Abstract
The triglyceride glucose body mass index (TyG-BMI) is a potential indicator for insulin resistance, but its association with mortality in diabetic patients is unclear. This study investigates the relationship between TyG-BMI and all-cause and cardiovascular mortality in diabetics. The study included 3109 diabetic patients from the National Health and Nutrition Examination Survey (2001–2018). Mortality data were obtained from National Death Index records until 31 December 2019. Multivariate Cox models analyzed the association between TyG-BMI and mortality. Non-linear correlations were assessed using restricted cubic splines, and a two-piecewise Cox model evaluated the relationship on both sides of the inflection point. Over a median 7.25-year follow-up, 795 total and 238 cardiovascular deaths occurred. A U-shaped link was found between initial TyG-BMI and mortality in diabetic patients. Low TyG-BMI (< 279.67 for all-cause, < 270.19 for CVD) reduced death risks (all-cause: HR 0.77, 95% CI 0.69–0.86; CVD: HR 0.64, 95% CI 0.48–0.86). High TyG-BMI (> 279.67 for all-cause, > 270.19 for CVD) increased these risks (all-cause: HR 1.26, 95% CI 1.10–1.44; CVD: HR 1.33, 95% CI 1.06–1.68). In the NHANES study population, a U-shaped association was observed between the baseline TyG-BMI index and all-cause mortality or CVD in diabetic patients.
Similar content being viewed by others
Diabetes has emerged as a significant global health issue in recent decades1,2. According to a recent survey by the International Diabetes Federation (IDF), 536.6 million people worldwide suffered from diabetes in 2021, and it is estimated that the number of diabetic patients worldwide will reach 700 million by 20451. It is reported that diabetes is associated with an increased risk of a variety of complications, including cardiovascular diseases, kidney diseases, and neuropathy3,4,5. In addition, the all-cause mortality and cardiovascular mortality of diabetic patients increased significantly6. Therefore, timely identification of more risk factors is crucial to prevent, delay or reduce the progression of diabetes and diabetes related deaths7.
Insulin resistance (IR) is a key risk factor for type 2 diabetes mellitus (T2DM), dyslipidemias, obesity, and cardiovascular disease (CVD)8. It's linked to CVD progression and cardiovascular outcomes prediction9. The triglyceride-glucose (TyG) index, calculated as Ln(fasting triglycerides [mg/dl] × fasting plasma glucose [mg/dl]/2), assesses IR10. This index, in contrast to complex methods like the hyperinsulinemic euglycemic clamp (HIEC) and Homeostasis Model of Insulin Resistance (HOMA-IR), is more accessible and cost-effective11. Studies demonstrate its close relationship with glucose clamp techniques, endorsing its clinical use as an IR marker12. It's noted that TyG is more strongly associated with IR than HOMA-IR13 and is linked to CVD14 and negative outcomes in ACS, heart failure (HF), and ischemic stroke15,16,17. Recently, some scholars have proposed triglyceride glucose-body mass (TyG-BMI) index, which combines glucose metabolism indicators, lipid metabolism indicators, and anthropometric indicators. After comparing traditional blood lipid parameters, glucose parameters, and obesity related indicators, it was found that TyG-BMI has high diagnostic value in identifying IR18. However, there remains debate over the TyG-BMI index's ability, as an IR marker, to forecast outcomes in diabetic patients. Hence, our goal is to investigate the prognostic significance of the TyG-BMI index in assessing the risk of all-cause and CVD mortality among diabetic patients.
Methods
Study population and design
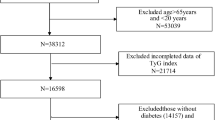
The National Health and Nutrition Examination Survey (NHANES) assesses the health and nutrition of U.S. adults and children. Overseen by the Centers for Disease Control and Prevention (CDC), NHANES's protocols are approved by the National Center for Health Statistics (NCHS) Research Ethics Review Board. Participant rights are protected through informed consent. Data for this study, spanning 2001–2018, is from NHANES's website (https://www.cdc.gov/nchs/nhanes/index.html). Diabetes was defined following ADA criteria as a self-reported diagnosis, insulin or hypoglycemic medication use, FBG ≥ 126 mg/dL, or HbA1c ≥ 6.5%19. We examined 3122 diabetic adults aged 20–85 years. Excluding 13 without baseline TyG-BMI index data, 3109 were included in our analysis (Fig. 1).
