Abstract
Posttraumatic stress disorder (PTSD) can develop after trauma exposure. Some studies report that women develop PTSD at twice the rate of men, despite greater trauma exposure in men. Lipids and their metabolites (lipidome) regulate a myriad of key biological processes and pathways such as membrane integrity, oxidative stress, and neuroinflammation in the brain by maintaining neuronal connectivity and homeostasis. In this study, we analyzed the lipidome of 40 adults with PTSD and 40 trauma-exposed non-PTSD individuals (n = 20/sex/condition; 19–39 years old). Plasma samples were analyzed for lipidomics using Quadrupole Time-of-Flight (QToF) mass spectrometry. Additionally, ~ 90 measures were collected, on sleep, and mental and physical health indices. Poorer sleep quality was associated with greater PTSD severity in both sexes. The lipidomics analysis identified a total of 348 quantifiable known lipid metabolites and 1951 lipid metabolites that are yet unknown; known metabolites were part of 13 lipid subclasses. After adjusting for BMI and sleep quality, in women with PTSD, only one lipid subclass, phosphatidylethanolamine (PE) was altered, whereas, in men with PTSD, 9 out of 13 subclasses were altered compared to non-PTSD women and men, respectively. Severe PTSD was associated with 22% and 5% of altered lipid metabolites in men and women, respectively. Of the changed metabolites, only 0.5% measures (2 PEs and cholesterol) were common between women and men with PTSD. Several sphingomyelins, PEs, ceramides, and triglycerides were increased in men with severe PTSD. The correlations between triglycerides and ceramide metabolites with cholesterol metabolites and systolic blood pressure were dependent upon sex and PTSD status. Alterations in triglycerides and ceramides are linked with cardiac health and metabolic function in humans. Thus, disturbed sleep and higher body mass may have contributed to changes in the lipidome found in PTSD.
Similar content being viewed by others
Introduction
Posttraumatic stress disorder (PTSD) is a psychiatric condition that may develop after exposure to an actual or threatened death, serious injury, or sexual violence. PTSD may be characterized by: (i) reexperiencing (e.g., intrusive thoughts, nightmares, flashbacks); (ii) avoidance; (iii) negative changes in cognition and mood (hopelessness, lack of emotions), and (iv) hyperarousal (trouble sleeping, risky or destructive behavior, angry outbursts) (DSM-5)1. Disturbed sleep is one of the most common complaints among individuals with PTSD2. Lower slow wave sleep duration and delta-band spectral power are more pronounced in men than women and correlate with PTSD status3. In contrast, greater rapid eye movement sleep is found in women with PTSD compared to healthy controls, a difference not seen in men3. PTSD is also known to affect physical health and has been associated with greater inflammation, metabolic syndrome, gastrointestinal illness, and even early mortality4,5,6,7,8,9,10,11,12,13. It is possible that disturbed sleep may play a role in these health impacts since sleep duration correlates with metabolic risk in PTSD14.
Epidemiological evidence suggests that women develop PTSD at twice the rate of men, despite greater trauma exposure in men15,16. Women are also at increased risk for stress-related physical comorbidities, including inflammatory, metabolic, and GI disorders15,17,18,19. Although some have suggested that greater exposure to interpersonal violence may contribute to higher rates of PTSD in women; evidence also implicates sex differences in the molecular mechanisms involved in stress regulation and disease processes. Trauma exposure can have significant effects on molecular, biochemical, and cellular systems that are associated with a complex array of PTSD symptoms and physiological comorbidities20,21. Lipids are emerging as an important contributor of health of the brain but the relationship between PTSD severity and lipid metabolites is unknown. While prior studies have found alterations in neuroendocrine, immune, and aging processes in PTSD4,5,6,7,8,9,10,11,12,13, our understanding of the role of metabolite disturbances in PTSD is limited. The structure and function of the complete set of lipids in each cell or organism is referred to as the “Lipidome”. Several classes of lipids that include fatty acids, diacylglycerols, triglycerides, phospholipids, sphingomyelin, ceramides, and acylcarnitine comprise the lipidome (Fig. 1a)22. Most of the classes of lipids are derived from fatty acids with Acyl-CoA as a key intermediary (Fig. 1).
