Abstract
Gut microbiota plays a crucial role in gastrointestinal tumors. Additionally, gut microbes influence the progression of esophageal cancer. However, the major bacterial genera that affect the invasion and metastasis of esophageal cancer remain unknown, and the underlying mechanisms remain unclear. Here, we investigated the gut flora and metabolites of patients with esophageal squamous cell carcinoma and found abundant Bacteroides and increased secretion and entry of the surface antigen lipopolysaccharide (LPS) into the blood, causing inflammatory changes in the body. We confirmed these results in a mouse model of 4NQO-induced esophageal carcinoma in situ and further identified epithelial–mesenchymal transition (EMT) occurrence and TLR4/Myd88/NF-κB pathway activation in mouse esophageal tumors. Additionally, in vitro experiments revealed that LPS from Bacteroides fragile promoted esophageal cancer cell proliferation, migration, and invasion, and induced EMT by activating the TLR4/Myd88/NF-κB pathway. These results reveal that Bacteroides are closely associated with esophageal cancer progression through a higher inflammatory response level and signaling pathway activation that are both common to inflammation and tumors induced by LPS, providing a new biological target for esophageal cancer prevention or treatment.
Similar content being viewed by others
Introduction
The incidence of esophageal cancer has demonstrated significant differences, with the north-central region of China being the main high-incidence area of esophageal squamous cell carcinoma (ESCC)1. The incidence of ESCC is comparatively insidious and often progresses rapidly, with a high incidence of invasion and distant metastasis, resulting in high mortality rates2.
The mechanisms of cancer metastasis are complex, and studies have revealed that disturbances in the intestinal microbiota may increase the secretion of certain metabolites that activate inflammatory and tumor-related signaling pathways in the body, thereby promoting cancer invasion and metastasis3. Patients with gastrointestinal cancers frequently exhibit pathological characteristics of intestinal dysbiosis, and the intestinal microbiota and its metabolites induce long-term chronic inflammatory activation of metastasis-related pathways in tumors4. Thus, the gut microbiota contributes to the development and progression of many gastrointestinal malignancies by modulating the cancer-inflammatory microenvironment. Esophageal cancer is a prevalent malignant tumor of the gastrointestinal tract, and studies have revealed that the occurrence of esophageal adenocarcinoma is closely associated with intestinal microbiota dysbiosis5. However, changes in the intestinal flora and their regulatory mechanisms for the invasion and metastasis of ESCC remain unknown.
This study aims to investigate the characteristics and mechanisms of action of the intestinal flora in ESCC. This study revealed a significantly increased abundance of the gram-negative bacterium Bacteroides in patients with ESCC and the mouse models of ESCC. Additionally, the blood of patients and mouse model demonstrated significant alterations in the lipopolysaccharide (LPS) and the inflammatory cytokines interleukin (IL)-6, IL-10, tumor necrosis factor-alpha (TNF-α), and transforming growth factor beta (TGF-β). LPS is the main functioning component of the outer membrane of Bacteroides6, and an increased abundance of Bacteroides increases the amount of free bacterial component LPS that enters the circulation7, thereby changing the inflammatory environment of the organism and ultimately providing favorable conditions for ESCC invasion and metastasis. We further confirmed the role and possible mechanism of LPS, the surface antigen of Bacteroides, in promoting invasion, metastasis, and epithelial–mesenchymal transition (EMT) in ESCC at the cellular level. The results of this study may provide a new target for ESCC treatment.
Result
Basic participant characteristics
This study included 30 patients with ESCC and 30 healthy controls. Table 1 shows the demographic characteristics of all included individuals. No significant differences in terms of age, gender, alcohol consumption, or smoking were found between the two groups of participants (p > 0.05).
Bacteroides induce inflammatory changes via LPS in patients with ESCC
We revealed that the alphadiversity (species richness) of gut flora species in the two populations demonstrated a reasonable progressive amount of sample sequencing data, and the betadiversity (microbial composition) exhibited a large difference in gut microbial structure between the two populations (Fig. S1A–B). We used the LEfSe analysis of effect size method to determine differentially enriched species and identified Family Bacteroidaceae, Order Bacteroidales, Class Bacteroidia, and Order Lactobacillales in the fecal flora of patients with ESCC compared to healthy controls. Additionally, Family Peptostreptococcaceaeg and Order Peptostreptococcales Tissierellaleh increased in abundance, whereas Family Ruminococcaceae and Order Veillonellales Selenomonadales decreased in abundance, with a clear dominance of gram-negative Bacteroides (Fig. 1A). More specifically, we observed an increased abundance of Bacteroides with ESCC progression (Fig. 1B). Moreover, we detected LPS levels in the serum of clinical subjects using enzyme-linked immunosorbent assay (ELISA) and revealed significantly elevated LPS in the serum of patients with esophageal cancer (Fig. 1C). We further analyzed the data using Spearman’s correlation analysis to elucidate the relationship between Bacteroides and LPS and found that LPS was significantly and positively correlated with Bacteroides spp. (R = 0.4599, P < 0.001) (Fig. 1D).