Assessment of covariates
We obtained patient histories at baseline. Demographics including age, sex, race/ethnicity, education level, family income, smoking status, comorbidities (diabetes, CVD, hypertension, chronic obstructive pulmonary disease). The diagnosis of CVD was established by self-reported physician diagnoses obtained during an individual interview using a standardized medical condition questionnaire. The participants were asked, “Has a doctor or other health expert ever informed you that you have CHF/CHD/angina pectoris/MI/stroke?” A person was regarded as having CVD if he or she replied “yes” to any of the above questions. Body mass index (BMI) was calculated using weight (kg) divided by height (m^2). Participants' race/ethnicity included White, Black, Mexican, or Other, and education levels were divided into less than high school, high school/equivalent, or college and above. Income and poverty were classified into three categories: 0–1.0, 1.0–3.0, or > 3.0. Smoking status was identified as never, former, or current smoker. Drinking was categorized into heavy (≥ 3 drinks daily for women, ≥ 4 for men, or binge drinking [≥ 4 drinks for women, ≥ 5 for men] on 5 + days/month), moderate (≥ 2 drinks daily for women, ≥ 3 for men, or binge drinking ≥ 2 days/month), mild (not meeting the above), nondrinker, or history of daily binge drinking.
Assessment of TyG-BMI index
The TyG-BMI index was derived by the formula: ln[TG (mg/dL) × FPG (mg/dL)/2] × BMI (kg/m^2), where TG stands for triglycerides and FBG for fasting blood glucose. Triglycerides were quantified using enzymatic assays conducted on a Roche Modular P analyzer, while FBG levels were determined through a hexokinase-mediated enzymatic method on a Roche/Hitachi Cobas C 501 analyzer. To facilitate comparative analysis, the study population was stratified into quartiles (Q1 to Q4) based on the TyG-BMI index distribution, with the lowest quartile (Q1) serving as the reference cohort.
Ascertainment of mortality
To determine mortality in the follow-up group, we used the NHANES public-use linked mortality file as of December 31, 2019. This file is connected to the National Death Index (NDI) through a probability matching algorithm by NCHS. We defined CVD mortality using the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision codes I00 to I09, I11, I13, I20 to I51, and I60 to I6920.
Statistical analysis
Statistical analyses were performed using R software (version 4.2.1; https://www.r-project.org). To accommodate NHANES's complex sampling method, sample weights, clustering, and stratification were integrated into all analyses21. Participants were segmented into quartiles (Q1-Q4) according to their TyG-BMI index. We presented continuous variables as means and SDs and categorical variables as frequencies and percentages. Differences in baseline characteristics by TyG-BMI quartiles were assessed using one-way ANOVA for continuous and Pearson chi-square test for categorical variables.
All-cause and CVD mortality rates were computed for each TyG-BMI quartile during the follow-up. To assess the predictive power of TyG-BMI, we employed various Cox proportional hazards regression models. These comprised an unadjusted model (Model 1), a model adjusted for age, race, and gender (Model 2), and a fully adjusted model (Model 3) that also included variables such as tobacco and alcohol use, education level, hypertension, COPD, CVD, eGFR, HDL, FINS and family income-poverty ratio. The relationship between the TyG-BMI index and mortality was analyzed using multivariable adjusted Cox models, employing restricted cubic splines and penalized spline methods for smooth curve fitting. Non-linear relationships prompted threshold estimation through likelihood maximization, followed by a two-piecewise Cox model on either side of the inflection point. Kaplan–Meier plots and log-rank tests compared survival rates among different TyG-BMI index categories. We also performed subgroup analyses, taking into account variables like gender, age (either < 60 or ≥ 60 years), race (White, Black, Mexican, or Other), COPD, CVD and hypertension. A p-value below 0.05 was considered statistically significant.