Lipid biosynthesis pathways and lipid metabolites. (a) Schematic of pathways involved in the synthesis of various lipid subclasses from Acetyl-Co-A. Glucose is converted to Acetyl-Co-A via glycolysis in the mitochondrial membrane (tricarboxylic acid cycle: TCA). Introduction of a double bond in the Δ9 position of the acyl chain and subsequent elongation of the carbon chain leads to the generation of mono and poly unsaturated fatty acids (PUFA), respectively. Essential omega FA cannot be synthesized by human cells and must be obtained from the diet. Sphingolipids contain polar heads derived from serine, phosphocholine, or phosphoethanolamine. Cholesterol is also synthesized from Acetyl-Co-A and is the structural backbone and starting material for all steroid hormone biosynthesis. (b) Biosynthesis pathway of 13 major lipid subclasses identified using MToF mass spectrometry. COX1/2 prostaglandin-endoperoxide synthase, CDP-DAG cytidine diphosphate-diacylglycerol, CER ceramide, DAG/DG diacylglycerol, FA fatty acid, LPA lysophosphatidic acid, LPC lysophosphatidylcholine, LPE lysophosphatidylethanolamine; LPS lysophosphatidylserine, PA phosphatidic acid, PC phosphatidylcholine, PE phosphatidylethanolamine, PG phosphatidylglycerol, PGE2 prostaglandin E2, PGH2 prostaglandin H2, PI phosphatidylinositol, PIPx phosphatidylinositol phosphate, PS phosphatidylserine, S1P sphingosine-1-phosphate, SP sphingosine, TAG/TG triacylglyceride. Adapted from Baenke et al.22.
Technological developments such as mass spectrometry allow for the examination of levels of multiple analytes simultaneously and discovery of novel pathways and hence offers a great advantage to detect patterns of alterations in a complex disorder such as PTSD. The use of non-targeted metabolomics to better understand the pathophysiology of PTSD has great potential since it considers all known, and even unknown, molecules and pathways all at once. Metabolomics is a global approach to understanding regulation of metabolic pathways and networks of physiologically relevant interactions. The metabolome is regulated by gene-environment interactions and reflects the intermediary state between genotype and phenotype. Gene mutations, single nucleotide polymorphisms, and mutations in proteins are associated with PTSD, but none of these alone explains the complex manifestation of PTSD and comorbid health conditions. A multi-omics approach has been used to identify potential biomarkers that range from DNA methylation, proteins, miRNA, lipids, and other metabolites in warzone male veterans with PTSD23. Metabolomic profiling has also led to the identification of key differences in glycolysis and fatty acid pathways that were associated with mitochondrial dysfunction in men with PTSD24. Characterization of metabolomic pathways can help elucidate the discovery of yet unknown biological mechanisms of disease.
Several studies have examined the lipidome of PTSD. One study examined peripheral blood serum samples of 20 PTSD civilian patients and 18 healthy non-trauma controls and found alterations in lipid-derived metabolites were altered in PTSD25. Unfortunately, sex differences were not examined in that study. PTSD was associated with alterations in lactate and pyruvate, pathways related to glycolysis, decreases in unsaturated fatty acids involved in inflammatory processes, and metabolism, possibly pointing to mitochondrial alterations in male combat trauma-exposed veterans from the Iraq and Afghanistan conflicts (n = 52 PTSD; n = 51 controls). Two glycerophospholipids, phosphatidylethanolamine (18:1/0:0) and phosphatidylcholine (18:1/0:0) that may relate to inflammation, mitochondrial dysfunction, membrane breakdown, oxidative stress, and neurotoxicity were found in a study of male Croatian war veterans (n = 50 with PTSD, 50 healthy controls; and a validation group of n = 52 with PTSD, n = 52 healthy controls)26. Alterations in compounds related to bile acid metabolism, fatty acid metabolism and pregnenolone steroids, which are involved in innate immunity, inflammatory process and neuronal excitability were associated with PTSD in male World Trade Center responders (n = 56 participants with PTSD, n = 68 controls)27.
