Abstract
This study investigated whether Ki–Patlak derived from a shortened scan time for dynamic 18F-NaF PET/CT in chronic kidney disease (CKD) patients undergoing hemodialysis can provide predictive accuracy comparable to that obtained from a longer scan. Twenty-seven patients on chronic hemodialysis, involving a total of 42 scans between December 2021 and August 2023 were recruited. Dynamic 18F-NaF PET/CT scans, lasting 60–90 min, were immediately acquired post-injection, covering the mid-twelfth thoracic vertebra to the pelvis region. Ki–Patlak analysis was performed on bone time–activity curves at 15, 30, 45, 60, and 90 min in the lumbar spine (L1–L4) and both anterior iliac crests. Spearman’s rank correlation (rs) and interclass correlation coefficient were used to assess the correlation and agreement of Ki–Patlak between shortened and standard scan times. Bone-specific alkaline phosphatase (BsAP) and tartrate-resistant acid phosphatase isoform 5b (TRAP5b) were tested for their correlation with individual Ki–Patlak. Strong correlations and good agreement were observed between Ki–Patlak values from shortened 30-min scans and longer 60–90-min scans in both lumbar spine (rs = 0.858, p < 0.001) and anterior iliac crest regions (rs = 0.850, p < 0.001). The correlation between BsAP and Ki–Patlak in the anterior iliac crests was weak and statistically insignificant. This finding suggests that a proposed shortened dynamic 18F-NaF PET/CT scan is effective in assessing bone metabolic flux in CKD patients undergoing hemodialysis, offering a non-invasive alternative approach for bone turnover prediction.
Similar content being viewed by others
Introduction
The diagnosis of renal osteodystrophy (ROD) is typically based on bone turnover, mineralization, and volume (TMV system), which can be assessed using bone histomorphometry from biopsy1. ROD in patients with chronic kidney disease (CKD) and end-stage renal disease (ESRD) is determined clinically by bone turnover, which is defined as the rate of skeleton remodeling or the rate of bone formation and resorption. Low bone turnover is associated with reduced osteoblast and osteoclast activities, whereas high bone turnover is associated with increased osteoblast and osteoclast activities2. Both low and high bone turnover may lead to bone abnormalities and increased fracture risk. Although bone biopsy is considered the gold standard for the specific classification of the different subtypes of ROD, it is an invasive and painful procedure that is limited to a single bone site and requires considerable expertise to interpret specimens. Furthermore, assessing the impact of treatment on bone turnover requires multiple sequential biopsies because a single biopsy is insufficient to record the changes that occur over time3.
Bone metabolic flux (Ki) obtained from fluorine-18-labeled sodium fluoride (18F-NaF) acquired using positron emission tomography/computed tomography (PET/CT) imaging is used as an alternative approach for ROD classification4,5,6. Ki represents the net regional plasma clearance of 18F-NaF and the bone formation rate. The uptake mechanism of 18F-NaF relies on the diffusion of ion exchange from the plasma into the extracellular fluid space within bones and is deposited on the surface of newly formed hydroxyapatite crystals at sites of bone formation, with increasing 18F-NaF uptake in locations where osteoblasts and osteoclasts are activated. 18F-NaF is rapidly excreted by the kidneys7,8,9. Several studies have shown that Ki is positively and significantly correlated with bone turnover parameters obtained using bone biopsies5,9. Although Ki can serve as an imaging biomarker for assessing regional bone metabolism at one or multiple skeletal sites with a single injection, obtaining the Ki value traditionally requires a long dynamic scan that lasts at least 60 min. This prolonged image acquisition time may cause patient discomfort and movement during clinical procedures. Therefore, several published studies have examined the possibility of reducing the scan time required to estimate Ki values. Recently, Puri et al.10 found that Ki values obtained from a short scan of 12-min using the Hawkins two-tissue compartmental model achieved similar statistical accuracy to those obtained from the 60-min dynamic scan. However, their short scan method did not study in the lumbar spine, which is also an important site for 18F-NaF PET imaging for bone turnover assessment. Peters et al.11 examined a correlation between the 30-min Ki value and the mean standardized uptake value (SUV) at 30-min and 60-min, offering clinically relevant dynamic parameters for the diagnosis of pseudarthrosis. However, a 60-min dynamic imaging was not performed in their study because the long scan time was impractical in patients with significant back pain. Therefore, the comparison of Ki between 30-min and 60-min dynamic scans was not investigated. Siddique et al.12 estimated the Ki from a series of 4-min static scan, simulated from a dynamic scan acquired in a time window 30–60 min after injection. While they found that bone plasma clearance obtained from a single static image can provide an accurate and robust estimate of Ki comparable to the conventional Hawkins 60-min dynamic scan, this approach requires taking several venous blood samples to estimate the arterial input function (AIF).