Ethical approval
The protocol for NHANES received approval from the National Center for Health Statistics and Ethics Review Board, with all participants giving written informed consent.
Results
Baseline characteristics of study participants
Table 1 outlines the initial characteristics of the study group (n = 3109), divided by TyG-BMI index quartiles. The average age was 58.93 years, and males constituted 51.19%. Baseline lab results are detailed in Table 2. Participants with higher TyG-BMI indices were generally younger, had higher obesity levels, and showed greater prevalence of hypertension compared to the lowest quartile.
Relationships of TyG-BMI index with mortality
Kaplan Meier curves indicated lower all-cause and CVD mortality in patients with elevated TyG-BMI compared to lower levels (p < 0.0001) (Fig. 2). There were 795 all-cause and 238 CVD deaths during follow-up (Table 3). We used three Cox models to assess the link between TyG-BMI levels and mortality risk. In Model 3, adjusted for various factors, multivariate-adjusted HRs and 95% CIs for all-cause mortality across TyG-BMI quartiles (119.61–241.61, 241.61–282.74, 282.74–334.26, 334.26–710.26) were 1.00 (reference), 0.72 (0.59,0.88), 0.56 (0.45,0.70), and 0.86 (0.68,1.09) (P = 0.046); for CVD mortality, they were 1.00 (reference), 0.65 (0.44,0.97), 0.56 (0.33,0.92), and 1.15 (0.78,1.69) (P = 0.646).
The detection of nonlinear relationships
Prior analysis identified a non-linear relationship between initial TyG-BMI index and mortality risks from all causes and CVD, using of Cox proportional hazards regression with restricted cubic splines for smooth curves. Adjusted results revealed U-shaped patterns between TyG-BMI index and both all-cause (Fig. 3A) and CVD mortality (Fig. 3B). Using both standard and two-piecewise Cox models, we located inflection points at 279.67 for all-cause and 270.19 for CVD mortality (log-likelihood ratio P values < 0.001) (Table 4). Before reaching the above threshold, the baseline TyG-BMI index had the lowest risk (Table 4 and Fig. 3). For each standard deviation increase in the TyG-BMI index, the corresponding all-cause mortality and CVD mortality rates decreased by 23% (HR 0.77, 95% CI 0.69–0.86) and 36% (HR 0.64, 95% CI 0.48–0.86), respectively. However, after reaching the above threshold, each standard deviation increase in the TyG-BMI index increased the risk of all-cause mortality (HR 1.26, 95% CI 1.10–1.44) and CVD mortality (HR 1.33, 95% CI = 1.06–1.68).
Association between TyG-BMI index and all-cause (A) and CVD mortality (B) in patients with diabetes. Each hazard ratio was computed with a TyG-BMI index level of A 279.67 and B 270.19 as the reference. Adjusted for age, gender, race, tobacco use, alcohol use, education, hypertension, COPD, CVD, eGFR, family income-poverty ratio, HDL, FINS. The solid line and red area represent the estimated values and their corresponding 95% CIs, respectively (TyG-BMI Index: Triglyceride glucose-body mass index; CVD: cardiovascular disease).
Subgroup analyses
The benefit of a higher TyG-BMI index (≥ 279.67) compared to a lower one (< 279.67) on all-cause mortality in diabetic patients was uniform across subgroups like age, gender, CVD history, and hypertension (Table 5). There was no significant interaction between TyG-BMI index and these variables. However, a stronger inverse relationship was noted between TyG-BMI index and all-cause mortality in diabetic patients under 60. Similarly, a higher TyG-BMI index (≥ 270.19) showed consistent benefits over a lower index (< 270.19) in CVD mortality across subgroups stratified by age, race, COPD, and hypertension history (Table 6). No significant interaction was found between TyG-BMI index and these groups. However, among diabetic women, a more distinct inverse correlation was observed between TyG-BMI index and all-cause mortality.
