Abstract
Air pollution and heavy metal exposure are emerging public health concerns. Prenatal exposure to air pollutants and heavy metals has been implicated in the development of congenital heart disease (CHD). However, the relationship between exposure to airborne heavy metals and CHD has not yet been investigated. Therefore, in this large population-based study, we investigated the association between air pollutants, including airborne heavy metals, and the risk of CHD using national health insurance claims data from South Korea. Data regarding 1,129,442 newborns and their mothers were matched with air pollutant levels during the first 8 weeks of gestation. In the five-air pollutant model, we found significant positive correlations between prenatal exposure to sulfur dioxide (SO2; odds ratio [OR] 6.843, 95% confidence interval [CI] 5.746–8.149) and cadmium (Cd; OR 1.513, 95% CI 1.187–1.930) and the risk of ventricular septal defects in newborns. This study highlights the association between prenatal exposure to air pollutants, including airborne heavy metals, and an elevated CHD risk. Further research is essential to validate and expand these findings, with the ultimate goal of enhancing public health outcomes.
Similar content being viewed by others
Introduction
Air pollution caused by excessive concentrations of specific gases and solid particles has become a growing concern because of its potential adverse health effects1. Numerous studies have reported that exposure to air pollution is associated with various diseases, including respiratory, cardiovascular, and neurological diseases2. Mounting evidence suggests that prenatal exposure to air pollution has deleterious effects on fetuses3.
Congenital heart disease (CHD) is a major birth defect that affects approximately 1.8% of live births worldwide4. Although the prognosis of CHD has improved with the development of early diagnosis and treatment, it remains one of the leading causes of fetal death and a high social burden5. The etiology of CHD is complex and not fully understood6; however, environmental factors have been suggested as important contributors7. In particular, exposure to environmental factors during early pregnancy has a significant impact on cardiac abnormalities because most of morphological heart development occurs at 3–8 weeks of gestation8,9.
Several studies have reported that prenatal exposure to air pollutants, such as particulate matter < 2.5 μm in diameter (PM2.5), sulfur dioxide (SO2), nitrogen dioxide (NO2), ozone (O3), and carbon monoxide (CO), is associated with an increased risk of CHD10. Maternal exposure to heavy metals has also been identified as a risk factor for CHD11,12. However, the relationship between heavy metal components in the air and the incidence of CHD remains unclear.
Therefore, in this study, we aimed to investigate the association between CHD risk and early prenatal exposure to air pollutants, including airborne heavy metals, using national health insurance claims data from South Korea.
Methods
Study population and data collection
This study was approved by the Institutional Review Board of Korea University Ansan Hospital (2021AS0217) and was conducted in accordance with the Declaration of Helsinki. The data used in this study were extracted from the database of the South Korean National Health Insurance (NHI) claims. The South Korean NHI service provides a universal health insurance program covering almost its entire population. The database of the South Korean NHI claims contains information of all insurance claims, including diagnostic code (using the International Classification of Disease-10 [ICD-10]) and personal socioeconomic status. Personal information was encoded and provided by Korean NHI Sharing Service in a manner that prevented researchers from identifying specific individuals. Due to the retrospective, database-based study design, informed consent was waived. This waiver was confirmed by the Institutional Review Board of Korea University Ansan Hospital.
Infants born between January 2016 and December 2020 and their mothers were identified using the diagnostic code “P07: Disorders related to short gestation and low birth weight” and “Z38.0–Z38.5: Singletons and twins according to place of birth”. Subjects with incomplete medical data, which means the absence of any demographic data such as maternal age, occupational status, household income, birth date, and infant sex, were excluded for statistical processing. Additionally, multiple births exceeding twins were also excluded because higher-order multiple births are significant risk factors in themselves, and the codes “Z38.6–Z38.8: Other multiple liveborn infant” may not distinguish between triplets, quadruplets, or higher multiples. We believe that these exclusions likely lead to minimal selection bias.