Investigations regarding the shortened scan time of 18F-NaF Ki–Patlak especially in CKD patients undergoing hemodialysis, as published in previously studies remain limited. It is worthwhile to explore efforts to shorten the scan time for Ki value estimation for noninvasive biomarker-based bone turnover prediction, focusing on the anterior iliac crest (the site of bone biopsy) and the lumbar spine. Therefore, this study investigated the reduction of scan time for Ki value assessment at both the lumbar spine and anterior iliac crests for dynamic 18F-NaF PET/CT imaging for patients with CKD undergoing hemodialysis instead of a routine 60-min or 90-min dynamic scan. Furthermore, this study aimed to validate that a shorter scan time can provide Ki equivalent to that obtained from a prolonged scan.
Material and methods
Subjects
This study was approved by the Institutional Review Board of the Faculty of Medicine, Chulalongkorn University (IRB No.0625/65, COA No. 1644/2022). Twenty-seven Thai patients with ESRD on hemodialysis were recruited in this prospective study between December 2021 and August 2023. The patient group consisted of 15 females and 12 males, with a mean age of 54.8 ± 10.4 years, body weight of 57.6 ± 12.0 kg, body mass index of 23.0 ± 5.1 kg/m2, and injected activity of 18F-NaF of 132 ± 41.3 MBq. Patient’s demographic data included in this study are presented in Table 1. Thirteen patients underwent two scans, and one patient underwent three scans in order to evaluate the treatment responses, with a minimum of 3 months between each PET/CT examination. The remaining patients underwent one scan each. Therefore, the total number of scans to be analyzed in this study was 42. The inclusion criteria were as follows: patients who had undergone hemodialysis treatment for > 6 months with stable thrice-weekly hemodialysis, and those who exhibited a low bone mineral density T-score at the total hip or femoral neck, less than ˗1. Patients with a history of post orthopedic surgeries or those with significant bone disorders that affect the lumbar vertebrae (L1–L4) and iliac crests, current diagnosis of active malignancy, current or history of corticosteroids use for > 6 months, and history of organ transplantation were excluded from this study. Biochemical testing for bone turnover biomarkers (BTMs), such as parathyroid hormone (PTH), bone-specific alkaline phosphatase (BsAP), and tartrate-resistant acid phosphatase isoform 5b (TRAP5b), was performed before or after hemodialysis sessions.
Dynamic PET/CT acquisition
All patients underwent 18F-NaF PET/CT dynamic scans using a digital PET/CT Siemens Biograph Vision 600 scanner (Siemens Healthineers, Erlangen, Germany) at the Division of Nuclear Medicine, King Chulalongkorn Memorial Hospital. This PET/CT system has an axial field of view (FOV) of 26.3 cm for lutetium oxyorthhosilicate (LSO) crystals and is combined with a 64-slice CT scanner. In this study, two acquisition protocols were used: 90- and 60-min dynamic scan protocols. Eight scans were performed using continuous bed motion (CBM) acquisition on a whole-body 90-min dynamic scan, and 34 scans were performed using the 60-min dynamic scan. For the 90-min scan, a 6-min dynamic single-bed list-mode PET acquisition centered at the heart region, in which the FOV encompasses the arch of the aorta to the second lumbar vertebra (L2) for extracting the image-derived input function (IDIF), started immediately after intravenous injection of 18F-NaF. A subsequent set of 16 multi-pass dynamic whole-body PET scans covering areas from the base of the skull to the upper thigh using CBM of approximately 5 min each with table speed 2.4 mm/s was then acquired. A 4-min pause was added between these two steps before starting dynamic whole-body scans following the Siemens multiparametric PET suite protocol13. For the 60-min scan, a dynamic single-bed PET acquisition with FOV encompassing the mid-twelfth thoracic vertebra (T12) to the pelvis was acquired, followed by a subsequent routine whole-body static scan of approximately 10 min.