Discussion
In this retrospective cohort study, we found that a U-shaped association between TyG-BMI index and mortalities among diabetic patients was also explored with a turning point at 279.67 for all-cause mortality and 270.19 for CVD mortality. Subgroup analyses of demographics and comorbidities were consistent with these core findings.
Although HOMA-IR is a classic marker of IR22, its costliness and complexity have prevented its wide usage. TyG-BMI index is a new indicator proposed in recent years that reflects IR. It combines glucose and lipid metabolism indicators with anthropometric indicators, and has a strong correlation with HOMA-IR, allowing it to make early predictions of IR18. The TyG-BMI index, unlike HOMA-IR, doesn't require insulin measurement and is suitable for all patients on insulin therapy. Recent research has linked the TyG-BMI index with heart failure, NAFLD, and hyperuricemia23,24,25. Compared to other IR parameters, a NNHANES study with 9884 participants validated TyG-BMI as a feasible IR evaluation tool18. Additionally, an investigation into metabolic indicators and atherosclerotic cardiovascular disease (ASCVD) risk highlighted a notable association between TyG-BMI index and ASCVD risk, particularly in females26. Furthermore, Du showed that TyG-BMI index was linearly correlated with ischemic stroke without threshold or saturation effects in the general population27. These results, therefore, indicate the potential value of TyG-BMI index as a predictive indicator for metabolic diseases and for risk stratification in patients with diabetes.
Although our study demonstrated a link between TyG-BMI index and mortality in patients with diabetes, the underlying mechanism is still unclear. IR, a key feature of T2DM, is known as a CVD risk factor28. It contributes to CVD development in both the general and diabetic populations and is associated with poorer cardiovascular outcomes in CVD patients29. Studies indicate that patients with IR are at a heightened risk for metabolic disorders, which are associated with adverse cardiovascular disease outcomes30. Persistent hyperglycemia and abnormal blood lipids caused by IR may lead to oxidative stress and inflammatory reactions, thereby damaging endothelial cell function31. Continuous IR can increase hypertension and lead to vascular and renal damage32. In addition, the levels of free fatty acids (FFA) in plasma can increase with the increase of TG levels, thereby promoting the development of obesity-related IR and CVD33. These pathologies collectively may lead to the onset and advancement of coronary heart disease and worsen prognosis.
Our study adds unique insights to the evolving research on the TyG-BMI index, revealing its intricate link with all-cause and CVD mortality risks. We observed that lower baseline TyG-BMI levels (< 279.67 for all-cause, < 270.19 for CVD mortality) significantly influence its relationship with mortality risks. Notably, for those with baseline TyG-BMI below these thresholds, every unit increase in the index is associated with a 24% decrease in all-cause mortality risk and a 36% decrease in CVD mortality risk, post-adjustment for confounders. For example, it is widely recognized that low blood sugar elevates counter-regulatory hormones such as adrenaline, which can cause vasoconstriction and platelet aggregation, leading to an increase in cardiovascular and cerebrovascular stroke events34. Similarly, studies on patients with heart failure suggest that low TG levels have been identified as a predictive factor for their cardiac death35. In addition, research shows that BMI is associated with mortality in a U-shaped curve regardless of the severity of diabetes36. Hence, maintaining a balanced TyG-BMI index is crucial, as both excessively high and low levels could lead to adverse health outcomes.
In subgroup analysis, the impact of TyG-BMI index on all-cause mortality was more obvious in female younger than 60 years old. A plausible reason for this observation is that older men often have multiple illnesses, making a single indicator insufficient to fully assess their effects. Another key discovery from our study is the more pronounced link between the TyG-BMI index and CVD mortality risk in women compared to men. This could be due to estrogen's role in premenopausal cardiovascular protection, which improves insulin sensitivity. A cohort study reported that elevated fasting serum insulin levels and HOMA-IR are linked with hypertension in women, but this association is not seen in men37. This study has certain limitations. Due to its single center and observational nature, this study cannot determine causal relationships. Despite adjusting for multiple factors and conducting subgroup analyses, unaccounted confounding factors might influence the prognosis. Furthermore, our analysis concentrated solely on the baseline TyG-BMI index's prognostic value, leaving the effects of TyG-BMI index variations during follow-up on mortality prediction undetermined. Nevertheless, this does not negate the observed association between TyG-BMI index and increased/decreased risk of mortality among patients with diabetes. Further investigations are warranted to confirm such correlation.
