Abstract
Neoadjuvant chemotherapy (NACT) is the standard treatment for locally advanced, high-risk breast cancer. Pathological complete response (pCR) improves survival. Peripheral blood-derived indices reflecting systemic inflammation and nutritional status have long been used as predictive and prognostic markers in solid malignancies. This retrospective study investigates whether eight commonly used indices in patients receiving NACT affect pCR and survival. This study includes 624 locally advanced breast cancer patients who received NACT. The biomarker indices were calculated from peripheral blood samples taken two weeks before starting chemotherapy. The indices’ optimal cut-off values were determined using ROC Curve analysis. During a median follow-up period of 42 months, recurrence was detected in 146 patients, and 75 patients died. pCR was observed in 166 patients (26.6%). In univariate analysis, NLR, PLR, SII, PNI, HALP, and HRR were statistically significantly associated (p = 0.00; p = 0.03; p = 0.03; p = 0.02; p = 0.00; p = 0.02 respectively), but in multivariate analysis, only NLR was significantly predictive for pCR(p = 0.04). In multivariate analysis, the HGB/RDW score significantly predicted DFS(p = 0.04). The PNI score was identified as a marker predicting survival for both OS and PFS (p = 0.01, p = 0.01, respectively). In conclusion, peripheral blood-derived indices have prognostic and predictive values on pCR and survival. However, further studies are needed to validate our findings.
Similar content being viewed by others
Introduction
Breast cancer (BC) is the leading cause of cancer incidence and mortality among women1. With the expansion of screening programs and the increase in average life expectancy, more patients are diagnosed at early stages2. Despite promising clinical outcomes achieved through early diagnosis, advancements in surgical techniques, and multimodal treatments, breast cancer continues to be a leading cause of cancer-related deaths in women3. However, recurrence is still observed in many patients despite surgical resection, neoadjuvant, and adjuvant treatments4.
Neoadjuvant chemotherapy (NACT) is a significant treatment option for locally advanced breast cancer5. Pathological complete response (pCR) is associated with lower recurrence rates and indicates more favorable survival outcomes6.
The relationship between chronic inflammation and cancer is a popular topic in oncology. The value of inflammatory markers is being investigated both diagnostically and prognostically. Studies have shown inflammation contributes to tumor formation and progression7,8,9. Neutrophils, monocyte-derived macrophages, and platelets negatively impact the tumor microenvironment by promoting tumoral angiogenesis and tumor growth, thus having poor prognostic significance, whereas tumor-infiltrating lymphocytes indicate positive outcomes10,11,12,13.
Cancer-related anemia (CRA) can occur at any stage of cancer, from early to terminal, though it typically appears in advanced stages. This condition can arise secondary to chronic inflammation related to cancer, independent of antineoplastic therapy14,15. CRA has been shown to have a negative prognostic impact on disease-free survival (DFS) and overall survival (OS) in various types of cancer16,17,18. Red cell distribution width (RDW), reflecting changes in erythrocyte volume, has begun to be used as a prognostic marker in many malignancies19,20. Patients’ albumin levels, influenced by nutritional and inflammatory status, are adverse prognostic factors in various types of cancer24,25. Different laboratory indices derived from peripheral blood cells can provide information about the status of the intratumoral immune system26,27,28,29,30,31,32,33,34.
Our study aims to determine the predictive and prognostic values of commonly used indices when evaluated independently of their classical prognostic and predictive values and to assess whether they have any superiority. There is a lack of consistency in the results obtained from the commonly used indices across different studies. For this purpose, we selected and included eight widely used indices in the study, and we aimed to evaluate the predictive and prognostic value in complete response and survival in patients receiving neoadjuvant chemotherapy independently of classical prognostic factors.
Materials and methods
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Ethics/Institutional Review Board approval of research with the number 2023/514/246/27, dated 29.03.2023.
Our study retrospectively reviewed the oncological records of 692 patients diagnosed with breast cancer between January 2010 and November 2022, who underwent pathological evaluation after NACT and had complete follow-up files in our clinic. Exclusions are shown in Fig. 1.