The largest study to date examined differences in severity and progression of PTSD using a multi-omics analysis of metabolomics, proteomics and DNA methylome assays in 159 active-duty male participants with relatively recent onset PTSD (< 1.5 years) and 300 male veterans with chronic PTSD (> 7 years)28. Their findings indicated that active-duty participants with recent PTSD had alterations in signaling and metabolic pathways involved in cellular remodeling, neurogenesis, molecular safeguards against oxidative stress, polyunsaturated fatty acid metabolism, immune regulation, post-transcriptional regulation, cellular maintenance, and markers of longevity. In contrast, Veterans with chronic PTSD showed evidence of alterations associated with chronic inflammation, neurodegeneration, cardiovascular and metabolic disorders, and cellular attrition. These findings suggest that time since trauma and/or age may play an important role, as molecular alterations in the younger cohort reflected homeostatic or compensatory responses, whereas the older cohort had alterations indicative of more chronic disease. The implications of these findings may be relevant for women in the menopausal transition; however, too few women were included in this latter study to allow for an analysis of sex differences.
We are not aware of any study that has systematically ascertained sex differences in the status of lipid metabolites in serum samples of women and men with PTSD and trauma-exposed non-PTSD individuals. In this study, we aimed to examine alterations of lipid metabolites and the contribution of sleep measures in both men and women with PTSD using integrated systems analysis approach.
Results
Demographic data and clinical characteristics
By design, PTSD and control subjects were sex-and age-matched, and had no significant differences in education, or race/ethnicity across all four groups (Table 1) and as previously reported14. In our cohort, men and women with PTSD did not differ in terms of CAPS scores, rates of current MDD, or history of childhood trauma (defined by the presence of two or more categories of childhood trauma as compared to one or none)3. Eleven control subjects reported a lifetime history of a traumatic criterion A1 event, no current or lifetime history of PTSD as assessed by the CAPS. However, women with PTSD had higher PTSD Symptom Checklist (PCL) scores than men with PTSD (Fig. 2a). Additionally, none of the control subjects reported a history of two or more categories of childhood trauma. There were no differences between PTSD and control women in use of hormonal birth control or group differences in smoking of tobacco. Men with moderate and severe PTSD symptoms had higher BMI scores compared with women with similar scores and men with low PCL score (controls) had the lowest BMI (Fig. 2a). Clinical data from a total of 98 different measures, including sleep measures (Supplementary Table S1), were analyzed together with lipid metabolites in an integrated systems approach.
Clinical characteristics of PTSD cohort. (a) Bar charts showing the average BMI, PCL and PSQI scores were analyzed based on PTSD severity according to terciles derived from PCL scores (Low PCL; no PTSD), 33–67% (Moderate PCL; moderate PTSD symptom severity) and 67–100% (High PCL; severe PTSD symptom severity) scores in unadjusted analysis. (b,c) Scatter plots for the four measures most significantly correlated with top 4 measures in the dataset (including clinical and lipid metabolites) after adjusting for BMI and PSQI. Trendlines, or an nth-degree polynomial trendline if its goodness-of-fit is either 50% greater than, or if it explains at least half of the variance not explained by the (n − 1)th-degree polynomial, were fit to the data. R2, r and p denote goodness-of-fit, Pearson’s correlation coefficient and significance of the correlation, respectively. Numbers on X-axis in bar charts in (a) denote the actual number of patients in which the measures were detected. N/group: Low PCL ♀: 16; Moderate (Mod) PCL ♀: 15; High PCL♀: 15; Low PCL ♂: 15; Mod PCL ♂: 15; High PCL ♂: 14. GGT glutamyltransferase, ALT/SGPT serum glutamic-pyruvic transaminase. Adjusted for BMI and PSQI.
Correlation of lipid subclasses with anthropomorphic and clinical measures of BMI and triglycerides
In our cohort, we analyzed PTSD severity according to terciles derived from PCL scores. Participants were found to have low (17–28), moderate (28–35) or high PCL scores (> 35) corresponding to no PTSD symptoms, moderate, or high PTSD symptoms, respectively (Fig. 2a). While perceived sleep quality as determined by Pittsburgh Sleep Quality Index (PSQI) scores did not differ between men and women, PSQI scores were highest in men and women with high PTSD symptoms (PCL scores > 35) (Fig. 2a). BMI was highly correlated with % body fat as ascertained using a DEXA scan (Fig. 2b). BMI was also highly correlated in men and women with high PTSD symptoms, but no correlation with BMI was evident in women with no PTSD symptoms (Fig. 2b). A correlation between BMI and the clinical measure of triglycerides was also evident in women and men (Fig. 2b); at low PCL scores, the correlations in women and men were r = 0.723, p = 0.00156 vs r = 0.614, p = 0.0149, respectively, whereas at high PCL scores, the correlations were not significant (female: r = 0.467, p = 0.079 vs male: r = 0.498, p = 0.070; Fig. 2b). While triglyceride levels were correlated with systolic blood pressure levels in men and women with low PCL scores, this correlation was not significant in individuals with higher PCL scores (Fig. 2c). Triglyceride levels correlated with plasma gamma-glutamyltransferase (GGT) levels, but serum glutamic-pyruvic transaminase (ALT/SGPT) levels were only correlated in women with high PCL scores.