List-mode data were reconstructed and re-binned into 42 timeframes for 90-min scans (12 × 5 s, 6 × 10 s, 8 × 30 s, 4-min pause, 16 × 300 s), and 35 timeframes for 60-min scans (12 × 5 s, 6 × 10 s, 6 × 30 s, 11 × 300 s). All images were reconstructed using the ordered subset expectation maximization (OSEM) algorithm with a Gauss filter and the point spread function (PSF) TrueX (Gauss and all pass filter) algorithm with four iterations and five subsets and a matrix size of 220 × 220. Low-dose CT protocol for attenuation correction (120 kV, CARE Dose 4D automatic tube-current modulation, pitch 0.8, rotation time 0.5 s, 3.0 mm slice thickness, 780 mm axial FOV) encompassing the base of the skull to the upper thigh was used to acquired CT images for both protocols.
Kinetic modeling and data analysis
PMOD (version 4.002; PMOD Technologies LLC, Switzerland) was used to draw the volume of interest (VOI) on dynamic images for data analysis and Ki–Patlak kinetic model fitting. Motion correction of the dynamic PET images was performed by averaging across the early time-frame series to create a reference for performing rigid body transform across all frames of the dynamic PET. Two input data were required for Ki quantification using the Patlak analysis method, that is, IDIF and bone time–activity curve (BTAC) obtained from the dynamic images.
Image-derived input function (IDIF)
In this study, a noninvasive method, IDIF, was used for alternative arterial blood sampling14,15. The VOIs were drawn within the abdominal aorta at the level of the twelfth thoracic spine (T12) to the second lumbar vertebra (L2) of the dynamic PET image using 3D cuboid with dimensions between 3 × 3 × 5 mm to 5 × 5 × 25 mm in the early phase time frames (approximate twelve 5-s), which can be used to visualize 18F-NaF concentration within the aorta and allows an accurate quantification of IDIF for calculating Ki16. To maintain the consistency of the measurement, the same VOI was duplicated for all PET time frames. Thus, all individual IDIFs derived from 42 studies were generated at 0–90 min for eight studies and 0–60 min for thirty-four studies.
To obtain the IDIF for the short scan method, the time frames of 0–15, 0–30, 0–45, and 0–60 min were selected from 90-min PET dynamic data in PMOD to generate IDIF15min, IDIF30min, IDIF45min, and IDIF60min, respectively. Similarly, for 60-min dynamic data, IDIF15min, IDIF30min, and IDIF45min were generated from PET images at 0–15, 0–30, and 0–45 min, respectively.
Bone time–activity curves (BTACs)
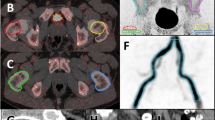
In this study, BTAC were obtained by measuring the activity concentration of 18F-NaF within the VOI regions of lumbar vertebrae and both iliac crests. To ensure consistency in contouring across participants, for the lumbar spine, the 3D cuboidal VOIs sized approximately 1–4 cm3 were placed within the middle of each vertebral body L1 to L4, avoiding the end plates and the disk space, as these could influence the activity measurement. For both iliac crests, the 3D spherical VOIs were defined by contouring on the anterior iliac crest at approximately the same region as the bone biopsy, with the VOI size ranging from 0.1–0.3 cm3. The contouring locations of all VOIs were then verified by a nuclear medicine physician. Illustration of the lumbar vertebrae and iliac crest VOIs contouring are shown in Fig. 1. For bone VOIs, six BTACs were generated from either 60-min or 90-min PET dynamic images from each scan. BTAC15min, BTAC30min, and BTAC45min were generated from the 60-min dynamic data at 0–15, 0–30, and 0–45 min, respectively, whereas BTAC15min, BTAC30min, BTAC45min, and BTAC60min were generated from the 90-min dynamic data at 0–15, 0–30, 0–45, and 0–60 min, respectively, for the shortened dynamic scan method.