Patients’ medical records were reviewed for demographic characteristics (Table 1), histological features (Table 2), pathological data (Table 3), and laboratory results. Immunohistochemical staining from diagnostic biopsies was used to classify patients with > 1% expression levels of ER or PR as hormone positive. HER-2 status was determined as positive in patients with a score of 3 or a suspiciously positive score of 2 with positive FISH results. Patients with < 1% ER and PR expression levels and concurrently negative for HER-2 were classified as triple-negative. The response to neoadjuvant chemotherapy was assessed through pathology reports of surgery. Staging was based on the American Joint Committee on Cancer (AJCC) Staging Manual, eighth edition. Complete blood counts and biochemical values were reviewed before starting NACT. NLR, MLR, HRR, PLR, SII, HALP, PNI, and PIV were calculated using absolute neutrophil, lymphocyte, monocyte, platelet counts, hemoglobin, albumin, and red cell distribution width values. These calculations were made as follows:
-
NLR = neutrophil count (103/mm3)/lymphocyte count (103/mm3)
-
MLR = monocyte count (103/mm3)/lymphocyte count (103/mm3)
-
PLR = platelet count (103/uL)/lymphocyte count (103/mm3)
-
SII = platelet count (103/uL) × neutrophil count (103/mm3)/lymphocyte count (103/mm3)
-
PNI = serum albumin (g/L) + 5 × lymphocyte count (103/mm3)
-
HALP = hemoglobin (g/dL) × albumin (g/L) × lymphocyte count (103/mm3)/platelets (103/uL)
-
PIV = neutrophil count (103/mm3) × platelet count (103/uL) × monocyte count (103/mm3)/lymphocyte count (103/mm3)
-
HRR = hemoglobin (g/dL)/RDW %
pCR was defined as the absence of tumor cells in pathology samples from mastectomy or excised tumors and lymph nodes following neoadjuvant chemotherapy. DFS was calculated as the time (in months) from curative surgery to recurrence and/or death (whichever occurred first). OS was calculated as the time (in months) from diagnosis to death or the last check-up time for patients who did not die.
Statistical analyses were performed using IBM SPSS Statistics for Windows, Version 25.0 (Statistical Package for the Social Sciences, IBM Corp., Armonk, NY, USA). Descriptive statistics were presented as n and % for categorical variables and mean ± SD or median (min–max) for continuous variables. ROC Curve analysis results were provided for the investigated NLR, MLR, HRR, PLR, SII, HALP, PNI, and PIV indices’ ability to predict mortality. Kaplan Meier method was used to compare OS and DFS durations among various clinical parameter groups. Univariate and Multivariate Logistic Regression results were provided for the risk of pCR on various clinical factors. Finally, Multivariate Cox regression results were provided for the impact of different clinical variables on OS and DFS. A p-value of < 0.05 was considered statistically significant.
Consent to participate
Patient data were obtained retrospectively from patient records after obtaining written informed consent from the patients or their relatives.
Results
Of the 692 patients screened, 624 met the inclusion and exclusion criteria and were included in the study. The average age of the patients was 50 years (range 22–82 years), with three (0.5%) being male. There were 330 premenopausal patients (52.9%). The general characteristics of the patients and tumors are presented in Table 1.
pCR was observed in 166 patients (26.6%) following NACT. Among the 293 hormone-positive patients, complete response was seen in 22(7.5%), partial response in 191(65.1%), and no response to NACT in 80(27.3%) patients. The highest pCR rate was observed in HER2-positive disease (47.9%), with complete response in 117 (47.9%) of 244 HER-2-positive patients. Among 87 triple-negative patients, a complete response was seen in 27 (31.0%). Laboratory values were calculated according to NLR, MLR, PLR, SII, PIV, PNI, HALP, and HRR indices, and cut-off values for pCR were determined using ROC Curve analysis (Table 2). These values were examined along with their sensitivity and specificity. While Univariate analysis found NLR, PLR, SII, PNI, HALP, and HRR (p = 0.00; p = 0.03; p = 0.03; p = 0.02; p = 0.00; p = 0.02, respectively) to be statistically significantly associated, only the NLR index was found to be statistically significant in predicting pCR in Multivariate analysis (p = 0.04).