We next ascertained which lipid subclasses were associated with age, BMI, and weight. In sex segregated analyses of the 13 lipid subclasses, age was not associated with any lipid subclasses in women but 3 subclasses (fatty acids (FA), lysophosphatidylethanolamine (LPE), and phosphatidylethanolamine (PE)) were correlated with age in men (Table 2). BMI was associated with 9 lipid subclasses in men and only 3 in women, whereas only PI was associated with body weight in men and 2 subclasses (diglycerides (DG) and triglycerides (TG)) in women (Table 2). In sex aggregated analysis, only PE correlated with age, whereas 9/13 lipid subclasses correlated with BMI, and 6/13 lipid subclasses correlated with body weight (Table 2). In BMI unadjusted analysis, total blood cholesterol and calculated low-density lipoprotein (LDL) cholesterol exhibited significant correlations with 12/13 and 11/13 lipid subclasses, respectively in sex aggregated analyses (Table 3) with cholesterol esters and sphingomyelin subclasses exhibiting r > 0.82 in both women and men (Table 2). Triglycerides measured with mass spectrometry in our dataset correlated highly with the clinical measure of TGs measured by routine assays (r ≥ 0.95) in both women and men (Table 3). TGs in our dataset correlated very strongly with calculated VLDL (r ≥ 0.94) and cholesterol: HDL ratio (r ≥ 0.61) in both sexes, but less strongly with total cholesterol, HDL and VLDL levels (Table 3). In addition to TG, 8 other lipid subclasses correlated with blood TG levels in men and 5 subclasses in women with DG (r ≥ 0.89) and ceramides exhibiting strong correlations. In women, except for LPC, all other subclasses associated with VLDL levels (Table 3).
Sex differences in correlations of PTSD symptom severity with lipid subclasses
Next, we ascertained the correlation of lipid subclasses with the general health and PTSD symptom severity measures (Table 2). Regression analyses revealed sex differences in the correlation of 13 lipid subclasses with various PTSD, other biological and anthropomorphic measures. In sex aggregated analysis, 5/12 lipid subclasses were associated with PCL scores. In women, only PE was associated with the PCL (r = 0.36), whereas 7 subclasses were significantly associated in men (Table 2). The same 7 lipid subclasses in men were also associated with BMI, whereas in women, ceramide, DG, and TG were associated with BMI. In men, LPE and PE were positively associated with age, whereas FA was associated negatively. No lipid subclasses were associated with age in women. In sex aggregated analysis, 6 lipid subclasses were associated with body weight, but sex-specific analyses revealed that while DGs and TGs were associated with body weight in women, only PI was associated with body weight in men (Table 2).
Integrated and systems lipidome and clinical measures analyses
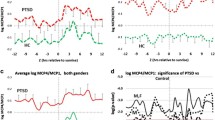
The lipid panel identified a total of 413 known lipids of which 348 were present in all 80 individuals; known metabolites were part of 13 classes of lipids namely, acylcarnitine, cholesteryl esters (CE), ceramides (Cer), glucosylceramide (GlcCer), diglycerides (DG), fatty acids (FA), LPC, lysophosphatidylethanolamine (LPE), PC, PE, PI, sphingomyelin (SM), and TGs. PCA revealed that individuals with moderate and high PCL scores fell into discrete groups with women and men showing clear separation (Fig. 3a), suggesting variability in the datasets of men and women with PTSD, despite similar total PTSD symptom severity levels. Volcano plots revealed that men with high PCL scores experienced significant alterations in 8 lipid subclasses, whereas phosphatidylethanolamines were the only changed lipid subclass in women with high PCL scores (Fig. 3b–d). PCA donut charts revealed subclasses of lipids that comprise various components to explain > 90% variability in the lipidome in men with moderate and high PCL scores (Fig. 3c).