All infants diagnosed with CHD were identified using the code “Q20–28: Congenital malformation of the circulatory system.” The following codes were used to detect several major CHDs: “Q21.0: Ventricular septal defect (VSD),” “Q21.1: Atrial septal defect (ASD),” “Q25.0: Patent ductus arteriosus,” “Q22.1: pulmonary valve stenosis (PS),” “Q23.0: Aortic valve stenosis,” “Q21.2: Atrioventricular septal defect,” “Q23.2: Mitral stenosis,” “Q25.1: Coarctation of the aorta,” “Q21.3: Tetralogy of Fallot (TOF),” “Q20.3: Transposition of the great arteries,” and “Q25.5: Pulmonary atresia with intact ventricular septum.”
Infants born between December and February were classified as winter births, March and May as spring births, June and August as summer births, and September and November as fall births. The gestational age of premature infants was determined using the codes “P07.2: Extreme immaturity (less than 28 completed weeks)” and “P07.3: Other preterm infants (28–36 completed weeks).” Twin births were detected using the code "Z38.3-Z38.5: Twins according to place of birth."
Air pollutant data were obtained from the public database of the Korean Ministry of Environment (https://www.airkorea.or.kr/eng/). The included air pollutants were PM2.5, SO2, NO2, O3, CO, and airborne heavy metals such as lead (Pb), cadmium (Cd), chromium (Cr), copper (Cu), manganese (Mn), iron (Fe), nickel (Ni), and arsenic (As). The concentrations of air pollutants were measured using the following methods: PM2.5 (β-Ray absorption method), SO2 (Pulse UV fluorescence method), NO2 (Chemiluminescence method), O3 (UV photometric method), CO (Non-dispersive infrared method), and airborne heavy metals (Atomic absorption spectrophotometry). PM2.5, SO2, NO2, O3, and CO were measured at 405 monitoring stations nationwide at 5-min intervals. The hourly averages were calculated and reported if more than 75% of the measurements were valid. These hourly averages were then used to calculate monthly average values. Airborne heavy metals were measured at 57 monitoring stations across the country, with air samples collected for 24 h on 5 days each month (during the second week of the month). The monthly average values were calculated from these daily measurements. The country was divided into 16 regions (Seoul, Incheon, Gyeonggi, Gangwon, Chungbuk, Chungnam, Daejeon, Sejong, Jeonbuk, Jeonnam, Gwangju, Gyeongbuk, Gyeongnam, Daegu, Ulsan, and Busan), and monthly average values were calculated for each region. These region-based air pollution data for up to 8 weeks of gestation were matched with NHI data based on the mother’s NHI registration region at childbirth.
Statistical analysis
Data are presented as numbers (%), mean ± standard deviation, median (interquartile range), or odds ratio (OR) (95% confidence interval [CI]). The demographic data of the study population with and without CHD were compared using the chi-square test or Fisher's exact test.
OR and 95% CI were calculated using multivariable logistic regression analysis to assess the impact of air pollutants on CHD incidence, adjusting for confounding factors, including maternal age, infant sex, birth season, and household income. This approach was chosen based on previous studies with similar methodologic structure13,14. Confounding factors were selected based on their potential biological or socioeconomic risks associated with CHD7. Considering their clinical significance and evidence from previous studies15,16, pollutants that exhibited OR exceeding 1.000 with statistical significance in single air pollutant models were chosen for the construction of a multiple air pollutant model. Accordingly, SO2, CO, Pb, Cd, and As were selected to construct a five-air pollutant model for the risk of VSD.
All statistical analysis were performed using SAS® ver. 9.4 (Statistical Analysis Software 9.4; SAS Institute Inc., Cary, NC, USA). Differences were considered statistically significant at p < 0.05.
Results
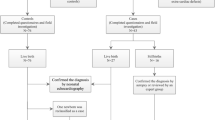
We identified 1,261,648 in children born in South Korea between January 2016 and December 2020 from the NHI claims database. A total of 132,206 newborns with incomplete medical records were excluded and 1,129,442 were enrolled in the study (Fig. 1). During the matched period, about 2.37% of the monthly averages from each monitoring station had valid measurements less than 75%. There were no regions where the valid values fell below 75% when calculating the regional averages.