Expression of Bacteroides, LPS, and inflammatory factors in normal population and patients with esophageal cancer. (A) Evolutionary branching diagram showing species differentially enriched in the gut microbiota. Red indicates greater expression in ESCC than in controls, green denotes greater expression in controls than in ESCC, and yellow denotes no significant differences. (B) Comparison of the abundance of Bacteroides, n = 30. (C) Serum LPS levels, n = 30. (D) Correlation between Bacteroides and LPS levels with Spearman correlation. (E) Serum inflammatory cytokine levels, including IL-6, IL-10, TNF-α, and TGF-β, n = 30. * vs. controls, # vs. ESCC (I), Δ vs. ESCC (II), Y vs. ESCC (III), *p < 0.05, **p < 0.01, ***p < 0.001.
Meanwhile, changes in the levels of endogenous bioactive cytokines, including IL-6, IL-10, TNF-α and TGF-β, which are usually diagnosed as indicators of cancer progression, were further assessed by ELISA of serum IL-6, IL-10, TNF-α, and TGF-β levels in clinical subjects, and we found significant differences in serum inflammatory cytokines in patients with ESCC compared with healthy controls (Fig. 1E).
Inflammatory changes induced by Bacteroides via LPS in mouse ESCC models
We developed a 4NQO-inducible mouse model of ESCC to further investigate the association between fecal microorganisms and serum inflammatory factors. Figure 2A shows esophageal carcinoma in situ and invasive carcinoma in mice at week 32. The fecal flora of experimental mice was investigated using 16S rRNA technology. Alphadiversity (species richness) of mouse fecal flora species demonstrated a progressively reasonable amount of sequencing data from the samples, and betadiversity (microbial composition) exhibited large differences in the structure of gut microorganisms between the two groups of mice (Fig. S2A–B). Among them, Family Bacteroidaceaeb, Family Prevotellaceaec, Family Lachnospiraceaeh, Order Lachnospiralesi, Family Oscillospiraceaej, Family Ruminococcaceaek, Order Oscillospiralesl, Class Clostridium, Family Enterobacteriaceae with increased abundance of Order Enterobacterales, Family Erysipelotrichaceaed, Order Erysipelotrichalese, Family Clostridiaceaef, and Order Clostridialesg decreased in abundance. Interestingly, Bacteroides abundance was significantly higher in the resolving flora of the model mice than in the control group, which is similar to the findings of the clinical trial (Fig. 2B–C). Meanwhile, the serum LPS level of mice in the model group was significantly increased (Fig. 2D), and Spearman’s correlation analysis revealed that LPS was significantly and positively correlated with Bacteroides (R = 0.8977, P < 0.0001) (Fig. 2E).
Expression of Bacteroides, LPS, and inflammatory factors in the experimental mouse. (A) Hematoxylin and eosin examination of histopathological changes. (B) Evolutionary branching diagram illustrating species differentially enriched in the gut microbiota. (C) Comparison of abundance of Bacteroides, n = 8. (D) Serum LPS levels, n = 8. (E) Spearman’s analysis method to analyze the correlation between Bacteroides and LPS levels in mice. (F) Inflammatory cytokines, including IL-6, IL-10, TNF-α, and TGF-β in mice, n = 8. **p < 0.01, ***p < 0.001.
Moreover, compared with the normal controls, significant changes in serum IL-6, IL-10, TNF-α, and TGF-β levels were observed in the ESCC mouse model and were consistent with the changes in inflammatory cytokines in patients with ESCC (Fig. 2F).
Gut barrier damage increases circulating LPS
Immunohistochemistry (IHC) was used to detect mouse intestinal barrier-associated proteins, zonula occludens 1 (ZO-1) and occludin. The results revealed a significant decrease in ZO-1 and occludin-positive staining in the model group compared to that in the control group (Fig. 3). This indicates a damaged intestinal barrier in mice, increased permeability, and elevated LPS entry into the blood through the intestinal mucosal barrier.
Disruption of the intestinal barrier and increased permeability in esophageal cancer mice. Histopathological changes in the rectum were examined by H&E under a microscope, H&E: hematoxylin and eosin. ZO1 and Occludin staining of mouse rectum tissue, brown for positive staining; ZO1 and Occludin: Mouse intestinal barrier-associated proteins. Observed under a 40 × microscope.
Increased tumor proliferation and EMT and activation of TLR4/Myd88/NF-κB signaling pathway in model mice
IHC revealed that the proliferation marker protein Ki67, invasion and metastasis marker proteins N-cadherin, and snail of ESCC tumor tissue were significantly increased in the model group (Fig. 4A). Additionally, IHC detected the expression and distribution of TLR4, Myd88, and NF-κB. The three indicators demonstrated increased expression in esophageal epithelial tissue compared to that in the control group (Fig. 4B). This indicates that EMT progression in mouse esophageal cancer may be associated with the TLR4/Myd88/NF-κB signaling pathway.