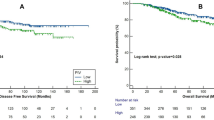
The median follow-up period was 42 months, during which 75 (12%) patients died, and recurrence was observed in 146 (23.3%) patients. Laboratory values were calculated according to NLR, MLR, PLR, SII, PIV, PNI, HALP, and HRR indices with determined cut-off values for DFS and OS (Table 3). While Univariate analysis found significance in all indices, Multivariate analysis observed that the HGB/RDW score statistically significantly predicted DFS (p = 0.04). It was found that the NLR (neutrophil-to-lymphocyte ratio) was not a good predictor for DFS (disease-free survival) and OS (overall survival) in hormone-positive and triple-negative subtypes of breast cancer (p = 0.250, p = 0.087, p = 0.698, and p = 0.389 respectively). However, in the HER2-positive subtype, it was not predictive for DFS (p = 0.073) but found to be significantly prognostic for OS (p = 0.032). In the entire patient group, high NLR was observed to be a poor prognostic factor for DFS and OS in univariate analysis (p = 0.004 and p = 0.027, respectively). However, in multivariate analysis, this significance was not observed (p = 0.953 and p = 0.440, respectively). The PNI score was identified as a marker predicting survival for both OS and PFS (p = 0.01, p = 0.01, respectively) (Fig. 2a and b).
Discussion
In our study, we explored the relationship between laboratory indices and survival outcomes, DFS, and pCR in breast cancer patients undergoing NACT. Our approach was unique because we simultaneously analyzed multiple indices in a selected patient group to establish the relationship between survival and pCR. We found that the NLR index predicted pCR. While HRR PNI was predictive for DFS, only PNI significantly correlated with overall survival (OS). Our study stands out in the literature for investigating the relationship of 8 commonly used and long-established indices with pCR and DFS/OS in the same patient cohort.
Clinical studies have been conducted to analyze the indices derived from laboratory values. However, these studies have reported conflicting findings with respect to different indices and even for the same index across various studies. The superiority of different indices also remains debatable, with contradictory results reported in various studies35,36,37,38,39. For example, one study reported NLR to be predictive of neoadjuvant response and an independent prognostic marker for survival39. On the other hand, another study demonstrated that NLR was not predictive of neoadjuvant response and was not an independent prognostic marker for survival36. These inconsistencies suggest that laboratory-derived indices may not be reliable prognostic and predictive biomarkers in breast cancer, particularly when evaluated independently of established prognostic and predictive markers like lymph node status and hormone status.
Inflammation plays a crucial role in all stages of carcinogenesis and progression, as well as in the ineffectiveness of anti-cancer treatments40,41,42. Numerous studies have shown that systemic inflammation in cancer patients is associated with poorer survival, thereby motivating the clinical use of inflammatory markers as prognostic indicators43,44.
As the first line of defense, the acute inflammatory response represents innate and adaptive immune responses against any damage or foreign entities perceived by the body, such as infections. Most infiltrating inflammatory cells in acute inflammation are neutrophils45,46. While acute inflammation contributes to cancer cell death by inducing an anti-tumor immune response, chronic inflammation caused by treatment promotes therapeutic resistance and cancer progression47.
A meta-analysis by Zhou et al., encompassing 17 clinical studies and 5504 patients, demonstrated that a lower NLR value significantly predicted both pCR and improved DFS and OS43. According to a meta-analysis by Cullinane et al., which involved eight clinical studies and 1586 patients, a low NLR value was found to be consistent with better PCR. However, the results did not reach statistical significance in terms of improved 5-year DFS, despite the fact that patients with lower NLR levels showed some improvement48. In another meta-analysis by Xue et al., a higher NLR value was associated with worse pathological complete response, but the prognostic value of NLR for DFS and OS could not be demonstrated49. On the other hand, a study by Feeney G. found that low preoperative NLR was predictive of complete response50. However, some studies have proven the opposite. For example, in two different studies, no significant predictive relationship was observed between NLR level and complete response. Additionally, both studies observed that the NLR value was not an independent prognostic index for DFS36,37. In our study, an NLR value below 2.25 was significantly associated with good pCR in univariate and multivariate analyses. But it was not predictive of PFS and OS. There are conflicting findings in studies regarding the NLR index. While some studies suggest that it is useful for predicting complete response, disease-free survival (DFS), and overall survival (OS), it is only available in studies that show its predictive ability for complete response or survival. However, other studies are showing that it is not predictive of complete response, DFS and OS. The reliability of these indices obtained from peripheral blood is questionable. The use of these indices should be considered independently of clinicopathological features. If these indices are to be used, the patient’s inflammation and nutritional status should be considered. Given that NLR primarily reflects acute inflammation, it is associated with pCR, an early-stage malignancy condition, but not with long-term indicators such as DFS and OS.