Ki–Patlak analysis
All BTACs and IDIFs were generated using the PMOD view module and subsequently transferred to the PMOD kinetic module to estimate the regional Ki values at the lumbar spine and iliac crest VOIs. For the Patlak analysis, Ki was obtained from Eq. (1) as follows17:
where \({C}_{Bone}(T)\) represents the total amount of 18F-NaF in bone VOI at time T after injection, \({C}_{Blood}(t)\) is the total amount of 18F-NaF in whole blood at each time point (t), Ki is the plasma clearance that describes the rate of entry into the peripheral compartment, and V0 is the volume of distribution of 18F-NaF, which shows the proportion of bone to blood activity in the bone VOI17. \({K}_{i}\underset{0}{\overset{T}{\int }}{C}_{Blood}\left(t\right)dt\) represents the amount of 18F-NaF trapped in the bound bone pool, whereas \({V}_{0}{C}_{Blood}(T)\) represents the amount of 18F-NaF in the unbound bone pool. Equation (1) can be divided by \({C}_{Blood}(T)\) to obtain a linear relationship with a slope equal to Ki and an intercept equal to V0. The Ki slope corresponds to the 18F-NaF trapping ratio in bone VOI.
For the initial parameter fitting in this study, the Patlak plot model allows fitting a linear regression to data starting at t*, which can be specified manually from 0 to 60 with a default weighting constant. The results are the regression slope (Ki) and intercept (V). A maximum value of 10% was set as the error criterion.
Statistical analysis
The Wilcoxon test was used to assess the statistical significance of the difference between Ki–Patlak values in the lumbar spine and both anterior iliac crests. P-values of 0.05 were used to denote statistical significance for this test. Correlations between Ki–Patlak values obtained from the four lumbar vertebrae and both iliac crests were assessed using Spearman’s rank correlation test (rs). The intraclass correlation coefficient (ICC) was used to evaluate the agreement between Ki–Patlak derived from a shortened scan time and Ki–Patlak derived from a long routine scan time.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the Faculty of Medicine, Chulalongkorn University (IRB No.0625/65, COA No. 1644/2022).
Consent to participate
Informed consent was obtained from all individual participants included in this study.
Results
Routine Ki–Patlak values
The mean routine Ki–Patlak values from 42 dynamic 18F-NaF PET/CT scans were 0.068 ± 0.022 mL/min/mL in the lumbar spine and 0.069 ± 0.027 mL/min/mL in both anterior iliac crests as shown in Table 2. No significant difference in the means of routine Ki–Patlak values was observed between the two regions for the 60 min routine scan time (p = 0.866). According to bone turnover-based classification established by Aaltonen et al.17, the specific cutoff values with a threshold between 0.038 and 0.055 mL/min/mL were used to distinguish low turnover from non-low turnover, and high turnover from non-high turnover in the lumbar spine and anterior iliac crest regions. In this study, 33 scans (78.6%) were classified as high and non-low turnovers, 4 scans (9.5%) were classified as normal turnover, and 5 scans (11.9%) were classified as low and non-high turnovers for both regions. The Ki–Patlak values in the lumbar spine were compared with those in both anterior iliac crests using Spearman’s correlation test, which showed a significant correlation (rs = 0.772, p < 0.001). Additionally, a strong correlation was observed between the average Ki–Patlak values in the L1-L4 and each lumbar region, and between Ki–Patlak values in both anterior iliac crests and each iliac crest region.