Demographic data of the study population are shown in Table 1. In total, 60,527 infants were diagnosed with CHD, with an overall incidence of 5.4% in this study population. The proportion of infants with CHD whose mothers were aged > 40 years was higher than that of infants without CHD (19.0% vs. 15.2%, p < 0.001). Infants with CHD were more likely to be born in winter than those without it (28.7% vs. 26.1%, p < 0.001). There were more infants with CHD born prematurely (30.7% vs. 4.0%, p < 0.001) and as twins (5.6% vs. 2.2%, p < 0.001) compared with those without CHD. No other statistically significant differences were found between the two groups.
Table 2 summarizes the air pollutant concentrations, including those of airborne heavy metals, over the whole study period. The number of cases of specific types of CHD is shown in Table 3. ASD was the most common form of CHD, followed by patent ductus arteriosus, VSD, and PS.
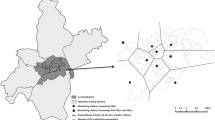
The impact of each air pollutant, including airborne heavy metals, on the risk of CHD was assessed using single air pollutant models (Table 4). The ORs for each air pollutant were below 1 across all CHD subtypes. However, pollutants such as SO2, CO, Pb, Cd, and As were linked to an increased risk of VSD. In the case of ASD and PS, the ORs for all other air pollutants, with the exception of O3, were less than 1. TOF displayed no statistical significance in association with any pollutant except for Fe. A five-air pollutant model for VSD risk was developed using SO2, CO, Pb, Cd, and As (Table 5). In this model, SO2 (OR 6.843, 95% CI 5.746–8.149) and Cd (OR 1.513, 95% CI 1.187–1.930) showed a positive correlation with the risk of VSD. Graphical descriptions of the spatial distribution of the mean SO2/Cd concentration and number of CHDs/VSDs are shown in Fig. 2.
The spatial distribution of the overall mean concentrations of SO2 and Cd and the number of congenital heart diseases in South Korea. (a) SO2 with number of total CHD; (b) SO2 and number of VSD; (c) Cd and number of total CHD; (d) Cd and number of VSD. The mean concentration of SO2/Cd is shown in the grey scale, and the number of patients with total CHD/VSD is indicated by the size of each circle. CHD, congenital heart disease; VSD, ventricular septal defect.
Discussion
This study provides evidence that prenatal exposure to specific air pollutants, including airborne heavy metals, is associated with an increased incidence of several CHD subtypes. Specifically, our findings indicated that higher concentrations of SO2 and Cd were linked to an increased risk of VSD in the context of the five-air pollutant model.
Air pollution has emerged as a significant public health concern and is exacerbated by gradual deterioration due to industrialization and urbanization1,17. Major air pollutants and airborne heavy metal components are predominantly emitted by industrial facilities and vehicles, and subsequently spread into the atmosphere18,19. Consequently, large populations across extensive geographical areas can be affected simultaneously by these air pollutants. Adverse health effects associated with air pollution extend beyond simple inconveniences, such as throat, eye, and skin irritation, encompassing severe health problems and even mortality1. Air pollution has been linked to increased all-cause mortality, likely owing to the multisystemic effects of air pollutants. Several studies have established positive correlations between air pollution and various health conditions including cardiovascular diseases (such as myocardial infarction, heart failure, and arrhythmias), respiratory diseases (such as asthma or chronic obstructive pulmonary disease), cancer, and neuropsychiatric diseases (such as stroke, dementia, Parkinson’s disease, and autism spectrum disorder)2,20,21.