Chronic inflammation is implicated in every step of tumor formation, including malignant cellular transformation, proliferation, invasion, angiogenesis, and metastasis51. It plays a central role in the emergence and progression of tumors and contributes to resistance to chemotherapy and radiotherapy40,41. Chronic inflammation is characterized by simultaneous tissue destruction and healing, with macrophages and lymphocytes as the primary immune cells infiltrating chronic inflammation areas, also playing a role in forming immunological memory45.
Malnutrition can affect the progression and survival of cancer patients. Several studies have linked malnutrition in cancer to poor prognosis, reduced quality of life (QoL), and lower activity levels, as well as increased adverse symptoms related to treatment and reduced tumor response to therapy52,53. Serum albumin levels are closely associated with malnutrition and are independent predictors of inflammation and mortality21.
In a study by Mantzorou et al., which included breast cancer patients, PNI was found to be predictive for disease progression and prognosis52. Similarly, a study by Chen et al. observed that a high PNI value was an independent significant prognostic index for better DFS and OS in breast cancer patients receiving NACT25. Another study involving over 1100 patients found that pre-treatment PNI was a reliable predictor of pCR in breast cancer patients54. However, in the study by Yang et al., it was observed that pCR was not a predictive marker in breast cancer patients receiving PNI and NACT55. Lastly, a study by Oba T. divided patients receiving NACT into two groups based on their PNI before treatment. There was no difference in DFS between the high PNI and low PNI groups56. In our study, however, high PNI values did not correlate with pCR but showed a close correlation with better DFS and OS. The limitations present in NLR are also present in PNI, and as such, peripheral indices should be considered in conjunction with clinicopathological characteristics. As mentioned earlier, lymphocytes are more prominent in chronic inflammation, and albumin levels are also at the forefront in malnutrition and chronic inflammation. Therefore, the effects of PNI, calculated using albumin and lymphocyte counts, emerge in the longer term rather than in determining the immediate pCR after NACT.
Although studies support the predictive value of indices calculated from peripheral blood values, it is unclear which index possesses the most accurate predictive power. The literature presents conflicting publications on this matter8,29,43.
Our study’s limitations include its retrospective nature and being a single-center experience. However, its strengths lie in having a large patient cohort and investigating eight commonly used indices for pCR and DFS/OS relationships in the same group.
Conclusions
There are conflicting results regarding the indices commonly used today. When using these indices, it is important to consider the clinicopathologic and demographic aspects of the patient, especially those with breast cancer. These indices, which are readily available, cost-effective, and acceptable, might assist oncologists in choosing relevant therapies for patients, it is crucial to persist in their development. Nevertheless, it is important to consider that the indices obtained from peripheral blood are significantly affected by both the patient’s inflammatory and nutritional condition. Our study identified a statistically significant relationship between pre-treatment lower NLR and pCR, which is closely associated with acute inflammation. We also demonstrated a statistically significant relation between high PNI, DFS, and OS, reflecting its close association with nutritional status and chronic inflammation. However, prospective randomized studies with larger patient populations across multiple centers are necessary to generalize these results.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71(3), 209–249 (2021).
Wilkinson, L. & Gathani, T. Understanding breast cancer as a global health concern. Br. J. Radiol. 95(1130), 20211033 (2022).
Waks, A. G. & Winer, E. P. Breast cancer treatment: A review. JAMA 321(3), 288–300. https://doi.org/10.1001/jama.2018.19323 (2019).
Tabor, S., Szostakowska-Rodzos, M., Fabisiewicz, A. & Grzybowska, E. A. How to predict metastasis in luminal breast cancer? Current solutions and future prospects. Int. J. Mol. Sci. 21(21), 8415 (2020).
Kerr, A. J. et al. Adjuvant and neoadjuvant breast cancer treatments: A systematic review of their effects on mortality. Cancer Treat Rev. 105, 102375 (2022).