Routine Ki–Patlak values and biochemical markers
A weak correlation was observed between BsAP and Ki–Patlak values in both anterior iliac crests (rs = 0.459, p = 0.002). No correlation was observed between TRAP5b and Ki–Patlak values in lumbar spine (rs = 0.094, p = 0.553) and both anterior iliac crests (rs = 0.284, p = 0.068) or between BsAP and Ki–Patlak values in the lumbar spine (rs = 0.079, p = 0.621).
Shortened scan Ki–Patlak and routine Ki–Patlak values
The mean Ki–Patlak values from the shortened scan time in L1–L4 and both anterior iliac crests are presented in Table 2. No significant differences in the means of 30-min and 45-min Ki–Patlak values were observed between the two regions (p = 0.179 and p = 0.970). Figure 2 shows the distribution of Ki–Patlak values calculated from shortened scan times at 15, 30, 45, and 60 min at the lumbar spine and both anterior iliac crests obtained from 42 dynamic 18F-NaF PET/CT scans. The correlations of the shortened scan Ki–Patlak values in the lumbar region and at both iliac crests are shown in Fig. 3A–F. A strong correlation was observed between a 60-min Ki–Patlak and the shortened scan Ki–Patlak at 15 min (rs = 0.672, p < 0.001), 30 min (rs = 0.858, p < 0.001), and 45 min (rs = 0.941, p < 0.001) in the lumbar region. This correlation was also significant between a 60-min Ki–Patlak and the shortened scan Ki–Patlak at 15 min (rs = 0.736, p < 0.001), 30 min (rs = 0.850, p < 0.001), and 45 min (rs = 0.894, p < 0.001) at the anterior iliac crest region. Figure 4A–4F show the Bland–Altman plot of the mean difference between the shortened scan and long scan Ki–Patlak values in the lumbar spine and anterior iliac crest regions (Table 3).
Bland–Altman plots demonstrating the relationship between the shortened scan and long scan Ki–Patlak values in the lumbar spine and iliac crest regions. Limits of agreement are indicated by dotted lines with 95% confidence interval (light blue regions), while bias is represented by a solid line with 95% confidence interval (gray region).
No significant difference of routine dynamic scan Ki–Patlak values was found among different bone turnover categories in the two regions as shown in Table 4. These findings correspond with the Ki–Patlak values obtained from the shortened scan time, as shown in Fig. 5. When verifying the Ki–Patlak values obtained from shortened scan with routine 60-min scan, the results presented in Table 4 and the consistency observed with Ki–Patlak from the shortened scan in the anterior iliac crests, as illustrated in Fig. 5, demonstrate that the selected cutoff values for bone turnover-based classification hold promise for distinguishing different turnover categories in both regions. However, further validation studies are required to extend these finding to provide a more comprehensive understanding of the utility and accuracy of the proposed threshold in clinical applications in CKD patients with hemodialysis.