Heavy metals are naturally present on the Earth. However, since industrialization, anthropogenic activities have significantly increased the exposure of living organisms to heavy metals22. Even minimal exposure to toxic heavy metals, such as Pb, Cd, Cr, and As, can be detrimental to human health23,24. Some heavy metals, including Zn, Cu, Fe, and Mn, are present in trace amounts in the body and play essential roles in biologic metabolism25. Excessive exposure to heavy metals can lead to severe health problems. Heavy metals can disrupt normal cellular metabolism, induce oxidative stress, and trigger inflammatory response26,27,28, ultimately resulting in carcinogenic effects and organ damage29.
Maternal exposure to air pollution has been shown to have adverse effects on fetal outcomes, including low birth weight, premature birth, stillbirth, spontaneous abortion, and malformations30,31,32,33. Furthermore, several reports suggested that prenatal exposure to air pollution may have lifelong adverse effects and increase the long-term risk of metabolic, respiratory, allergic, and neurological disorders3,34,35. Similarly, prenatal exposure to heavy metals appears to be correlated with poor fetal outcomes and congenital malformations36,37.
Numerous studies have investigated the relationship between prenatal exposure to air pollution and the risk of CHD. However, these studies have yielded inconsistent results depending on various factors, including the specific pollutants examined, the subtypes of CHD assessed, and the timing of hazardous exposure38. In our study, we observed that prenatal exposure to high SO2 concentrations during the first 8 weeks of gestation was associated with an increased risk of VSD. This finding aligns with that of a study by Gianicolo et al., which reported an association between prenatal exposure to the 90th percentile of SO2 levels during the 3rd to 8th weeks of gestation and overall CHD and VSD39. However, a recent meta-analysis has reported a negative association between SO2 levels and several CHD subtypes40. An umbrella review of 11 systematic reviews suggested that the positive relationship between an increased risk of coarctation of the aorta and high NO2 concentration was rated as having moderate certainty in four meta-analyses, while other findings were rated as having very low or low certainty, possibly due to methodologic heterogeneity38. Therefore, drawing definitive conclusions remains challenging and requires further research.
Several animal and epidemiological studies have indicated a positive relationship between prenatal exposure to heavy metals, particularly Pb, Cd, and As, and the risk of CHD12,25. Our analysis of the effects of these heavy metals on CHD risk revealed a statistically significant positive correlation only with Cd. Preliminary animal studies have reported various morphological abnormalities in the hearts of zebrafish embryos41,42 and chicks exposed to Cd43. Similarly, investigations of maternal blood44 and hair45 have revealed higher Cd levels in mothers of infants with CHD than in mothers of those without.
The pathogenesis of CHD is complex and involves intricate interactions between genetic and environmental factors. Prenatal exposure to air pollution and heavy metals has been suggested as a potential contributing factor to the development of CHD. Several mechanisms have been proposed to explain the influence of prenatal exposure to air pollution and heavy metals on the pathogenesis of CHD.
One plausible mechanism involves the induction of oxidative stress and systemic inflammation caused by air pollution and heavy metals27,46,47. Exposure to air pollutants and heavy metals can generate reactive oxygen species and trigger inflammatory responses in maternal and fetal tissues. These processes can result in cellular damage and disrupt normal cardiac development. Air pollutants and heavy metals may also induce epigenetic changes32,48,49, potentially modifying the expression of genes involved in cardiac development and increasing the risk of CHD. Given the multitude of genes involved in heart development and the sequential development of heart structures over time50,51,52, it is conceivable that the specific subtype of CHD is determined by which genes are affected and when they are affected by environmental contaminants38. Another emerging concept is the heart–placenta axis8,53, which suggests that common genes and signaling pathways affect the development of the heart and placenta. Impaired placental blood flow and reduced fetal heart function may synergistically compromise fetal wellbeing, including development. However, research on the individual effects of these proposed mechanisms for each pollutant is considerably lacking, and more preclinical studies are needed in the future.