Conclusion
Our study's findings suggest that the TyG-BMI index is an effective predictor of all-cause and CVD mortality risks in diabetic patients, highlighting a non-linear relationship between the TyG-BMI index and mortality. Consequently, utilizing the TyG-BMI index for risk assessment and prognosis prediction in this patient group could be advantageous. Further research is needed to determine if interventions focused on the TyG-BMI index can enhance clinical outcomes in these patients.
Data availability
Upon a reasonable request, the datasets utilized and assessed in this study can be acquired from the corresponding author.
Abbreviations
- BMI:
-
Body mass index
- CDC:
-
The Centers for Disease Control and Prevention
- CHF:
-
Congestive heart failure
- CI:
-
Confidence interval
- CVD:
-
Cardiovascular disease
- DM:
-
Diabetes mellitus
- eGFR:
-
Estimated glomerular filtration rate
- FINS:
-
Fasting insulin
- FFA:
-
Free fatty acid
- FPG:
-
Fasting plasma glucose
- HDL:
-
High density lipoprotein
- HF:
-
Heart failure
- HR:
-
Hazard ratio
- IR:
-
Insulin resistance
- MI:
-
Myocardial infarction
- NCHS:
-
National Center for Health Statistics
- NDI:
-
National Death Index
- NHANES:
-
National Health and Nutrition Examination Survey
- SD:
-
Standard deviation
- TyG:
-
Triglyceride glucose index
- TyG-BMI:
-
Triglyceride glucose body mass index
References
Sun, H. et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 183, 109119. https://doi.org/10.1016/j.diabres.2021.109119 (2022).
Chan, J. C. N. et al. The lancet commission on diabetes: Using data to transform diabetes care and patient lives. Lancet (London, England). 396(10267), 2019–2082. https://doi.org/10.1016/s0140-6736(20)32374-6 (2021).
Laddha, A. P. & Kulkarni, Y. A. Tannins and vascular complications of diabetes: An update. Phytomedicine 56, 229–245. https://doi.org/10.1016/j.phymed.2018.10.026 (2019).
Bai, P., Barkmeier, A. J., Hodge, D. O. & Mohney, B. G. Ocular sequelae in a population-based cohort of youth diagnosed with diabetes during a 50-year period. JAMA Ophthalmol. 140(1), 51–57. https://doi.org/10.1001/jamaophthalmol.2021.5052 (2022).
Avogaro, A. & Fadini, G. P. Microvascular complications in diabetes: A growing concern for cardiologists. Int J Cardiol. 291, 29–35. https://doi.org/10.1016/j.ijcard.2019.02.030 (2019).
Raghavan, S. et al. Diabetes mellitus-related all-cause and cardiovascular mortality in a national cohort of adults. J Am Heart Assoc. 8(4), e011295. https://doi.org/10.1161/jaha.118.011295 (2019).
Defronzo RA. Banting lecture. From the triumvirate to the ominous octet: A new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 58(4):773–95. https://doi.org/10.2337/db09-9028
Tello-Flores, V. A. et al. Role of long non-coding RNAs and the molecular mechanisms involved in insulin resistance. Int J Mol Sci. https://doi.org/10.3390/ijms22147256 (2021).
Hill, M. A. et al. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metab Clin Exp. 119, 154766. https://doi.org/10.1016/j.metabol.2021.154766 (2021).
Simental-Mendía, L. E. et al. The triglycerides and glucose index is strongly associated with hepatic steatosis in children with overweight or obesity. Eur J Pediatr. 180(6), 1755–1760. https://doi.org/10.1007/s00431-021-03951-1 (2021).