Discussion
The Ki–Patlak derived from dynamic 18F-NaF PET/CT in patients on hemodialysis shows a significant correlation with histomorphometric parameters obtained from bone biopsies5,6. This highlights the potential of using bone plasma clearance Ki–Patlak of 18F-NaF PET/CT as an alternative noninvasive biomarker method to evaluate bone turnover changes in ROD. Typically, kinetic modeling of 18F-NaF requires a long scan time with consequent patient discomfort, limiting its use in clinical implementation. This study investigated the feasibility of Ki–Patlak derived from a shortened scan time for dynamic 18F-NaF PET/CT to predict bone turnover in CKD patients undergoing hemodialysis, compared to those obtained from a traditional long dynamic scan in the lumbar spine (L1–L4) and both anterior iliac crests. The results showed that Ki–Patlak values in the L1–L4 and anterior iliac crests derived from the 30-min scan were reliable, supported by the high correlation with Ki–Patlak values at the 60-min scan and the high ICC for Ki–Patlak values at 30- and 60-min scans in both regions. The observed correlation can be attributed to the kinetics of 18F-NaF, indicating that high-contrast bone imaging can be achieved as early as 30–45 min after injection. Therefore, a 30-min Ki–Patlak can effectively capture bone turnover changes, aligning with the prediction of both 60-min and 90-min Ki–Patlak. Based on the findings in this study, the results were consistent with previous literatures, particularly the study of Fuglø et al.3 who highlighted the potential of a 30-min dynamic 18F-NaF PET/CT scan, spanning from the 5th lumbar vertebra to the proximal femur, for assessing bone turnover. They demonstrated a strong correlation between Ki values obtained through five non-invasive methods and those derived from the arterial input function method. Peters et al.11 found a strong correlation between the 30-min Ki value and the mean SUV at 30- and 60-min, showing clinically significant dynamic parameters for diagnosing pseudarthrosis. Furthermore, Siddique et al.12 indicated the feasibility of estimating the Ki values from a 4-min static 18F-NaF PET scan acquired between 30–60 min post-injection. In contrast, it is noteworthy that an uptake period less than or equal 15 min may not provide sufficient data for quantitative methods in clinical interpretation.
There was a slightly lower Ki–Patlak in anterior iliac crests compared to lumbar spine. Additionally, the distribution of Ki–Patlak in the anterior iliac crests for all scan times exhibited a wider range than that in the lumbar spine. These results align with the findings of Puri et al.19 who mentioned that Ki values are influenced by bone blood flow, and lower bone blood flow in the hip is a significant factor for Ki quantification. Nevertheless, no significant differences were observed in the mean routine Ki–Patlak and shortened scan Ki–Patlak values between the lumbar spine and anterior iliac crest regions, except at 15 min.
Regarding biochemical markers, a weak correlation was observed between BsAP and Ki–Patlak values, particularly at the anterior iliac crest. Additionally, no significant correlation was found between TRAP5b and either Ki–Patlak values or between BsAP and Ki–Patlak values in the lumbar spine. It is noted that circulating bone turnover markers, such as BsAP and TRAP5b, may reflect overall skeletal metabolism, including cortical and trabecular bones. In contrast, Ki–Patlak derived from PET scans represents regional metabolism within specific skeletal regions. However, 28 out of 42 scans (66.67%) can provide the BsAP and TRAP5b biomarker testing results agreed with routine Ki–Patlak values from both regions, indicating the potential benefits of using biochemical testing as a noninvasive validation method for bone turnover prediction.
When measuring Ki using various methods with clinical potential, the derivation of an individual input function (IF) for each participant is required16. In this study, the abdominal aorta region was used to derive the IF because several prior studies have indicated that the IDIF can be extracted from the aorta rather than the left ventricle14,20,21. However, partial volume corrections were not performed in this study primarily because all participants were adults, and the diameter of the abdominal aorta vessel significantly exceeded the resolution of the digital PET scanner21. In line with this decision, Lodge et al.22 suggested that the partial volume effect (PVE) is unlikely to introduce significant error in aortic VOI measurements, and PVE can be neglected for large vessels.
The significance of this study lies in the extensive number of scans conducted in Thai CKD patients, and the assessment of bone metabolic flux at the lumbar vertebrae and both anterior iliac crests, corresponding to the biopsy site, using dynamic scanning in both regions. Most previous studies of bone tracer kinetics employed the dynamic scanning to estimate Ki at selected skeletal site, typically either the lumbar spine or hip, as the information was often constrained by the field of view of conventional PET/CT scanner3,9,10,11,12. Despite the primary limitation of this study being the lack of bone histomorphometric parameters obtained from bone biopsy, attributed to patient discomfort and challenges in operation room setup, additional investigations were conducted to further explore correlations between biochemical testing (TRAP5b and BsAP) and Ki–Patlak values23. The clinical implications of these findings are that shortened scan Ki–Patlak may aid treatment decisions and decrease the need for invasive bone biopsies in patients with CKD. The challenges in establishing a normal value range from histomorphometric biopsy results may arise from several factors, such as race, age, and sex, which can introduce variations18. Healthy volunteers are required to assess the normal range of Ki in future studies. Furthermore, in this study, we did not investigate sex differences in Ki–Patlak values in bone turnover prediction. This limitation is acknowledged, and further research is suggested to explore potential sex-dependent variations in bone turnover rate estimation. This would allow for a more comprehensive analysis and understanding of the factors influencing bone metabolism.