Expression of TLR4/Myd88/NF-κB signaling pathway and EMT-related proteins in ESCC tissues. (A) Immunohistochemistry (IHC) staining of Ki67, E-cadherin, and snail in mouse esophageal tissues at 40 × magnification. (B) IHC staining of TLR4, MyD88, and NF-κB in mouse esophageal tissues at 40 × magnification.
LPS promotes KYSE-150 cell proliferation and migration invasion
Studies have revealed that Pseudomonas fragilis is the most pathogenic of the genus Pseudomonas8. We obtained LPS for in vitro experiments by culturing P. fragilis. We used different LPS concentrations (0, 125, 250, and 500 ng/mL) to coculture with KYSE-150 cells for 6, 12, and 24 h. The proliferative ability of KYSE-150 cells was significantly improved after LPS treatment dose- and time-dependent manner (Fig. 5A). Additionally, LPS of 500 ng/mL was used to treat KYSE-150 cells for 12 and 24 h to observe their migration and invasion abilities. The results revealed significantly improved migration and invasion abilities of KYSE-150 cells in the LPS intervention group compared with the control group (Fig. 5B–E).
LPS promotes KYSE-150 cell proliferation, migration, and invasion. (A) Cell viability at different LPS concentrations (0, 125, 250, and 500 ng/mL) at 6,12, and 24 h. (B) Cell migration ability with LPS at 500 ng/ml. (C) Cell invasion ability of LPS at 500 ng/ml for 12 or 24 h. **p < 0.01,***p < 0.001,****p < 0.0001.
LPS promotes EMT in KYSE-150 cells and activates the TLR4/Myd88/NF-κB pathway
We investigated the expression of key EMT proteins in LPS-treated KYSE-150 cells and found that the epithelial marker E-cadherin was significantly downregulated, and the mesenchymal markers N-cadherin and snail were significantly upregulated at both 12 and 24 h (Fig. 6A, C).
LPS promotes EMT activation of TLR4/Myd88/NF-κB pathway in KYSE-150 cells. (A, C) E-cadherin and snail expression after LPS treatment. (B, D) TLR4, myd88, p-NF-κBp65, and NF-κBp65 protein expression after LPS treatment, n = 3. *p < 0.05, **p < 0.01, ***p < 0.001. Original blots are presented in plementary Figs 3, 4, 5, 6, 7 and 8.
We explored the molecular mechanisms by which LPS promoted KYSE-150 cell proliferation, migration, and invasion. We examined the expression of TLR4/Myd88/NF-κB pathway-related proteins after LPS treatment of KYSE-150 cells. Western blot analysis revealed significantly elevated TLR4 and myd88 at 12 versus 24 h, and p-NF-κBp65 was significantly increased at 24 h. However, NF-κBp65 exhibited no significant changes. This indicated that the TLR4/Myd88/NF-κB pathway was activated by LPS in the order of signal transduction (Fig. 6B, D).
Discussion
Gut microbiota plays an important role in cancer development and is frequently characterized by altered microbiota in cancer. Gut microbiota alterations play an important role not only in colorectal cancer9 but also in extraintestinal tumors, including melanoma10, hepatocellular carcinoma11, and gastric cancer12. A related study on esophageal cancer by Natasha et al. revealed that a high-fat diet accelerates esophageal allopatric hyperplasia by altering the esophageal microenvironment and gut microbiome5. Additionally, Qunate et al. revealed that metabolites of intestinal microorganisms are important mediators in accelerating the progression of esophageal cancer13.
Previous studies revealed the involvement of microbiota changes in the progression of ESCC in patients with esophagitis and ESCC14. We performed microbiota analysis of fresh feces from patients with ESCC using the 16S rRNA technique to investigate the critical role of major strain changes and their metabolites in ESCC development, and the results revealed a significantly elevated abundance of gram-negative bacteria, Bacteroides, in all cases. Interestingly, both gastric and colorectal cancers demonstrated a significant increase in the abundance of Bacteroides15,16. Our step studies revealed a positive correlation between serum LPS and Bacteroides abundance in patients with ESCC and the ESCC mouse model.
Meanwhile, experiments revealed an increased inflammatory infiltration level in the rectal tissues of mice in the model group and decreased expression of tight junction proteins, occluding, and ZO-1. Occludin and ZO-1 are the most abundant components of intercellular tight junction formation17. This indicates intestinal barrier damage and increased intestinal permeability in mice18,19. LPS can not only damage the intestinal tract but also trigger bacterial translocation, impair the intestinal barrier, and lead to abnormal expression of intestinal tight junction proteins. This allows LPS to penetrate the intestinal wall20. The increase in LPS in the blood further activates the host immune response21, dysregulating the cancer-inflammatory microenvironment, which plays an important role in extraintestinal diseases22,23. Therefore, we hypothesized that the increased abundance of Bacteroides in the intestinal flora plays an important role in ESCC development and that its surface antigen-LPS may be a key factor.