Pacini, G. & Mari, A. Methods for clinical assessment of insulin sensitivity and beta-cell function. Best Pract Res Clin Endocrinol Metab. 17(3), 305–322. https://doi.org/10.1016/s1521-690x(03)00042-3 (2003).
Guerrero-Romero, F. et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity: Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 95(7), 3347–3351. https://doi.org/10.1210/jc.2010-0288 (2010).
Vasques, A. C. et al. TyG index performs better than HOMA in a Brazilian population: a hyperglycemic clamp validated study. Diabetes Res Clin Pract. 93(3), e98–e100. https://doi.org/10.1016/j.diabres.2011.05.030 (2011).
Park, K. et al. Elevated TyG index predicts progression of coronary artery calcification. Diabetes Care. 42(8), 1569–1573. https://doi.org/10.2337/dc18-1920 (2019).
Zhao, Q. et al. Impacts of triglyceride-glucose index on prognosis of patients with type 2 diabetes mellitus and non-ST-segment elevation acute coronary syndrome: results from an observational cohort study in China. Cardiovasc Diabetol. 19(1), 108. https://doi.org/10.1186/s12933-020-01086-5 (2020).
Huang, R. et al. Prognostic value of triglyceride glucose (TyG) index in patients with acute decompensated heart failure. Cardiovasc Diabetol. 21(1), 88. https://doi.org/10.1186/s12933-022-01507-7 (2022).
Shi, W. et al. Value of triglyceride-glucose index for the estimation of ischemic stroke risk: Insights from a general population. Nutr Metab Cardiovasc Dis: NMCD. 30(2), 245–253. https://doi.org/10.1016/j.numecd.2019.09.015 (2020).
Yan, S., Wang, D. & Jia, Y. Comparison of insulin resistance-associated parameters in US adults: A cross-sectional study. Hormones (Athens, Greece). 22(2), 331–341. https://doi.org/10.1007/s42000-023-00448-4 (2023).
Zou, X., Zhou, X., Zhu, Z. & Ji, L. Novel subgroups of patients with adult-onset diabetes in Chinese and US populations. Lancet Diabetes Endocrinol. 7(1), 9–11. https://doi.org/10.1016/s2213-8587(18)30316-4 (2019).
Organization WH. International Statistical Classification of Diseases and related health problems: Alphabetical index. vol 3. World Health Organization; 2004.
Control CfD, Prevention. The National Health and Nutrition Examination Survey (NHANES) Analytic and Reporting Guidelines. Atlanta, GA: CDC. 2006;
Tam, C. S. et al. Defining insulin resistance from hyperinsulinemic-euglycemic clamps. Diabetes Care. 35(7), 1605–1610. https://doi.org/10.2337/dc11-2339 (2012).
Yang, S. et al. Association between triglyceride glucose-body mass index and heart failure in subjects with diabetes mellitus or prediabetes mellitus: A cross-sectional study. Front Endocrinol. 14, 1294909. https://doi.org/10.3389/fendo.2023.1294909 (2023).
Wang, H. et al. Comparison of different insulin resistance surrogates to predict hyperuricemia among U.S. non-diabetic adults. Front Endocrinol. 13, 1028167. https://doi.org/10.3389/fendo.2022.1028167 (2022).
Song, K. et al. Comparison of the triglyceride glucose index and modified triglyceride glucose indices to predict nonalcoholic fatty liver disease in youths. J Pediatrics. 242, 79–85. https://doi.org/10.1016/j.jpeds.2021.11.042 (2022).
Huang, Y. C. et al. Comparison of innovative and traditional cardiometabolic indices in estimating atherosclerotic cardiovascular disease risk in adults. Diagnostics (Basel, Switzerland). https://doi.org/10.3390/diagnostics11040603 (2021).