Conclusions
This study shows that the proposed shortened scan time for performing 18F-NaF PET dynamic measurements of Ki–Patlak bone metabolic flux at the lumbar spine and iliac crest regions can evaluate bone turnover in patients with CKD equivalent to that derived from a long dynamic scan. The implications of these findings are significant, potentially offering guidance for alternative treatment decisions, reducing the impact of patient movement during scans, and minimizing the need for invasive bone biopsies.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AIF:
-
Arterial input function
- BsAP:
-
Bone-specific alkaline phosphatase
- BTAC:
-
Bone time-activity curve
- BTMs:
-
Bone turnover biomarkers
- CBM:
-
Continuous bed motion
- CKD:
-
Chronic kidney disease
- ESRD:
-
End-stage renal disease
- 18F-NaF:
-
Fluorine–18–labeled sodium fluoride
- IDIF:
-
Image-derived input function
- Ki :
-
Bone metabolic flux
- PTH:
-
Parathyroid hormone
- PVE:
-
Partial volume effect
- ROD:
-
Renal osteodystrophy
- SUV:
-
Standardized uptake value
- TMV:
-
Turnover, mineralization, and volume
- TRAP5b:
-
Tartrate-resistant acid phosphatase isoform 5b
References
KDIGO 2017 Clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int. Suppl. 7, 1–59 (2017).
Hsu, C. Y., Chen, L. R. & Chen, K. H. Osteoporosis in patients with chronic kidney diseases: A systemic review. Int. J. Mol. Sci. 21, 6846 (2020).
Fuglø, D., Drachmann, A. L. P., Heltø, K. M. M., Marner, L. & Hansen, D. Bone turnover in patients with chronic kidney disease stage 5D and healthy controls - a quantitative [18F]fluoride PET study. Mol. Imaging Biol. 25, 815–823 (2023).
Puri, T. et al. Utility of a simplified [18F] sodium fluoride PET imaging method to quantify bone metabolic flux for a wide range of clinical applications. Front. Endocrinol. (Lausanne) 14, 12881 (2023).
Aaltonen, L. et al. Correlation between 18F-sodium fluoride positron emission tomography and bone histomorphometry in dialysis patients. Bone. 134, 115267 (2020).
Messa, C. et al. Bone metabolic activity measured with positron emission tomography and [18F]fluoride ion in renal osteodystrophy: correlation with bone histomorphometry. J. Clin. Endocrinol. Metab. 77, 949–955 (1993).
Czernin, J., Satyamurthy, N. & Schiepers, C. Molecular mechanisms of bone 18F-NaF deposition. J. Nucl. Med. 51, 1826–1829 (2010).
Blake, G. M., Siddique, M., Frost, M. L., Moore, A. E. B. & Fogelman, I. Quantitative PET imaging using (18)F sodium fluoride in the assessment of metabolic bone diseases and the monitoring of their response to therapy. PET Clin. 7, 275–291 (2012).
Vrist, M. H. et al. Bone turnover, mineralization, and volume estimated by 18F-sodium fluoride PET/CT and biomarkers in chronic kidney disease: Mineral and bone disorder compared with bone biopsy. Am. J. Nephrol. 53, 490–502 (2022).
Puri, T., Siddique, M. M., Frost, M. L., Moore, A. E. B. & Blake, G. M. A short dynamic scan method of measuring bone metabolic flux using [18F]NaF PET. Tomography. 7, 623–635 (2021).