Our study revealed that elevated serum IL-6, TNF-α, and TGF-β and decreased IL-10 levels revealed that patients with ESCC and model mice demonstrated increased immune levels, and these inflammatory cytokines are considered important mediators associated with inflammation and cancer. Imbalances in pro- and antiinflammatory signaling factors promoted inflammatory cancer transformation and cancer progression24. IL-6 is considered a pleiotropic molecule that promotes tumor transformation and can, particularly, promote tumor cell invasion and metastasis25. IL-10 is involved in suppressing inflammatory responses, promoting tissue repair, and maintaining immune tolerance26. Under normal conditions, IL-10 is involved in maintaining immune tolerance in the gut microbiota27, which limits intestinal inflammation and tumor formation28. Many studies revealed that the effects of TNF-α and TGF-β include the promotion of EMT invasion and metastasis29,30,31,32.
Research revealed that LPS generated a broad inflammatory cascade response by binding to TLR433. TLR4 is not only a key hub that connects innate and adaptive immunity but also a sensor for recognizing microbial ecological dysregulation, which reflects the dysregulation of the body’s immunity as well as the microbiota and is closely related to tumors34,35. Sato et al. revealed that high TLR4 expression predicts poor prognosis in patients with advanced thoracic ESCC after esophagectomy36. Upon LPS stimulation, TLR4 uses MyD88 junction protein to transmit signals that activate NF-κB and induce inflammatory cytokines37,38. Recent studies revealed that TLR4/Myd88/NF-κB is a signaling pathway that improves IL-6, TNF-α, and TGF-β production39,40,41. The results of this study indicate significantly increased protein expression of key nodes of the TLR4/Myd88/NF-κB pathway in the esophagus of mice modeled for esophageal cancer in situ, and the same changes in protein expression were observed in esophageal cancer cells after LPS treatment in vitro. This indicates that the TLR4/Myd88/NF-κB signaling pathway is involved in esophageal cancer disease progression and is closely associated with LPS.
EMT is abnormally activated in human cancers, providing a special capacity for tumor cell movement that contributes to tumor cell invasion42. Some evidence indicates that the TLR4/Myd88/NF-κB signaling pathway, as well as inflammatory cytokines, influenced the EMT process43,44,45,46. We performed in vivo and in vitro experiments to further validate the effect of LPS on the EMT process in esophageal cancer and revealed the accelerated EMT process progression in ESCC, which provides invasive and migratory properties to cancer cells.
This study has several limitations. The human gut microbiota is a diverse ecosystem that is closely related to cancer; however, we only investigated the promotional effects and possible mechanisms of Bacteroides, which were the most significant differences in our study. And the roles of gut microorganisms in ESCC still need to be collected from more clinical samples and experimental data and investigated in depth. Additionally, due to ethical issues, we were unable to obtain intestinal tissue samples from esophageal cancer patients and normal volunteers to compare the extent of intestinal barrier disruption between the two groups.
Conclusions
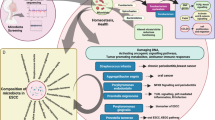
Overall, the increased abundance of Bacteroidetes was associated with ESCC development. Additionally, Bacteroidetes may further initiate the EMT process through LPS-mediated alteration of the inflammatory environment and TLR4/Myd88/NF-κB signaling pathway, thereby promoting the invasion and metastasis of ESCC (Fig. 7).
Materials and methods
Clinical samples and ethical statement
This study included 30 patients newly diagnosed with esophageal cancer (ESCC group) and 30 healthy controls from the Department of Physical Examination (control group) of the Fourth Hospital of Hebei Medical University in 2021. Inclusion criteria47 were as follows: (1) patients aged > 18 years, (2) patients with primary esophageal cancer, (3) patients who had not received radiotherapy, chemotherapy, and/or surgery, and (4) all healthy controls with normal bowel habits. Exclusion criteria48 were as follows: (1) patients with diabetes and depression, (2) patients who had taken antibiotics, H2 receptor antagonists, proton pump inhibitors, and probiotics within the past 1 month, (3) patients with other cancers, and 4) dental bacterial diseases. The study was conducted in strict accordance with the protocol approved by the Ethics Committee of the Fourth Hospital of Hebei Medical University, and all subjects provided written informed consent (2022KY057). The ESCC group referred to the “Chinese Society of Clinical Oncology (CSCO) Esophageal Cancer Diagnosis and Treatment Guidelines-2020” developed by the CSCO, and diagnosed squamous cell carcinoma of the esophagus by histopathology.