Sex differences in lipid subclasses in PTSD patients. (a) Principal component analysis (PCA) biplot showing clustering of samples by PCL scores and biological sex. (b) Volcano plots showing significant differences in 13 lipid subclasses in moderate and high PCL groups by sex compared with respective low PCL groups. The size of a point represents its quantifiability, calculated as 0.375 + 0.625 × sqrt([percent of cells positive]) × [percent of samples positive] × [maximum point size]; the opacity of points represents expression. The color intensity of points represents the size of the change, with increased symbols drawn in red and decreased symbols in blue. Green lines denote (from top to bottom) p < 0.001, 0.01 and 0.05 by Welch’s t-test. (c) PCA donut plots showing the primary components necessary to explain at least 90% of the variance in moderate (left) and high (right) PCL scores in male patients compared with low PCL scores for 13 lipid subclasses most correlated with each of the primary components versus low PCL scores of the same sex. No lipid subclass was significantly different in women with moderate PCL scores and only phosphatidylethanolamine (PE) was significantly increased in women with high PCL scores compared with women with low PCL scores. (d) Venn diagram contrasting significant differences in measures between female (top left) and male (top right) PTSD participants each compared to non-PTSD participants and listing the most changed symbols for each Venn diagram segment, sorted by ascending p-value (not shown), with log2 fold change versus low PCL score non-PTSD participants shown. Venn diagram contrasting significantly changed measures between male and female PTSD participants with moderate PCL (bottom left) and high PCL (bottom right) scores. Lipid subclass measures with red lines were increased and those with blue lines were decreased. Adjusted for BMI and PSQI.
Men with greater PTSD symptom severity have many more differences in the lipidome than women with similar severity levels
Next, we performed an integrated analysis with 348 individual lipids and 93 clinical measures and examined differences in individual lipid metabolites and clinical measures in women and men with moderate and high PCL scores compared to women and men with low PCL scores, respectively. PCA donut charts revealed that component 1 explained 35% and 44% of the variability in the datasets (including lipidome and clinical measures) in women and men, respectively (Fig. 4a). A detailed distribution of the first two PCA components is shown in scatter plots (Fig. 4b). Women with moderate and high PCL scores shared only ~ 1.86% (8/430) of the measures; ~ 3.2% (14/430) measures were significant only in the PCL moderate group and ~ 5.6% (24/430) measures were significantly altered in women with high PCL scores compared with women with low PCL scores (Fig. 4c). In contrast to women, men with moderate and high PCL scores shared ~ 10% (44/431) measures, with ~ 4.2% (18/431) measures specifically altered in men with moderate PCL scores and ~ 21% (92/431) measures specifically altered in men with high PCL scores compared with men with low PCL scores (Fig. 4d). Pearson correlations of all clinical and lipid metabolites with each measure are shown in Supplementary Table S2. Men with moderate and high PCL scores demonstrated many more differences in individual lipid metabolites than women with similar PCL scores (Fig. 5a,b). Men with high PCL scores had 22% (94/431) significantly different lipid metabolites, whereas women had only ~ 5% (18/430) of significantly different measures with only 0.5% (3/431) measures (2 phosphatidylethanolamine (PE) and cholesterol) shared between women and men (Fig. 5c). When the analysis was performed including various clinical measures, the significantly shared measures increased to 8 from 5 (Fig. 5c, left).
Integrated and systems lipidome and clinical measure analysis. (a) PCA donut plots showing the primary components necessary to explain at least 90% of the variance in females (top) and males (bottom) with high PCL scores compared with low PCL scores for all 348 individual lipid metabolites from 13 lipid subclasses and the 7 symbols most correlated with each of the first two primary components. (b) PCA biplots for the first two components with the color of points denoting log2 fold change versus low PCL scores of the same sex. PCA biplot showing clustering of samples by PCL scores and biological sex. Venn diagram contrasting significantly changed measures between female (c) and male (d) patients with moderate and high PCL scores compared to non-PTSD patients with low PCL scores and listing the most changed symbols for each Venn diagram segment, sorted by ascending p-value (not shown), with log2 fold change versus low PCL score non-PTSD patients shown. Lipid metabolites and clinical measures with red lines were increased and those with blue lines were decreased. Adjusted for BMI and PSQI.