In our study population, we observed significant differences in maternal age, birth season, and rates of premature and twin births between the CHD and non-CHD groups. These findings are consistent with those of previous studies, underscoring the significant role played by maternal conditions in CHD development. Previous studies have reported associations between underlying maternal diseases, such as diabetes and hypertension, and an increased risk of CHD54. Other risk factors for CHD include premature birth, multiple births, and advanced maternal age55. Additionally, seasonal variations in the incidence of CHD have been noted, which may be attributed to seasonal differences in factors such as UV exposure, infection, and nutrition7 South Korea exhibits distinct seasonal trends in air pollution56, which may serve as noteworthy contributing factors. These demographic factors were used as confounding factors in our study and were adjusted for in the logistic regression analysis.
Despite the strengths of our study’s large population-based design, certain limitations related to the uncontrolled confounding factors must be acknowledged. First, the air pollution data used in our study were matched based on the mother's NHI registration region, which may not have accurately represented the actual living environment. Individual variations, such as time spent outdoors, use of masks or air purifiers, residential movement during pregnancy and other personal factors also pose challenges in assessing true exposure levels. It would be valuable to assess precise individual exposure using specialized methods, such as personal monitoring devices or global positioning systems, in future research. Additionally, our study focused solely on air pollution as a source of exposure to heavy metals without considering other potential sources such as soil, water, and food. Future research should explore not only the degree of exposure but also the concentration of heavy metals in biological samples, such as blood or placental tissue. There are other factors not addressed in this study, such as maternal drug history, underlying diseases, family history, and paternal information. If these additional confounding factors were considered together, it would be possible to assess the impact of air pollution more accurately.
In this study, non-surviving fetuses with cardiac malformations were not included due to the limitations of the NHI claim database. Given that cardiac malformations are more frequently observed in stillbirths than in live births57,58, our study may underestimate the true association between air pollution and severe forms of CHD. Additional research on cardiac malformations in stillbirths is expected to help illuminate the relationship between air pollution and heart development.
Multivariable logistic regression was selected to assess the impact of multiple pollutants and to adjust for confounders, based on the methodology of previous studies with a similar structure15,16. The OR, as calculated by multivariable logistic regression, approximates the risk ratio when prevalence is low59. However, it is also known that ORs can tend to exaggerate the actual risk60. Therefore, when interpreting the ORs derived from the results of this study as an indicator of actual risk, it is necessary to proceed with caution.
Surprisingly, many indicators suggest that air pollution might have a protective effect on the development of CHD. However, as previously described, this retrospective database-based study has several limitations, making it difficult to draw any definitive conclusions based on our findings. Therefore, instead of overinterpreting the findings of our study, attention should be given to the possibility of an association between air pollution, including airborne heavy metals, and CHD risk, acknowledging that there could be varying patterns according to CHD subtypes.
To the best of our knowledge, this is the first study to examine the relationship between prenatal exposure to air pollutants, including airborne heavy metals, and the risk of CHDs. However, the complex etiology of CHD requires further investigation. Future research should encompass preclinical and prospective studies to further clarify the effects of prenatal exposure to air pollutants on fetal heart development and to investigate the biological mechanisms of how air pollution impacts fetal heart development. Such studies are crucial in highlighting the importance of reducing level of air pollution or preventing prenatal exposure to air pollution, providing scientific information for policy-making, and ultimately improving public health outcomes.
Conclusions
This study underscores the relationship between prenatal exposure to air pollutants, including airborne heavy metals, and an increased risk of CHD. Our findings indicate that high levels of SO2 and Cd are associated with an increased incidence of VSD. Further preclinical and prospective studies are needed to expand this knowledge base, ultimately paving the way for effective prevention against CHD and improving public health outcomes.
Data availability
The datasets generated in this study cannot be made publicly available due to restrictions imposed by South Korean government authorities to ensure ethical privacy protection. Access to the NHI database is restricted to preauthorized researchers during specific research period. The export of these datasets is permitted only in processed form, not in their original datasets. Additional details can be accessed on the NHI Sharing Service's website at https://nhiss.nhis.or.kr. Other data related to this study can be obtained from the corresponding author on reasonable request.