Animal models and experimental design
Female C57BL/6 mice (6 weeks old) were purchased from Beijing Wei Tong Li Hua Laboratory Animal Technology (License number: SCXK [Beijing] 2016–0011). The mice were housed in the Experimental Animal Center of the Fourth Hospital of Hebei Medical University (SPF level) following national and international guidelines. The Institutional Laboratory Animal Care Guidelines of the Fourth Hospital of Hebei Medical University approved the study (IACUC-4th Hos Hebmu-2022,003).
Freshly prepared 4NQO (N8141, Sigma-Aldrich) stock solution (in propylene glycol) was added to drinking water at 100 µg/mL, and the water was changed weekly. Mice were randomly categorized into an experimental group provided with drinking water containing 4NQO (n = 8) and a control group provided with drinking water without 4NQO (n = 8), but with the same volume of propylene glycol. Mice were allowed access to drinking water at all times during treatment. The mice were provided regular water after 16 weeks of 4NQO treatment49,50. Mice fasted for 12 h at the end of the experiment, were anesthetized with an intraperitoneal injection of sodium pentobarbital (100 mg/kg), and then sacrificed by cervical dislocation.
Sample collection and 16S rRNA sequencing and analysis
Fresh fecal specimens were collected from the participants in sterile tubes, flash frozen in liquid nitrogen, and stored at − 80℃ refrigerator. Professional nurses performed intravenous blood collection in strict accordance with aseptic standard procedures. Serum was collected by centrifugation and stored at − 80 °C.
Samples were extracted from genomic DNA and amplified by polymerase chain reaction (PCR) applying primers specific to the 16SV34 region (upstream primer CCTAYGGGRBGCASCAG and downstream primer GGACTACNNGGGGTATCTAAT). The purified and gel electrophoresis-detected PCR products were recovered using a gel recovery kit (TIANGEN Biotech Co., Ltd., Beijing, China). The Illumina company library building kit (model: TruSeq DNA PCR Free Library Preparation Kit) was used to construct the library. NovaSeq6000 was used for computer sequencing, after qualifying the library for quantitative inspection. Changes in the abundance of bacteria were analyzed, and bacteria with large differences were selected as candidates.
ELISA assay
ELISA kits (Shanghai Fusheng Industrial Co., Ltd., China) were used for detecting LPS, IL-6, IL-10, TNF-α, and TGF-β levels. Briefly, sera from clinical participants and experimental mice were processed to obtain a suitable supernatant, which was later processed following the ELISA kit instructions, and absorbance values (OD) of the samples were measured at 450 nm to quantify the LPS, IL-6, IL-10, TNF-α, and TGF-β values by comparing the values relative to each standard curve.
Histochemical and immunohistochemical (IHC) staining
The esophageal and rectal tissues of mice were fixed with 4% paraformaldehyde, dehydrated, and paraffin-embedded at the end of the experiments to prepare 4-μm-thick sections, which were deparaffinized, hydrated, and other steps, and then subjected to hematoxylin and eosin staining with IHC staining.
The results in ESCC tissue included TLR4 (GB15186, Servicebio, China), Myd88 (GB11269, Servicebio, China), NF-κB (GB11269, Servicebio, China), Ki-67 (GB111141, Servicebio, China), E-cadherin (GB12083, Servicebio, China), and snail (GB11260, Servicebio, China) proteins, and rectal tissue used ZO1 (GB111402, Servicebio, China) and occludin (GB111401, Servicebio, China) proteins for IHC staining. Finally, two observers, who were unaware of each other, performed histologic evaluation and investigated the sections.
Preparation of dried powder of LPS from Mycobacterium fragilis.
The powder of Pseudomonas fragilis (BNCC336948, BeNa Culture Collection, China) preserved in the ampoule tube was passaged in Columbia blood agar plates (BNCC352241, BeNa Culture Collection, China), and the plates were incubated in an anaerobic gas-producing bag (Mitsubishi motors, MGC C-01, Japan) in an anaerobic jar (Mitsubishi motors, MGC C-31, Japan) for 24–48 h at 37℃ for two consecutive passages (motors, MGC C-01, Japan) in an anaerobic tank (Mitsubishi motors, MGC C-31, Japan) for 24 h. Single colonies were selected and passaged into liquid thioglycolate medium FT tubes (BNCC353538, BeNa Culture Collection, China). Collection, Chin) and cultured in a 37℃ constant temperature incubator (ZHICHENG, ZXDP-B2080, China) for 24 h. The liquid medium was centrifuged at 8000 g for 3 min at room temperature (22 °C) to harvest the B. fragilis precipitate, and LPS samples were extracted from the bacterial precipitates using an LPS extraction kit (EX1740, Servicebio, China), and finally desalted and lyophilized using 10-KD ultrafiltration tubes, and stored at − 80 °C in a refrigerator.
Cell culture
The human esophageal squamous cell carcinoma (ESCC) cell line KYSE150 was obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). KYSE150 Cells were cultured in RPMI-1640 medium (Gibco, USA) containing 10% fetal bovine serum (Gibco, USA). Cells were cultured in a 37℃, 5% CO2 humidified incubator (Thermo, US).