Sex differences in lipid metabolites in PTSD patients. Venn diagram contrasting significantly changed lipid metabolite measures for (a) all subgroups, (b) female and male patients with high PCL scores compared to non-PTSD participants with low PCL scores. The size of a point represents its quantifiability, calculated as 0.375 + 0.625 × sqrt([percent of cells positive]) × [percent of samples positive] × [maximum point size]; the opacity of points represents expression. (c) Venn diagram contrasting significantly changed lipid measures only (left) or lipid and clinical measures combined (right) between female and male participants with high PCL scores and listing the most changed symbols for each Venn diagram segment, sorted by ascending p-value (not shown), with log2 fold change versus low PCL score non-PTSD participants shown. Log2 fold change was calculated for each sample versus its corresponding value with low PCL score, with the average shown and *, ** and *** denoting p < 0.05, 0.01 and 0.001 by Welch’s t-test, respectively. Lipid metabolites and clinical measures with red lines were increased and those with blue lines were decreased. Adjusted for BMI and PSQI.
Sex differences in clinical and sleep measures in individuals with PTSD
Disturbed sleep is known to exacerbate many health conditions but whether sleep is associated with alterations in lipids subclasses in individuals with PTSD is not known. In sex aggregated analysis, 9/13 lipid subclasses correlated with PSQI scores and 4/13 were negatively correlated with self-reported total sleep time (TST). In sex segregated analysis, only 3/13 and 1/13 lipid subclasses were correlated with PSQI scores and TST in women with PTSD. In men with PTSD, 8/13 and 4/13 lipid subclasses were correlated with PSQI and TST, respectively (Table 2), matching our findings from sex aggregated analysis. A total of 4/13 lipid subclasses were significantly decreased in women with severe PTSD symptoms versus men with severe PTSD symptoms (Fig. S1a). Acylcarnitines were significantly less in women versus men regardless of PTSD status (Fig. S1a). We next determined which clinical laboratory values and sleep measures differed between men and women with PTSD compared with non-PTSD individuals. Using heat maps, we compared side-by-side, all significantly different clinical and sleep measures in men and women with moderate and high PCL scores compared with men and women with low PCL scores, respectively. Clinical and sleep measures that differed between women and men were also ascertained; 75% (22/29) clinical measures and 46% (6/13) sleep measures, including PCL scores were significantly different between women and men with severe PTSD symptoms (Fig. S1b,c). Eight of these 22 clinical measures were also different between women and men with no PTSD symptoms (Fig. S1b). BMI, age, total fat, cholesterol, triglycerides, creatinine, leptin, GGT were among some of the clinical measures (24/97) that significantly differed in men, but not women compared with their respective low PCL counterparts (Fig. 6a). GGT levels were increased ~ twofold in men with high PCL scores (log2 fold = 1), whereas GGT levels were increased in women with high PCL scores but did not reach statistical significance (Fig. 6a). Women with PTSD had lower body weight than women without PTSD, but the difference was significant only in women with moderate PCL scores vs low PCL scores (Fig. 6a). Total protein, human growth hormone, calcium, albumin, HDL cholesterol, and globulin levels trended to be higher in women with moderate and high PCL scores compared with women with low PCL scores (Fig. 6a); levels of globulin and HDL cholesterol were significantly different in women with high PCL vs low PCL scores, whereas as levels of total protein, calcium, and albumin were significantly different in women with moderate PCL vs low PCL scores. Human growth hormone levels were significantly increased in women with both moderate and high PCL scores compared with women with low PCL scores (Fig. 6a). Both women and men with moderate-to-high PCL scores reported poorer sleep quality with significantly worse PSQI scores (Fig. 6b). Several other measures of sleep, both objective and qualitative, such as non-REM delta sleep (ln_delta_nrem) and total epochs of sleep (a sleep duration variable) were significantly decreased in men with high PCL scores compared with men with low PCL scores (Fig. 6b). Women with high PCL scores reported significantly more waking after sleep onset (WASO) compared with women with low PCL scores and men with PTSD, regardless of severity (Fig. 6b). Women with high PCL scores also reported significantly more other anxiety-related awakening, as well as sleep onset latency (SOL), whereas women with PTSD, regardless of PCL score reported significantly more night awakening (nwak) (Fig. 6b). Women with moderate PCL scores experienced differences in only 10/430 lipid metabolites, 6 triglycerides and 1 PC (40:6 A) were decreased and 3 PE/PC were increased compared with women with low PCL scores (Fig. 6c). Women with high PCL scores experienced differences in 20/430 lipid metabolites with increases in TG, PC, PE, and CE subclasses, whereas LPCs decreased (Fig. 6d).
