses, modulation of Na and K ion homeostasis, enhancement of proline accumulation, and facilitation of root and shoot growth45,46,47. In addition, Ca assumes a pivotal role as a secondary messenger in orchestrating plant responses to adverse environmental conditions48. There exists an interrelationship between this essential macronutrient and GABA concerning the activation of plant antioxidant responses49. In essence, the accumulation of GABA under both biotic and abiotic stress conditions is intricately linked to the regulation of intracellular Ca2+ and its interaction with calmodulin (CaM).
Under non-optimal conditions such as drought, salinity, and temperature stress, plants exhibit an elevation in intracellular Ca2+ levels. This surge in Ca2+ concentration serves as a stimulatory cue for Ca2+/CaM to activate glutamate decarboxylase (GAD), culminating in the accumulation of GABA. Trobacher, et al.50 reported that the regulatory network involving Ca2+/CaM, GAD, and GABA depends on MdGAD1 and MdGAD2 in apple. Their activity and spectral properties are modulated by Ca2+/CaM balance and acidic pH. Moreover, the application of exogenous Ca has been demonstrated to activate GAD, thereby promoting the accumulation of GABA in carrots and pears51,52,53. This collective body of research underscores the interplay between Ca signaling and the regulation of GABA levels in plants, particularly in response to challenging environmental conditions.
Our findings indicated that PPO activity decreased in response to elevated CaCl2 and GABA levels. The reduced PPO activity provides evidence that GABA and CaCl2 mitigate oxidative damage by enhancing antioxidants as higher PPO is associated with senescence, decay, and quality loss in flowers54,55,56.
Our findings indicate applying GABA and Ca could help maintain postharvest quality as treated samples exhibited prolonged vase life, diminished petal MDA content, and higher petal protein concentration. This was in agreement with previous investigations. Gerbera cut flowers, when subjected to a 1 mM GABA treatment, displayed a reduction in electrolyte leakage, H2O2, MDA, lipoxygenase (LOX), and phospholipase D (PLD) activity. Simultaneously, these treated flowers exhibited an increase in proline content and enhanced antioxidant enzyme activities6,7. The application of GABA, both pre- and post-harvest, in the vase solution at ambient temperatures, resulted in enhanced quality and extended vase life of rose cut flowers, as reported by Mirzaei Mashhoud, et al. 5. Similar finding were reported by Babarabie, et al.13. They observed an improved vase life of tuberose flowers treated with GABA. In a study on Narcissus tazetta cv. ‘Shahla-e-Shiraz’, it was observed that the activity of PPO experienced significant inhibition in the presence of GABA. Moreover, GABA played a crucial role in enhancing the relative water content of narcissus petals during storage by mitigating alterations in the cellular membrane stability index12. Abdolmaleki, et al.57 proposed the use of CaCl2 as a method for enhancing the postharvest longevity of roses. Their research revealed that ‘Dolce Vita’ roses treated with a CaCl2 and/or salicylic acid solutions exhibited significantly prolonged vase life. Our findings and this observed improvement can be attributed to the previously mentioned interplay between Ca and GABA, orchestrating plant responses under stressful conditions. The synergistic action of Ca and GABA appears to optimize these responses. In this context, the bolstered antioxidant response induced by Ca and GABA can effectively mitigate the rate of senescence. This results in the preservation of crucial cellular structures such as proteins and membrane functionality for an extended duration, ultimately contributing to the overall longevity and quality of postharvest cut flowers.