Cell counting kit-8 (CCK-8) Assay
Cells were plated in 96-well plates at 2 × 103 cells/well. Cells were incubated in media with various LPS concentrations (0, 125, 250, and 500 ng/ml, Sigma, Germany) for 6, 12, and 24 h. The CCK-8 solution was added at a 1:10 dilution into each well. A microplate reader (Bio-Rad Laboratories, Hercules, USA) was used to measure absorbance at 450 nm. Additionally, the results were expressed as the percentage of CCK-8 conversion relative to control cell absorbance.
Scratch assay
The cells were inoculated in a 24-well culture plate overnight. We made straight scratches in middle of the well using 200-μl tips and gently washed three times with PBS to ensure get rid of floating cells. Cells were incubated in a medium containing 200 ng/mL LPS. Images were taken at 12 h and 24 h, and images were analyzed using Image J.
Invasion assays
Transwell system based with Matrigel, which was produced from BD, USA, was used to evaluate the invasiveness of tumor cells. Control cells or LPS treated cells were added to the upper chamber, and bovine serum-containing medium was added to the lower chamber. In addition, these cells were cultured in a CO 2 incubator for 12 h and 24 h. Cells in the upper chamber were removed, and those passing through the chamber were fixed with 4% paraformaldehyde, and stained with crystal violet, and then the photographs were taken.
Western blotting
WB analysis was conducted to evaluate protein expression levels. Cells were lysed using radioimmunoprecipitation assay buffer (G2002, Servicebio, China) on ice for 10 min. The enhanced bicinchoninic acid Protein Assay Kit (PC0020, Solarbio, China) was used to determine the quality of the total protein collected. The samples were then loaded onto an 8% SDS-PAGE gel and transferred onto a polyvinylidene fluoride membrane. Membranes were blocked with 5% bovine serum albumin in phosphate-buffered saline for 60 min. Subsequently, the membranes were horizontally cut following the molecular weight of the protein of interest, followed by incubation with primary antibodies against TLR4 rabbit pAb (1:2500, A5258, ABclonal), Myd88 rabbit pAb (1:250, A0980, ABclonal), P-NF-κB p105-S932 rabbit mAb (1:2500, AP1355, ABclonal), NF-κB rabbit pAb (1:250, A11160, ABclonal, China), snail rabbit pAb (1:750, A5243, ABclonal), E-cadherin rabbit pAb (1:1000, A3044, ABclonal), β-Actin rabbit mAb (1:50,000, AC026, ABclonal), and goat antirabbit IgG (H + L) as secondary antibody (1:5000, S1002, Ruipate) overnight at 4℃. The ECL detection kit (HY-K1005, MCE, USA) was used to detect protein expression, and ImageJ software was used for the analyses.
Statistical analysis
All statistical data were analyzed with SPSS 23.0 software. Data were expressed as mean ± standard deviation. Student T-test was used to analyze the statistical difference; p < 0.05 indicated that the difference was statistically significant.
Ethical approval
The research was conducted in strict accordance with the protocol approved by the Ethics Committee of the Fourth Hospital of Hebei Medical University, and written informed consent was obtained from all subjects (2022KY057). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The research was approved by the Institutional Laboratory Animal Care guidelines of Fourth Hospital of Hebei Medical University (IACUC-4th Hos Hebmu- 2022,003). This study was conducted appropriately and in accordance with local laws and institutional requirements. The experimental protocol was performed in accordance with the relevant guidelines and regulations of the Basel Declaration. The study is reported in accordance with ARRIVE guidelines (https://arriveguidelines.org).
Data availability
Analysis of alpha/beta diversity of gut flora between the two populations is shown in Supplementary Fig. S1. Analysis of alpha/beta diversity of gut flora between the two groups of mice in Supplementary Fig. S1. All raw reads are stored in the NCBI Sequence Read Archive (SRA) database under accession number PRJNA1091832 (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA1091832) and PRJNA1091827 (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA1091827) and were available upon reasonable request to the corresponding authors. All the original Western blots with replicates of the experiments are available in Supplementary Figs 3, 4, 5, 6, 7 and 8.
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71(3), 209–249 (2021).
Xu, F. et al. Epigenetic induction of tumor stemness via the lipopolysaccharide-TET3-HOXB2 signaling axis in esophageal squamous cell carcinoma. Cell Commun. Signal 18(1), 17 (2020).
Arifuzzaman, M. et al. Nutritional regulation of microbiota-derived metabolites: Implications for immunity and inflammation. Immunity 57(1), 14–27 (2024).
Caenepeel, C. et al. Dysbiosis and associated stool features improve prediction of response to biological therapy in inflammatory bowel disease. Gastroenterology 166(3), 483–495 (2024).
Münch, N. S. et al. High-fat diet accelerates carcinogenesis in a mouse model of barrett’s esophagus via interleukin 8 and alterations to the gut microbiome. Gastroenterology 157(2), 492-506.e2 (2019).