Du, Z., Xing, L., Lin, M. & Sun, Y. Estimate of prevalent ischemic stroke from triglyceride glucose-body mass index in the general population. BMC Cardiovasc Disord. 20(1), 483. https://doi.org/10.1186/s12872-020-01768-8 (2020).
Laakso, M. & Kuusisto, J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Rev Endocrinol 10(5), 293–302. https://doi.org/10.1038/nrendo.2014.29 (2014).
Uetani, T. et al. Impact of insulin resistance on post-procedural myocardial injury and clinical outcomes in patients who underwent elective coronary interventions with drug-eluting stents. Cardiovasc Intervent 5(11), 1159–1167. https://doi.org/10.1016/j.jcin.2012.07.008 (2012).
Tao, L. C., Xu, J. N., Wang, T. T., Hua, F. & Li, J. J. Triglyceride-glucose index as a marker in cardiovascular diseases: landscape and limitations. Cardiovasc Diabetol. 21(1), 68. https://doi.org/10.1186/s12933-022-01511-x (2022).
Gao, S., Ma, W., Huang, S., Lin, X. & Yu, M. Impact of triglyceride-glucose index on long-term cardiovascular outcomes in patients with myocardial infarction with nonobstructive coronary arteries. Nutr Metab Cardiovasc Dis: NMCD. 31(11), 3184–3192. https://doi.org/10.1016/j.numecd.2021.07.027 (2021).
da Silva AA, do Carmo JM, Li X, Wang Z, Mouton AJ, Hall JE. Role of hyperinsulinemia and insulin resistance in hypertension: Metabolic syndrome revisited. Can J Cardiol. 2020;36(5):671–682. https://doi.org/10.1016/j.cjca.2020.02.066
Samuel, V. T. & Shulman, G. I. The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. J Clin Invest. 126(1), 12–22. https://doi.org/10.1172/jci77812 (2016).
Galassetti, P. & Davis, S. N. Effects of insulin per se on neuroendocrine and metabolic counter-regulatory responses to hypoglycaemia. Clin Sci (London, England: 1979) 99(5), 351–362 (2000).
Kozdag, G. et al. Low serum triglyceride levels as predictors of cardiac death in heart failure patients. Texas Heart Inst J. 40(5), 521–528 (2013).
Lee, E. Y., Lee, Y. H., Yi, S. W., Shin, S. A. & Yi, J. J. BMI and all-cause mortality in normoglycemia, impaired fasting glucose, newly diagnosed diabetes, and prevalent diabetes: A cohort study. Diabetes Care. 40(8), 1026–1033. https://doi.org/10.2337/dc16-1458 (2017).
Arshi, B. et al. Sex-specific relations between fasting insulin, insulin resistance and incident hypertension: 8.9 years follow-up in a Middle-Eastern population. J Hum Hypertens. 29(4), 260–267. https://doi.org/10.1038/jhh.2014.70 (2015).
Acknowledgements
The authors extend their sincere thanks to both the participants and the staff of the NHANES for their invaluable contributions to this research.
Funding
Funding for this research was provided by the National Natural Science Foundation of China (Grant No. 81960081).
Author information
Authors and Affiliations
Contributions
Shucai Xiao: study conception and design; acquisition, analysis, and interpretation of data; drafting the manuscript; analysis and interpretation of data; Qin Zhang: drafting the manuscript and interpretation of the results; Hai-Yue Yang: drafting the manuscript and revising the manuscript; Ren-Qiang Yang and Jin-Ying Tong: study concept and design; supervision; revising the manuscript; All authors approved the final manuscript. Ren-Qiang Yang and Jin-Ying Tong, as the guarantor of this work, had complete access to all study data and assumes responsibility for the data's integrity and the accuracy of its analysis. All authors were involved in reviewing the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xiao, S., Zhang, Q., Yang, HY. et al. The association between triglyceride glucose-body mass index and all-cause and cardiovascular mortality in diabetes patients: a retrospective study from NHANES database. Sci Rep 14, 13884 (2024). https://doi.org/10.1038/s41598-024-63886-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-63886-z
- Springer Nature Limited