Peters, M. J. et al. Evaluation of a short dynamic 18F-fluoride PET/CT scanning method to assess bone metabolic activity in spinal orthopedics. Ann. Nucl. Med. 29, 799–809 (2015).
Siddique, M. et al. Estimation of regional bone metabolism from whole-body 18F-fluoride PET static images. Eur. J. Nucl. Med. Mol. Imaging. 39, 337–343 (2012).
Fahrni, G., Karakatsanis, N. A., Domenicantonio, G. D., Garibotto, V. & Zaidi, H. Does whole-body Patlak (18)F-FDG PET imaging improve lesion detectability in clinical oncology?. Eur Radiol. 29, 4812–4821 (2019).
Cook, G. J. R., Lodge, M. A., Marsden, P. K., Dynes, A. & Fogelman, I. Non-invasive assessment of skeletal kinetics using fluorine-18 fluoride positron emission tomography: Evaluation of image and population-derived arterial input functions. Eur. J. Nucl. Med. 26, 1424–1429 (1999).
Khamwan, K. et al. Dynamic 18F-FDG-PET kinetic parameters for epileptogenic zone localization in drug-resistant epilepsy. Front. Phys. 11, 1233059 (2023).
Puri, T., Frost, M. L., Moore, A. E. B., Cook, G. J. R. & Blake, G. M. Input function and modeling for determining bone metabolic flux using [18F] sodium fluoride PET imaging: A step-by-step guide. Med. Phys. 50, 2071–2088 (2023).
Assiri, R., Knapp, K., Fulford, J. & Chen, J. Correlation of the quantitative methods for the measurement of bone uptake and plasma clearance of 18F-NaF using positron emission tomography. Systematic review and meta-analysis. Eur. J. Radiol. 146, 110081 (2022).
Aaltonen, L. et al. Bone histomorphometry and 18F-sodium fluoride positron emission tomography imaging: comparison between only bone turnover-based and unified TMV-based classification of renal osteodystrophy. Calcif. Tissue Int. 109, 605–614 (2021).
Puri, T. et al. Differences in regional bone metabolism at the spine and hip: A quantitative study using (18)F-fluoride positron emission tomography. Osteoporos. Int. 24, 633–639 (2013).
Puri, T. et al. Validation of new image-derived arterial input functions at the aorta using 18F-fluoride positron emission tomography. Nucl. Med. Commun. 32, 486–495 (2011).
van der Weerdt, A. P. et al. Image-derived input functions for determination of MRGlu in cardiac (18)F-FDG PET scans. J. Nucl. Med. 42, 1622–1629 (2001).
Lodge, M. A. et al. Measurement of PET quantitative bias in vivo. J. Nucl. Med. 62, 732–737 (2021).
Jørgensen, H. S. et al. Diagnostic accuracy of noninvasive bone turnover markers in renal osteodystrophy. Am. J. Kidney Dis. 79, 667–76.e1 (2022).
Acknowledgements
The authors thank the entire staff at the Division of Nuclear Medicine at King Chulalongkorn Memorial Hospital for their technical support. We sincerely thank the Centre of Excellence for Metabolic Bone Disease in CKD patients, Faculty of Medicine, Chulalongkorn University, for recruiting the patients for this study. We also thank Siemens Healthineers for providing the multiparametric PET suite trial license for acquiring and analyzing PET/CT data.
Funding
This work was supported by the Fundamental Fund (fiscal year, 2023), the Thai Red Cross Society, and the Kidney Foundation of Thailand.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by J.P., K.K., T.S., B.P., V.S., and K.K. The first draft of the manuscript was written by V.S. and K.K, J.P, K.K, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sanoesan, V., Phannajit, J., Kingpetch, K. et al. Bone turnover prediction in patients with chronic kidney disease (CKD) undergoing hemodialysis using shortened dynamic 18F-NaF PET/CT Ki–Patlak. Sci Rep 14, 12536 (2024). https://doi.org/10.1038/s41598-024-63476-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-63476-z
- Springer Nature Limited