Sulit, A. K. et al. Bacterial lipopolysaccharide modulates immune response in the colorectal tumor microenvironment. NPJ. Biofilms. Microbiomes. 9(1), 59 (2023).
Kumari, P. et al. Host extracellular vesicles confer cytosolic access to systemic LPS licensing non-canonical inflammasome sensing and pyroptosis. Nat. Cell Biol. 25(12), 1860–1872 (2023).
Takashima Y., Kawamura H., Okadome K., et al. Enrichment of Bacteroides fragilis and enterotoxigenic Bacteroides fragilis in CpG island methylator phenotype-high colorectal carcinoma. Clin Microbiol Infect, (2024).
Han, J. et al. Gut microbiome: Decision-makers in the microenvironment of colorectal cancer. Front Cell Infect. Microbiol. 13, 1299977 (2023).
Routy, B. et al. Melanoma and microbiota: Current understanding and future directions. Cancer Cell 42(1), 16–34 (2024).
Jinato, T. et al. Distinct alterations of gut microbiota between viral- and non-viral-related hepatocellular carcinoma. Appl. Microbiol. Biotechnol. 108(1), 34 (2024).
Zhou, C. et al. The influence of Helicobacter pylori, proton pump inhibitor, and obesity on the gastric microbiome in relation to gastric cancer development. Comput. Struct. Biotechnol. J. 23, 186–198 (2024).
Quante, M. et al. Bile acid and inflammation activate gastric cardia stem cells in a mouse model of Barrett-like metaplasia. Cancer Cell 21(1), 36–51 (2012).
Lv, J. et al. Alteration of the esophageal microbiota in Barrett’s esophagus and esophageal adenocarcinoma. World J. Gastroenterol. 25(18), 2149–2161 (2019).
Zhang, J. et al. Unveiling intratumoral microbiota: An emerging force for colorectal cancer diagnosis and therapy. Pharmacol. Res. 203, 107185 (2024).
Abate, M. et al. A novel microbiome signature in gastric cancer: A two independent cohort retrospective analysis. Ann. Surg. 276(4), 605–615 (2022).
Yan, S. et al. Berberine regulates short-chain fatty acid metabolism and alleviates the colitis-associated colorectal tumorigenesis through remodeling intestinal flora. Phytomedicine 102, 154217 (2022).
Cheng, X. et al. A neutral polysaccharide from Persicaria hydropiper (L.) Spach ameliorates lipopolysaccharide-induced intestinal barrier injury via regulating the gut microbiota and modulating AKT/PI3K/mTOR and MAPK signaling pathways. J. Ethnopharmacol. 320, 117403 (2024).
Liu, Y. et al. (-)-Syringaresinol attenuates ulcerative colitis by improving intestinal epithelial barrier function and inhibiting inflammatory responses. Phytomedicine 124, 155292 (2024).
Sun, M. et al. Dehydrocostus lactone alleviates irinotecan-induced intestinal mucositis by blocking TLR4/MD2 complex formation. Phytomedicine 128, 155371 (2024).
Mishra, Y. et al. The role of the gut microbiome in gastrointestinal cancers. Cell Signal 115, 111013 (2024).
Wang, M. et al. Bacterial lipopolysaccharide-induced endothelial activation and dysfunction: A new predictive and therapeutic paradigm for sepsis. Eur. J. Med. Res. 28(1), 339 (2023).
Chen, S. et al. The role and mechanisms of gram-negative bacterial outer membrane vesicles in inflammatory diseases. Front Immunol. 14, 1157813 (2023).
Llopiz, D. et al. Enhancement of antitumor vaccination by targeting dendritic cell-related IL-10. Front Immunol. 2018, 9 (1923).
Rašková, M. et al. The role of IL-6 in cancer cell invasiveness and metastasis—overview and therapeutic opportunities. Cells. 11(22), 3698 (2022).
Barbi, J., Pardoll, D. & Pan, F. Treg functional stability and its responsiveness to the microenvironment. Immunol. Rev. 259(1), 115–139 (2014).
Cui, H. et al. The dynamic shifts of IL-10-producing Th17 and IL-17-producing Treg in health and disease: a crosstalk between ancient “Yin-Yang” theory and modern immunology. Cell Commun. Signal 22(1), 99 (2024).
Zegarra Ruiz, D. F. et al. Microbiota manipulation to increase macrophage IL-10 improves colitis and limits colitis-associated colorectal cancer. Gut. Microbes 14(1), 2119054 (2022).
Ebrahimi, N. et al. Harnessing function of EMT in cancer drug resistance: a metastasis regulator determines chemotherapy response. Cancer Metastasis Rev. 16, 1–23 (2024).
Wang, X., Eichhorn, P. J. A. & Thiery, J. P. TGF-β, EMT, and resistance to anti-cancer treatment. Semin. Cancer Biol. 97, 1–11 (2023).