References
Manisalidis, I., Stavropoulou, E., Stavropoulos, A. & Bezirtzoglou, E. Environmental and health impacts of air pollution: a review. Front. Publ. Health 20(8), 505570 (2020).
Bazyar, J. et al. A comprehensive evaluation of the association between ambient air pollution and adverse health outcomes of major organ systems: a systematic review with a worldwide approach. Environ. Sci. Pollut. Res. 26, 12648–12661 (2019).
Gheissari, R. et al. Health outcomes in children associated with prenatal and early-life exposures to air pollution: a narrative review. Toxics 10, 458 (2022).
Wu, W., He, J. & Shao, X. Incidence and mortality trend of congenital heart disease at the global, regional, and national level, 1990–2017. Medicine 99, e20593 (2020).
Zimmerman, M. S. et al. Global, regional, and national burden of congenital heart disease, 1990–2017: a systematic analysis for the Global Burden Of Disease Study 2017. The Lancet Child & Adolescent Health 4, 185–200 (2020).
Sun, R., Liu, M., Lu, L., Zheng, Y. & Zhang, P. Congenital heart disease: causes, diagnosis, symptoms, and treatments. Cell Biochem. Biophys. 72, 857–860 (2015).
Boyd, R., McMullen, H., Beqaj, H. & Kalfa, D. Environmental exposures and congenital heart disease. Pediatrics 149(1), e2021052151 (2022).
Huhta, J. & Linask, K. K. Environmental origins of congenital heart disease: The heart–placenta connection. Seminars Fetal Neonatal Med. 18, 245–250 (2013).
Gómez-Roig, M. D. et al. Environmental exposure during pregnancy: influence on prenatal development and early life: a comprehensive review. Fetal Diagn. Ther. 48, 245–257 (2021).
Nicoll, R. Environmental contaminants and congenital heart defects: a re-evaluation of the evidence. Int. J. Environ. Res. Publ. Health 15, 2096 (2018).
Li, S. et al. Relationship between maternal heavy metal exposure and congenital heart defects: a systematic review and meta-analysis. Environ. Sci. Pollution Res. 29, 55348–55366 (2022).
Wang, C. et al. Maternal exposure to heavy metals and risk for severe congenital heart defects in offspring. Environ. Res. 212, 113432 (2022).
David, O., Ingrid, M., Kristina, E. & Bertil, F. Traffic pollution at the home address and pregnancy outcomes in Stockholm. Sweden. BMJ Open 5, e007034 (2015).
Hwang, S. E. et al. Association between long-term air pollution exposure and insulin resistance independent of abdominal adiposity in Korean adults. Sci. Rep. 12, 19147 (2022).
Liu, Z. et al. Maternal lead exposure and risk of congenital heart defects occurrence in offspring. Reproduct. Toxicol. 51, 1–6 (2015).
Jin, X. et al. Maternal exposure to arsenic and cadmium and the risk of congenital heart defects in offspring. Reproduct. Toxicol. 59, 109–116 (2016).
Yang, N. et al. Economic growth and pollution emission in China: structural path analysis. Sustainability 10, 2569 (2018).
Querol, X. et al. Source origin of trace elements in PM from regional background, urban and industrial sites of Spain. Atmospheric Environ. 41, 7219–7231 (2007).
Choi, E. et al. Sources of airborne particulate matter-bound metals and spatial-seasonal variability of health risk potentials in four large cities, South Korea. Environ. Sci. Pollut. Res. 29, 28359–28374 (2022).
Rajagopalan, S. & Landrigan, P. J. Pollution and the Heart. New England J. Med. 385, 1881–1892 (2021).
Park, K. H. et al. Particulate matter induces arrhythmia-like cardiotoxicity in zebrafish embryos by altering the expression levels of cardiac development- and ion channel-related genes. Ecotoxicol. Environ. Safety 263, 115201 (2023).