In the present study, several correlations were identified among the studied parameters. SOD and CAT were positively correlated. SOD plays a crucial role in the first line of defense against oxidative stress by converting superoxide anions into H2O2. Hydrogen peroxide is then efficiently converted into water and molecular oxygen by CAT, acting as a potent ROS scavenger58. SOD exhibited a strong positive correlation with total antioxidant activity, underscoring its significant role in the plant's antioxidant system59. Conversely, PPO showed a negative correlation with total antioxidant activity, CAT, and SOD, aligning with previous studies that reported a decrease in PPO activity following abiotic stress, associated with improved antioxidant capacity35. Total phenolic compounds were negatively correlated with PPO, potentially due to the enzymatic activity of PPO oxidizing phenolic compounds60. A positive correlation was observed between total phenolic compounds and enzymatic antioxidants—SOD, CAT, and total antioxidant activity. This suggests that polyphenols play a role in the plant's mechanism against ROS, similar to SOD and CAT, indicating that higher polyphenolic compounds may lead to increased total antioxidant activity58,61. Ca exhibited positive correlations with SOD, CAT, and total antioxidant activity, while showing a negative correlation with PPO. Calcium is known to play a crucial role in the activation of antioxidant enzymes such as SOD and CAT62. Ca has an inhibitory effect on PPO activity63. This can result in an increased content of polyphenolic compounds and improved antioxidant responses, thereby enhancing vase life.
Conclusion
In conclusion, our findings demonstrated that the combined application of GABA and CaCl2 can synergistically enhance antioxidant activity and postharvest quality in cut rose flowers. Our results also revealed diminished PPO activity and MDA content along with higher protein levels and an extended vase life duration, indicating GABA and CaCl2 help mitigate the senescence. Overall, this research highlights the potential of using GABA and CaCl2 treatment as an effective postharvest strategy to prolong quality and vase life of cut roses by enhancing antioxidant capacity and delaying senescence.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Debener, T. & Linde, M. Exploring complex ornamental genomes: The rose as a model plant. Crit. Rev. Plant Sci. 28, 267–280 (2009).
Veluru, A. et al. Characterization of Indian bred rose cultivars using morphological and molecular markers for conservation and sustainable management. Physiol. Mol. Biol. Plants 26, 95–106 (2020).
Hosseini Farahi, M., Kholdebarin, B., Eshghi, S., Jamali, B. & Reza Roosta, H. Changes in plant growth substances, contents and flower quality of rose cv. ‘Dolce Vita’ in response to nitrogen sources under soilless culture conditions. J. Plant Nutr. 42, 1047–1060. https://doi.org/10.1080/01904167.2019.1578373 (2019).
Fanourakis, D. et al. Sources of vase life variation in cut roses: A review. Postharvest Biol. Technol. 78, 1–15 (2013).
Mirzaei Mashhoud, M., Aelaei, M. & Mortazavi, S. N. γ-aminobutyric acid (GABA) treatment improved postharvest indices and vase-life of ‘Red Naomi’ rose cut flowers. Acta Hortic. https://doi.org/10.17660/ActaHortic.2016.1131.5 (2016).
Mohammadi, M., Aelaei, M. & Saidi, M. Pre-harvest and pulse treatments of spermine, γ- and β-aminobutyric acid increased antioxidant activities and extended the vase life of gerbera cut flowers ‘Stanza’. Ornam. Hortic. 26, 306–316. https://doi.org/10.1590/2447-536x.v26i2.2120 (2020).
Mohammadi, M., Aelaei, M. & Saidi, M. Pre-harvest spray of GABA and spermine delays postharvest senescence and alleviates chilling injury of gerbera cut flowers during cold storage. Sci. Rep. 11, 14166. https://doi.org/10.1038/s41598-021-93377-4 (2021).
Molaei, M., Farahmand, H. & Nasibi, F. Vase life and antioxidant status of two carnations (Dianthus caryophyllus L.) cultivars affected by gamma aminobutyric acid (GABA) treatments. J. Hortic. Postharvest Res. 4, 497–508. https://doi.org/10.22077/jhpr.2021.4309.1207 (2021).
Vardien, W., Jacobs, G. & Hoffman, E. W. The efficacy of γ-aminobutyric acid (GABA) as a postharvest treatment inProtea, to enhance vase life and maintain quality. Acta Hortic. https://doi.org/10.17660/ActaHortic.2018.1201.55 (2018).
Mahjoory, F., Ebrahimzadeh, A., Hassanpouraghdam, M. B. & Aazami Mavaloo, M. A. Postharvest GABA application effects on some biochemical characteristics of anthurium cut flowers under cold storage conditions. J. Ornam. Plants 9, 115–127 (2019).