Spring, L. M. et al. Pathologic complete response after neoadjuvant chemotherapy and impact on breast cancer recurrence and survival: A comprehensive meta-analysis. Clin. Cancer Res. 26(12), 2838–2848 (2020).
Dupré, A. & Malik, H. Z. Inflammation and cancer: What a surgical oncologist should know. Eur. J. Surg. Oncol. 44(5), 566–570. https://doi.org/10.1016/j.ejso.2018.02.209 (2018).
Şahin, A. B. et al. Low pan-immune-inflammation-value predicts better chemotherapy response and survival in breast cancer patients treated with neoadjuvant chemotherapy. Sci. Rep. 11(1), 14662 (2021).
Hanahan, D. Hallmarks of cancer: New dimensions. Cancer Discov. 12(1), 31–46. https://doi.org/10.1158/2159-8290.CD-21-1059 (2022).
Ugel, S., Canè, S., De Sanctis, F. & Bronte, V. Monocytes in the tumor microenvironment. Ann. Rev. Pathol. 24(16), 93–122. https://doi.org/10.1146/annurev-pathmechdis-012418-013058 (2021).
Kim, J. & Bae, J. S. Tumor-associated macrophages and neutrophils in tumor microenvironment. Med. Inflamm. 2016, 6058147 (2016).
Huong, P. T., Nguyen, L. T., Nguyen, X. B., Lee, S. K. & Bach, D. H. The role of platelets in the tumor-microenvironment and the drug resistance of cancer cells. Cancers (Basel) 11(2), 240 (2019).
Azab, B. et al. Pretreatment neutrophil/lymphocyte ratio is superior to platelet/lymphocyte ratio as a predictor of long-term mortality in breast cancer patients. Med. Oncol. 30(1), 432 (2013).
Madeddu, C. et al. Pathogenesis and treatment options of cancer related anemia: Perspective for a targeted mechanism-based approach. Front. Physiol. 20(9), 1294 (2018).
Rodgers, G. M. 3rd. et al. Cancer- and chemotherapy-induced anemia. J. Natl. Compr. Cancer Netw. 10(5), 628–653 (2012).
Obermair, A. et al. The relationship of pretreatment serum hemoglobin level to the survival of epithelial ovarian carcinoma patients: A prospective review. Cancer 83(4), 726–731 (1998).
Zhang, Y. et al. Impact of preoperative anemia on relapse and survival in breast cancer patients. BMC Cancer 18(14), 844 (2014).
An, M. S. et al. T4 stage and preoperative anemia as prognostic factors for the patients with colon cancer treated with adjuvant FOLFOX chemotherapy. World J. Surg. Oncol. 13, 64 (2015).
Seretis, C., Seretis, F., Lagoudianakis, E., Gemenetzis, G. & Salemis, N. S. Is red cell distribution width a novel biomarker of breast cancer activity? Data from a pilot study. J. Clin. Med. Res. 5(2), 121–126 (2013).
Ichinose, J. et al. Prognostic significance of red cell distribution width in elderly patients undergoing resection for non-small cell lung cancer. J. Thorac. Dis. 8(12), 3658–3666 (2016).
Eckart, A. et al. Relationship of nutritional status, inflammation, and serum albumin levels during acute illness: A prospective study. Am. J. Med. 133(6), 713-722.e7 (2020).
Espinosa, E. et al. Serum albumin and other prognostic factors related to response and survival in patients with advanced non-small cell lung cancer. Lung Cancer 12(1–2), 67–76 (1995).
Ma, J. Y., Liu, G., Pan, L. Z., Hu, M. & Zhu, Z. Z. Clinical impact of pretreatment albumin-globulin ratio in patients with colorectal cancer: A meta-analysis. Medicine (Baltimore) 101(20), e29190 (2022).
Oñate-Ocaña, L. F. et al. Serum albumin as a significant prognostic factor for patients with gastric carcinoma. Ann. Surg. Oncol. 14(2), 381–389. https://doi.org/10.1245/s10434-006-9093-x (2007).
Chen, L. et al. Prognostic nutritional index (PNI) in patients with breast cancer treated with neoadjuvant chemotherapy as a useful prognostic indicator. Front. Cell Dev. Biol. 30(9), 656741 (2021).