Tchounwou, P. B., Yedjou, C. G., Patlolla, A. K. & Sutton, D. J. Heavy metal toxicity and the environment. Exp. Suppl. 101, 133–164 (2012).
Järup, L. Hazards of heavy metal contamination. British Med. Bull. 68, 167–182 (2003).
Rahman, Z. & Singh, V. P. The relative impact of toxic heavy metals (THMs) (arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: an overview. Environ. Monitor. Assessment 191, 419 (2019).
Liang, Y. et al. Exposure to essential and non-essential trace elements and risks of congenital heart defects: a narrative review. Front. Nutr. 14(10), 1121826 (2023).
Fu, Z. & Xi, S. The effects of heavy metals on human metabolism. Toxicol. Mech. Methods 30, 167–176 (2020).
Paithankar, J. G., Saini, S., Dwivedi, S., Sharma, A. & Chowdhuri, D. K. Heavy metal associated health hazards: an interplay of oxidative stress and signal transduction. Chemosphere 262, 128350 (2021).
Balali-Mood, M., Naseri, K., Tahergorabi, Z., Khazdair, M. R. & Sadeghi, M. Toxic mechanisms of five heavy metals: mercury, lead, chromium, cadmium, and arsenic. Front. Pharmacol. 13(12), 643972 (2021).
Rehman, K., Fatima, F., Waheed, I. & Akash, M. S. H. Prevalence of exposure of heavy metals and their impact on health consequences. J. Cellular Biochem. 119, 157–184 (2018).
Johnson, N. M. et al. Air pollution and children’s health-a review of adverse effects associated with prenatal exposure from fine to ultrafine particulate matter. Environ. Health Prev. Med. 26, 72 (2021).
Nyadanu, S. D. et al. Prenatal exposure to ambient air pollution and adverse birth outcomes: an umbrella review of 36 systematic reviews and meta-analyses. Environ. Pollut. 306, 119465 (2022).
Ghazi, T., Naidoo, P., Naidoo, R. N. & Chuturgoon, A. A. Prenatal air pollution exposure and placental DNA methylation changes: implications on fetal development and future disease susceptibility. Cells 10, 3025 (2021).
Klepac, P., Locatelli, I., Korošec, S., Künzli, N. & Kukec, A. Ambient air pollution and pregnancy outcomes: a comprehensive review and identification of environmental public health challenges. Environ. Res. 167, 144–159 (2018).
Ha, S. et al. Prenatal and early life exposures to ambient air pollution and development. Environ. Res. 174, 170–175 (2019).
Lee, K. S. et al. The effect of maternal exposure to air pollutants and heavy metals during pregnancy on the risk of neurological disorders using the national health insurance claims data of South Korea. Medicina 59, 951 (2023).
Khanam, R. et al. Prenatal environmental metal exposure and preterm birth: a scoping review. Int. J. Environ. Res. Public Health 18, 573 (2021).
Sabra, S., Malmqvist, E., Saborit, A., Gratacós, E. & Gomez Roig, M. D. Heavy metals exposure levels and their correlation with different clinical forms of fetal growth restriction. PloS ONE 12(10), e0185645 (2017).
Michel, S., Atmakuri, A. & von Ehrenstein, O. S. Prenatal exposure to ambient air pollutants and congenital heart defects: an umbrella review. Environ. Int. 178, 108076 (2023).
Gianicolo, E. A. L. et al. Congenital anomalies among live births in a high environmental risk area—A case-control study in Brindisi (southern Italy). Environ. Res. 128, 9–14 (2014).
Wan, X. et al. The association between maternal air pollution exposure and the incidence of congenital heart diseases in children: a systematic review and meta-analysis. Sci. Total Environ. 892, 164431 (2023).
Wold, M. et al. The longitudinal effects of early developmental cadmium exposure on conditioned place preference and cardiovascular physiology in zebrafish. Aquat. Toxicol. 191, 73–84 (2017).