Soleimani Aghdam, M., Naderi, R., Sarcheshmeh, M. A. A. & Babalar, M. Amelioration of postharvest chilling injury in anthurium cut flowers by γ-aminobutyric acid (GABA) treatments. Postharvest Biol. Technol. 110, 70–76. https://doi.org/10.1016/j.postharvbio.2015.06.020 (2015).
Heidari Krush, G. & Rastegar, S. γ-aminobutyric acid (GABA) inhibits the enzymatic browning of cut narcissus tazetta cv. ‘Shahla-e-Shiraz’ flowers during vase life. J. Plant Growth Regul. 42, 2602–2612. https://doi.org/10.1007/s00344-022-10730-1 (2022).
Babarabie, M., Zarei, H. & Eskandari, A. The impact of pre-harvest treatment with gamma-aminobutyric acid (GABA) and salicylic acid on vase life and post-harvest traits of tuberose cut flowers. Acta Scientiarum Polonorum Hortorum Cultus 18, 83–92. https://doi.org/10.24326/asphc.2019.4.8 (2019).
Hayat, F. et al. γ aminobutyric acid (GABA): A key player in alleviating abiotic stress resistance in horticultural crops: current insights and future directions. Horticulturae https://doi.org/10.3390/horticulturae9060647 (2023).
Li, Z., Yu, J., Peng, Y. & Huang, B. Metabolic pathways regulated by abscisic acid, salicylic acid and -aminobutyric acid in association with improved drought tolerance in creeping bentgrass (Agrostis stolonifera). Physiologia Plantarum 159, 42–58 (2017).
Fait, A., Fromm, H., Walter, D., Galili, G. & Fernie, A. R. Highway or byway: The metabolic role of the GABA shunt in plants. Trends Plant Sci. 13, 14–19 (2008).
Hawkesford, M. et al. Functions of macronutrients. In Marschner’s Mineral Nutrition of Higher Plants (ed. Marschner, P.) 135–189 (Academic Press, 2012).
van Ieperen, W. & van Gelder, A. Ion-mediated flow changes suppressed by minimal calcium presence in xylem sap in Chrysanthemum and Prunus laurocerasus. J. Exp. Bot. 57, 2743–2750 (2006).
Li, C., Tao, J., Zhao, D., You, C. & Ge, J. Effect of calcium sprays on mechanical strength and cell wall fractions of herbaceous peony (Paeonia lactiflora Pall.) inflorescence stems. Int. J. Mol. Sci. 13, 4704–4713 (2012).
Wehr, J. B., Menzies, N. W. & Blamey, F. P. C. Inhibition of cell-wall autolysis and pectin degradation by cations. Plant Physiol. Biochem. 42, 485–492 (2004).
Islam, M. M. et al. Exogenous application of calcium ameliorates salinity stress tolerance of tomato (Solanum lycopersicum L.) and enhances fruit quality. Antioxidants 12, 558 (2023).
Akintoye, H. A. et al. Effect of calcium chloride and salicylic acid solutions on the vase life of Heliconia spp. Acta Hortic. https://doi.org/10.17660/ActaHortic.2018.1225.27 (2018).
Nazari Deljou, M. & Gholipour, K. in International Symposium on Growing Media and Soilless Cultivation 1034. 539–543.
Bai, J. G. et al. Effects of exogenous calcium on some postharvest characteristics of cut gladiolus. Agric. Sci. China 8, 293–303 (2009).
Torre, S., Borochov, A. & Halevy, A. H. Calcium regulation of senescence in rose petals. Physiol. Plant. 107, 214–219 (1999).
Dhindsa, R. S., Plumb-Dhindsa, P. & Thorpe, T. A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 32, 93–101 (1981).
Chance, B. & Maehly, A. Assay of Catalases and Peroxidases (Elsevier, 1955).
Nakano, Y. & Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 22, 867–880 (1981).
Kumar, K. B. & Khan, P. A. Peroxidase and polyphenol oxidase in excised ragi (Eleusine coracana cv. pr 202) leaves during senescence. Indian J. Exp. Bot. 20, 412–416 (1982).
Jiang, W. B., Mayak, S. & Halevy, A. H. The mechanism involved in ethylene-enhanced ethylene synthesis in carnations. Plant Growth Regul. 14, 133–138 (1994).