Farag, C. M., Antar, R., Akosman, S., Ng, M. & Whalen, M. J. Addendum: What is hemoglobin, albumin, lymphocyte, platelet (HALP) score? A comprehensive literature review of HALP’s prognostic ability in different cancer types. Oncotarget 7(14), 748 (2023).
Yuce, E. et al. The effect of the change in hemoglobin-albumin-lymphocyte-platelet scores occurring with neoadjuvant chemotherapy on clinical and pathological responses in breast cancer. Bratisl. Lek. Listy. 124(1), 59–63 (2023).
Lou, C., Jin, F., Zhao, Q. & Qi, H. Correlation of serum NLR, PLR and HALP with efficacy of neoadjuvant chemotherapy and prognosis of triple-negative breast cancer. Am. J. Transl. Res. 14(5), 3240–3246 (2022).
Truffi, M. et al. Prognostic potential of immune inflammatory biomarkers in breast cancer patients treated with neoadjuvant chemotherapy. Cancers (Basel) 14(21), 5287 (2022).
Gasparri, M. L. et al. Low neutrophil-to-lymphocyte ratio and pan-immune-inflammation-value predict nodal pathologic complete response in 1274 breast cancer patients treated with neoadjuvant chemotherapy: A multicenter analysis. Ther. Adv. Med. Oncol. 15(15), 17588359231193732 (2023).
Büyükşimşek, M., Oğul, A., Mirili, C. & Paydaş, S. Inflammatory markers predicting pathological complete response in cases with breast cancer treated by neoadjuvant chemotherapy. Eur. J. Breast Health 16(4), 229–234 (2020).
Gu, Q., Zhao, J., Liu, Y., Chen, H. & Wang, L. Association between the systemic immune-inflammation index and the efficacy of neoadjuvant chemotherapy, prognosis in HER2 positive breast cancer-a retrospective cohort study. Gland Surg. 12(5), 609–618 (2023).
Yılmaz, A., Mirili, C., Tekin, S. B. & Bilici, M. The ratio of hemoglobin to red cell distribution width predicts survival in patients with gastric cancer treated by neoadjuvant FLOT: A retrospective study. Ir. J. Med. Sci. 189(1), 91–102 (2020).
Bozkaya, Y., Dilber, M., Bilgili, A. M. & Aktaş, C. A New prognostic parameter associated with recurrence in patients with nasopharyngeal cancer treated with chemoradiotherapy: The ratio of the hemoglobin-to-red cell distribution width. Cureus 15(6), e39907 (2023).
Eren, T. et al. Correlation between peripheral blood inflammatory indicators and pathologic complete response to neoadjuvant chemotherapy in locally advanced breast cancer patients. Medicine (Baltimore) 99(22), e20346 (2020).
Hu, Y. et al. Platelet/lymphocyte ratio is superior to neutrophil/lymphocyte ratio as a predictor of chemotherapy response and disease-free survival in luminal B-like (HER2-) breast cancer. Clin. Breast Cancer 20(4), e403–e409 (2020).
Ma, Y., Zhang, J. & Chen, X. Lymphocyte-to-monocyte ratio is associated with the poor prognosis of breast cancer patients receiving neoadjuvant chemotherapy. Cancer Manag. Res. 16(13), 1571–1580 (2021).
Zhou, Y. et al. Predictive significance of systemic immune-inflammation index in patients with breast cancer: A retrospective cohort study. Onco Targets Ther. 16(16), 939–960 (2023).
Wang, C. et al. Two hematological markers predicting the efficacy and prognosis of neoadjuvant chemotherapy using lobaplatin against triple-negative breast cancer. Oncologist 29(5), e635–e642 (2024).
Grivennikov, S. I., Greten, F. R. & Karin, M. Immunity, inflammation, and cancer. Cell. 140(6), 883–899. https://doi.org/10.1016/j.cell.2010.01.025 (2010).
Coussens, L. M. & Werb, Z. Inflammation and cancer. Nature 420(6917), 860–867. https://doi.org/10.1038/nature01322 (2002).
Crusz, S. M. & Balkwill, F. R. Inflammation and cancer: Advances and new agents. Nat. Rev. Clin. Oncol. 12(10), 584–596 (2015).
Zhou, Q. et al. Role of neutrophil-to-lymphocyte ratio as a prognostic biomarker in patients with breast cancer receiving neoadjuvant chemotherapy: A meta-analysis. BMJ Open. 11(9), e047957 (2021).