Mitovic, N. et al. Cadmium significantly changes major morphometrical points and cardiovascular functional parameters during early development of zebrafish. Environ. Toxicol. Pharmacol. 87, 103723 (2021).
McCauley, N. et al. In ovo exposure to cadmium causes right ventricle hyperplasia due to cell proliferation of cardiomyocytes. Toxicol. Lett. 366, 1–6 (2022).
Ou, Y. et al. Associations between toxic and essential trace elements in maternal blood and fetal congenital heart defects. Environ. Int. 106, 127–134 (2017).
Jin, X. et al. Maternal exposure to arsenic and cadmium and the risk of congenital heart defects in offspring. Reprod. Toxicol. 59, 109–116 (2016).
Yun, Y., Hou, L. & Sang, N. SO2 inhalation modulates the expression of pro-inflammatory and pro-apoptotic genes in rat heart and lung. J. Hazard. Mater. 185, 482–488 (2011).
Meng, Z. et al. Oxidative damage of sulfur dioxide inhalation on lungs and hearts of mice. Environ. Res. 93, 285–292 (2003).
Vilahur, N., Vahter, M. & Broberg, K. The epigenetic effects of prenatal cadmium exposure. Curr. Environ. Health Rep. 2, 195–203 (2015).
Vecoli, C., Pulignani, S. & Andreassi, M. G. Genetic and epigenetic mechanisms linking air pollution and congenital heart disease. J. Cardiovasc. Dev. Dis. 3(4), 32 (2016).
Gittenberger-de Groot, A. C., Bartelings, M. M., Poelmann, R. E., Haak, M. C. & Jongbloed, M. R. M. Embryology of the heart and its impact on understanding fetal and neonatal heart disease. Seminars Fetal Neonatal Med. 18, 237–244 (2013).
Zaidi, S. & Brueckner, M. Genetics and genomics of congenital heart disease. Circulat. Res. 120, 923–940 (2017).
Williams, K., Carson, J. & Lo, C. Genetics of congenital heart disease. Biomolecules 9, 879 (2019).
Linask, K. K. The heart-placenta axis in the first month of pregnancy: induction and prevention of cardiovascular birth defects. J. Pregnancy 2013, 320413 (2013).
Chou, H. H. et al. Association of maternal chronic disease with risk of congenital heart disease in offspring. Cmaj 188, E438-e446 (2016).
Liu, S. et al. Association between maternal chronic conditions and congenital heart defects: a population-based cohort study. Circulation 128, 583–589 (2013).
Yim, S. H. L., Gu, Y., Shapiro, M. A. & Stephens, B. Air quality and acid deposition impacts of local emissions and transboundary air pollution in Japan and South Korea. Atmos. Chem. Phys. 19, 13309–13323 (2019).
Dolk, H., Loane, M. & Garne, E. The prevalence of congenital anomalies in Europe. Adv Exp Med Biol 686, 349–364 (2010).
Son, S. L. et al. Stillbirth and fetal anomalies: secondary analysis of a case-control study. Bjog 128, 252–258 (2021).
Sperandei, S. Understanding logistic regression analysis. Biochem. Med. (Zagreb) 24, 12–18 (2014).
Knol, M. J., Le Cessie, S., Algra, A., Vandenbroucke, J. P. & Groenwold, R. H. Overestimation of risk ratios by odds ratios in trials and cohort studies: alternatives to logistic regression. Cmaj 184, 895–899 (2012).
Funding
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI21C0262).
Author information
Authors and Affiliations
Contributions
Study conception and design were performed by S.J., S.Z.Y. and Y.J.C. Data collection and analysis were performed by S.J., G.K., S.U.C. and Y.J.C. The first draft of the manuscript was written by S.J. and S.Z.Y. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jin, S., Yoon, S.Z., Choi, Y.J. et al. Prenatal exposure to air pollutants and the risk of congenital heart disease: a Korean national health insurance database-based study. Sci Rep 14, 16940 (2024). https://doi.org/10.1038/s41598-024-63150-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-63150-4
- Springer Nature Limited