Schuhwerk, H. & Brabletz, T. Mutual regulation of TGFβ-induced oncogenic EMT, cell cycle progression and the DDR. Semin. Cancer Biol. 97, 86–103 (2023).
Huang, Q. et al. Muscle-to-tumor crosstalk: The effect of exercise-induced myokine on cancer progression. Biochim. Biophys. Acta Rev. Cancer 1877(5), 188761 (2022).
Ciesielska, A., Matyjek, M. & Kwiatkowska, K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell Mol. Life Sci. 78(4), 1233–1261 (2021).
Mukherjee, S. et al. Toll-like receptor-guided therapeutic intervention of human cancers: Molecular and immunological perspectives. Front Immunol. 14, 1244345 (2023).
Le Noci, V. et al. Toll like receptors as sensors of the tumor microbial dysbiosis: Implications in cancer progression. Front Cell Dev. Biol. 9, 732192 (2021).
Sato, Y. et al. High TLR4 expression predicts a poor prognosis after esophagectomy for advanced thoracic esophageal squamous cell carcinoma. Esophagus 17(4), 408–416 (2020).
Wang, X. et al. Role of TLR4/MyD88/NF-κB signaling in the contrast-induced injury of renal tubular epithelial cells. Exp. Ther. Med. 20(5), 115 (2020).
Kawai, T. & Akira, S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 34(5), 637–650 (2011).
Vafaeipour, Z., Ghasemzadeh Rahbardar, M. & Hosseinzadeh, H. Effect of saffron, black seed, and their main constituents on inflammatory cytokine response (mainly TNF-α) and oxidative stress status: An aspect on pharmacological insights. Naunyn. Schmiedebergs Arch. Pharmacol. 396(10), 2241–2259 (2023).
Feitelson, M. A. et al. Short-chain fatty acids in cancer pathogenesis. Cancer Metastasis. Rev. 42(3), 677–698 (2023).
Mzyk, P. et al. Toll-like receptor 4 signaling in the trabecular meshwork. Front Cell Dev Biol 10, 936115 (2022).
Brabletz, S. et al. Dynamic EMT: a multi-tool for tumor progression. Embo J. 40(18), e108647 (2021).
Xu, R. et al. Fraxetin suppresses the proliferation, migration, and invasion of ovarian cancer cells by inhibiting the TLR4/STAT3 signaling pathway. Immunopharmacol. Immunotoxicol. 45(3), 287–294 (2023).
Lin, Y. et al. FADD phosphorylation contributes to development of renal fibrosis by accelerating epithelial-mesenchymal transition. Cell Cycle 22(5), 580–595 (2023).
Luo, L. et al. The TGFβ2-Snail1-miRNA(TGFβ2) circuitry is critical for the development of aggressive functions in breast cancer. Clin. Transl. Med. 14(2), e1558 (2024).
Sun, M. et al. Mechanisms of LPS-induced epithelial mesenchymal transition in bEECs. Theriogenology 216, 30–41 (2024).
Siegel, R. L. et al. Cancer statistics, 2023. CA Cancer J. Clin. 73(1), 17–48 (2023).
Zhang, X. et al. Modulating a prebiotic food source influences inflammation and immune-regulating gut microbes and metabolites: Insights from the BE GONE trial. EBioMedicine 98, 104873 (2023).
Liu, Z. et al. Esophageal squamous cancer from 4NQO-induced mice model: CNV Alterations. Int. J. Mol. Sci. 23(22), 14304 (2022).
Tang, X. H. et al. Oral cavity and esophageal carcinogenesis modeled in carcinogen-treated mice. Clin. Cancer Res. 10(1 Pt 1), 301–313 (2004).
Acknowledgements
The authors thank Figdraw for help with Fig. 7.
Funding
This study was supported partially by the National Natural Science Foundation of China (8227150213), Natural Science Foundation of Hebei Province (H2023206137), Science Research Project of Hebei Education Department (BJK2024113), and Hebei Province Traditional Chinese Medicine Scientific Research Subjects Programme (2024110).
Author information
Authors and Affiliations
Contributions
Qinghuan Li and Jing Li designed the experiments and conducted the study. Zhongbing Wu and Jianxin Guo wrote the manuscript. Zhongbing Wu revised the manuscript. Zhenhan Zhang and Shuang Gao . contributed to the animal experiment. Ming Huang and Yu Wang contributed to literature search, data collection, analysis and interpretation. Yushuang Zhang contributed to the sample collection in patients. Qinghuan Li performed sample collection and statistical analysis. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wu, Z., Guo, J., Zhang, Z. et al. Bacteroidetes promotes esophageal squamous carcinoma invasion and metastasis through LPS-mediated TLR4/Myd88/NF-κB pathway and inflammatory changes. Sci Rep 14, 12827 (2024). https://doi.org/10.1038/s41598-024-63774-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-63774-6
- Springer Nature Limited