Ishizuka, M., Nagata, H., Takagi, K., Iwasaki, Y. & Kubota, K. Combination of platelet count and neutrophil to lymphocyte ratio is a useful predictor of postoperative survival in patients with colorectal cancer. Br. J. Cancer. 109(2), 401–407 (2013).
Serhan, C. N. The resolution of inflammation: The devil in the flask and in the details. FASEB J. 25(5), 1441–1448 (2011).
Gajewski, T. F., Schreiber, H. & Fu, Y. X. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 14(10), 1014–1022 (2013).
Guthrie, G. J. et al. The systemic inflammation-based neutrophil-lymphocyte ratio: Experience in patients with cancer. Crit. Rev. Oncol. Hematol. 88(1), 218–230 (2013).
Cullinane, C. et al. Can the neutrophil to lymphocyte ratio predict complete pathologic response to neoadjuvant breast cancer treatment? A systematic review and meta-analysis. Clin. Breast Cancer 20(6), e675–e681 (2020).
Xue, L. B. et al. Prognostic role of high neutrophil-to-lymphocyte ratio in breast cancer patients receiving neoadjuvant chemotherapy: Meta-analysis. Medicine (Baltimore). 98(1), e13842 (2019).
Feeney, G. et al. Association of clinical biomarkers and response to neoadjuvant therapy in breast cancer. Ir. J. Med. Sci. 193(2), 605–613 (2024).
Mantovani, A. Cancer: Inflammation by remote control. Nature 435(7043), 752–753 (2005).
Mantzorou, M., Koutelidakis, A., Theocharis, S. & Giaginis, C. Clinical value of nutritional status in cancer: What is its impact and how it affects disease progression and prognosis?. Nutr. Cancer 69(8), 1151–1176 (2017).
Muscaritoli, M. et al. ESPEN practical guideline: Clinical nutrition in cancer. Clin. Nutr. 40(5), 2898–2913 (2021).
Qu, F. et al. Construction and validation of a prognostic nutritional index-based nomogram for predicting pathological complete response in breast cancer: A two-center study of 1,170 patients. Front. Immunol. 11(14), 1335546 (2024).
Yang, G., Liu, P., Zheng, L. & Zeng, J. Novel peripheral blood parameters as predictors of neoadjuvant chemotherapy response in breast cancer. Front. Surg. 4(9), 1004687 (2022).
Oba, T. et al. Neoadjuvant chemotherapy-induced decrease of prognostic nutrition index predicts poor prognosis in patients with breast cancer. BMC Cancer 20(1), 160 (2020).
Author information
Authors and Affiliations
Contributions
S.Y.: Conceptualization, Methodology, Investigation, Project administration, Data curation and management, Writing- original draft. A.D.: Data curation, Investigation, Writing- Review & Editing. G.A.: Data curation, Investigation, Writing- Review & Editing. Z.YY.: Data curation, Investigation, Writing- Review & Editing. H.B.: Software, Formal analysis, Writing- Review & Editing. O.K.: Data curation, Investigation, Writing- Review & Editing. S.T.: Data curation, Investigation, Writing- Review & Editing. S.O.: Data curation, Investigation, Writing- Review & Editing. U.O.: Data curation, Investigation, Writing- Review & Editing. H.S.Y.: Data curation, Investigation, Writing- Review & Editing. O.A.: Writing- original draft, Software, Formal analysis. S.CK.: Investigation, Writing- Review & Editing. D.I.: Supervision, Methodology, Writing- Review & Editing. H.S.: Data curation, Investigation, Writing- Review & Editing. T.B.: Data curation, Investigation, Writing- Review & Editing. ON.S.: Supervision, Project administration, Writing- Review & Editing. H.O.: Conceptualization, Supervision, Writing- Review & Editing. ME.Y.: Conceptualization, Supervision, Writing- Review & Editing. N.T.: Supervision, Project administration, Writing- Review & Editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yildirim, S., Dogan, A., Akdag, G. et al. The role of laboratory indices on treatment response and survival in breast cancer receiving neoadjuvant chemotherapy. Sci Rep 14, 12123 (2024). https://doi.org/10.1038/s41598-024-63096-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-63096-7
- Springer Nature Limited