Ali, M. B., Hahn, E.-J. & Paek, K.-Y. Effects of light intensities on antioxidant enzymes and malondialdehyde content during short-term acclimatization on micropropagated Phalaenopsis plantlet. Environ. Exp. Bot. 54, 109–120 (2005).
Jowkar, M., Hassanzadeh, N., Kafi, M. & Khalighi, A. Comprehensive microbial study on biocide application as vase solution preservatives for cut ‘Cherry Brandy’rose flower. Int. J. Hortic. Sci. Technol. 4, 89–103 (2017).
Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 (1976).
Tohidi, B., Rahimmalek, M. & Arzani, A. Essential oil composition, total phenolic, flavonoid contents, and antioxidant activity of Thymus species collected from different regions of Iran. Food Chem. 220, 153–161 (2017).
Sofo, A., Dichio, B., Xiloyannis, C. & Masia, A. Antioxidant defences in olive trees during drought stress: Changes in activity of some antioxidant enzymes. Funct. Plant Biol. 32, 45–53 (2005).
Aminzade, R., Ramezanian, A., Eshghi, S. & Hosseini, S. M. H. The potential of postharvest zinc treatment for preservation of pomegranate aril quality. Sci. Rep. 14, 1067. https://doi.org/10.1038/s41598-024-51437-5 (2024).
AL-Quraan, N. A. GABA shunt deficiencies and accumulation of reactive oxygen species under UV treatments: Insight from Arabidopsis thaliana calmodulin mutants. Acta Physiol. Plant. 37, 1–11 (2015).
Kalhor, M. S. et al. Title: Enhanced salt tolerance and photosynthetic performance: Implication of ɤ-amino butyric acid application in salt-exposed lettuce (Lactuca sativa L.) plants. Plant Physiol. Biochem. 130, 157–172. https://doi.org/10.1016/j.plaphy.2018.07.003 (2018).
Li, Z., Yu, J., Peng, Y. & Huang, B. Metabolic pathways regulated by γ-aminobutyric acid (GABA) contributing to heat tolerance in creeping bentgrass (Agrostis stolonifera). Sci. Rep. 6, 30338 (2016).
Soleimani Aghdam, M., Naderi, R., Jannatizadeh, A., Sarcheshmeh, M. A. A. & Babalar, M. Enhancement of postharvest chilling tolerance of anthurium cut flowers by γ-aminobutyric acid (GABA) treatments. Sci. Hortic. 198, 52–60 (2016).
Song, H., Xu, X., Wang, H., Wang, H. & Tao, Y. Exogenous gamma-aminobutyric acid alleviates oxidative damage caused by aluminium and proton stresses on barley seedlings. J. Sci. Food Agric. 90(99), 1410–1416. https://doi.org/10.1002/jsfa.3951 (2010).
Yang, A., Cao, S., Yang, Z., Cai, Y. & Zheng, Y. γ-aminobutyric acid treatment reduces chilling injury and activates the defence response of peach fruit. Food Chem. 129, 1619–1622. https://doi.org/10.1016/j.foodchem.2011.06.018 (2011).
Liu, C., Zhao, L. & Yu, G. The dominant glutamic acid metabolic flux to produce γ-amino butyric acid over proline in Nicotiana tabacum leaves under water stress relates to its significant role in antioxidant activity. J. Integr. Plant Biol. 53(58), 608–618. https://doi.org/10.1111/j.1744-7909.2011.01049.x (2011).
Sharma, D., Jamra, G., Singh, U. M., Sood, S. & Kumar, A. Calcium biofortification: Three pronged molecular approaches for dissecting complex trait of calcium nutrition in finger millet (Eleusine coracana) for devising strategies of enrichment of food crops. Front. Plant Sci. 7, 2028 (2017).
Henry, E. E. et al. Ions and organic solutes as implicated in the ameliorative effect of exogenous application of calcium on salt stressed tomato (Lycopersicon esculentum Mill.) Plants. Int. J. Plant Soil Sci. 33, 200–212 (2021).
Manaa, A. et al. Effect of salinity and calcium on tomato fruit proteome. OMICS: J. Integr. Biol. 17, 338–352 (2013).
Tanveer, K. et al. Effect of salt stress on tomato plant and the role of calcium. J. Plant Nutr. 43, 28–35 (2020).
Kim, N. H., Jacob, P. & Dangl, J. L. Con-Ca(2+) -tenating plant immune responses via calcium-permeable cation channels. New Phytol. 234, 813–818. https://doi.org/10.1111/nph.18044 (2022).
Jiang, Y. & Ding, P. Calcium signaling in plant immunity: A spatiotemporally controlled symphony. Trends Plant Sci. 28, 27 (2023).
Trobacher, C. P. et al. Calmodulin-dependent and calmodulin-independent glutamate decarboxylases in apple fruit. BMC Plant Biol. 13, 1–10 (2013).
Chi, Z. et al. Exogenous calcium chloride (CaCl2) promotes γ-aminobutyric acid (GABA) accumulation in fresh-cut pears. Postharvest Biol. Technol. 174, 111446 (2021).
Wang, K. et al. Effects of exogenous calcium chloride (CaCl2) and ascorbic acid (AsA) on the γ-aminobutyric acid (GABA) metabolism in shredded carrots. Postharvest Biol. Technol. 152, 111–117 (2019).
Wei, Q. et al. Calcium involved in the enrichment of γ-aminobutyric acid (GABA) in broccoli sprouts under fructose treatment. Plants 12, 224 (2023).
Hurrell, R. F. & Finot, P.-A. Nutritional and Toxicological Aspects of Food Safety (Springer, 1984).
Mayer, A. M. Polyphenol oxidases in plants and fungi: Going places? A review. Phytochemistry 67, 2318–2331 (2006).
Taranto, F. et al. Polyphenol oxidases in crops: Biochemical, physiological and genetic aspects. Int. J. Mol. Sci. 18, 377 (2017).
Abdolmaleki, M., Khosh Khui, M., Eshghi, S. & Ramezanian, A. Improvement in vase life of cut rose cv. “Dolce Vita” by preharvest foliar application of calcium chloride and salicylic acid. Int. J. Hortic. Sci. Technol. 2, 55–66. https://doi.org/10.22059/ijhst.2015.54264 (2015).
Mishra, N. et al. Achieving abiotic stress tolerance in plants through antioxidative defense mechanisms. Front. Plant Sci. 14, 1110622 (2023).
Khan, A. et al. Melatonin: Awakening the defense mechanisms during plant oxidative stress. Plants 9, 407 (2020).
Boeckx, T., Winters, A. L., Webb, K. J. & Kingston-Smith, A. H. Polyphenol oxidase in leaves: Is there any significance to the chloroplastic localization?. J. Exp. Bot. 66, 3571–3579 (2015).
Šamec, D., Karalija, E., Šola, I., Vujčić Bok, V. & Salopek-Sondi, B. The role of polyphenols in abiotic stress response: The influence of molecular structure. Plants 10, 118. https://doi.org/10.3390/plants10010118 (2021).
Jomova, K. et al. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 10, 2499–2574 (2023).
Kou, X. et al. Effects of CaCl2 dipping and pullulan coating on the development of brown spot on ‘Huangguan’pears during cold storage. Postharvest Biol. Technol. 99, 63–72 (2015).
Acknowledgements
This article is a part of the PhD thesis of the first author, which was submitted to the Department of Horticultural Sciences, Islamic Azad University, Yasuj Branch. We would like to thank the Research and Technology Vice-Chancellor of Islamic Azad University.
Author information
Authors and Affiliations
Contributions
Narges Ehsanimehr: Investigation, Formal analysis. Mehdi Hosseinifarahi, Supervision, methodology, Moslem Abdipour, Metodology, statically Analysis; Saeid Eshghi: Conceptualization, Writing—review & editing and Babak Jamali: Writing—original draft. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ehsanimehr, N., Hosseinifarahi, M., Abdipour, M. et al. Improving postharvest quality and vase life of cut rose flowers by pre-harvest foliar co-applications of γ-aminobutyric acid and calcium chloride. Sci Rep 14, 14520 (2024). https://doi.org/10.1038/s41598-024-64021-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-64021-8
- Springer Nature Limited