Significantly changed clinical and sleep measures in all PTSD patients and lipid measures in female PTSD patients. Heat map of the most-significantly changed clinical (a), sleep and PCL (b) measures in men and women with PTSD compared with their respective no PTSD (Low PCL scores) controls. Lipid metabolite measures identified in women with moderate PCL score (c) and high PCL score (d) compared with men and women with low PCL scores, respectively. Changed measures are shown for all 4 subgroups. log2 fold change was calculated for each metabolite versus its corresponding metabolite value in low PCL group, with the average shown and *, ** and *** denoting p < 0.05, 0.01 and 0.001 by Welch’s t-test, respectively. Labels next to symbols denote the percentage of samples in which metabolites were detected across all groups; group size (n) are shown above group names. Adjusted for BMI and PSQI.
In men with a moderate PCL score, 24/62 sphingomyelins (SM) were increased and were the most different subclass of lipids (Fig. 7a). Other increased lipid metabolites were from the subclasses acylcarnitine, ceramide, CEs, GlcCer, fatty acids, PCs, and LPC (Fig. 7b). In men with high PCL scores, PE (18/31), PC (14/134), LPC (3/29), SM (20/62), TG (18/34), and ceramide (14/20) along with a select number of fatty acids, acylcarnitine, LPE, PI, and DG were also different (Fig. 7c–f). Several SM, TG and Ceramides that were increased in men with a high PCL score, tended to decrease in women with moderate-to-high PCL scores, suggesting divergent responses to similar trauma symptom levels.
Significantly changed lipid measures in male PTSD patients. Heat map of the most-significantly changed lipid measures in (a,b) men with moderate PCL scores, and (c–f) men with high PCL scores compared with men with low PCL scores, respectively. Changed measures are shown for all 4 subgroups. Many of the lipid metabolites increased in men with high PCL scores were decreased in women. Log2 fold change was calculated for each metabolite versus its corresponding metabolite value in the low PCL group, with the average shown and *, ** and *** denoting p < 0.05, 0.01 and 0.001 by Welch’s t-test, respectively. Labels next to symbols denote the percentage of samples in which metabolites were detected across all groups; group sizes (n) are shown above group names. Adjusted for BMI and PSQI.
Most alterations in individual metabolites in individuals with moderate and high PCL scores correlate with cholesterol and other lipids, but not PCL or PSQI
We next determined the correlations between the top changed individual lipid metabolites with clinical measures and other lipid metabolites. TG (44:1) was the most changed/decreased (log2 fold = − 1.59, p < 0.05) in women with moderate PCL scores versus women with low PCL scores (Fig. 6c). TG (44:1) correlated most significantly in all groups with LPC (14:0) (r = − 0.665, p = 10−8.2); however, the correlation was stronger in men with high PCL scores than in women (Fig. 8a, ♂: r = 0.855, p = 10−4 vs ♀: r = 0.684, p = 0.0049, respectively). TG (44:1) also correlated with VLDL cholesterol in men and women with high, but not low PCL scores (Fig. 8a, ♂: r = 0.701, p = 0.007 and ♀: r = 0.533, p = 0.04). TG (56:8) was one of the top and most significantly increased metabolites in women with high PCL scores (log2 fold = 0.536, p < 0.05, Fig. 6D) and it correlated with VLDL cholesterol in women, but not men with high PCL scores (Fig. 8b, ♀: r = 0.821, p = 10−3.8 and ♂: r = 0.407, p = 0.167). PC (40:6)B was the most correlated lipid metabolite with TG (56:8) in all patients (r = 0.664, p = 10−8.1), however, individuals with low PCL scores displayed a very strong and robust correlation between TG (56:8) and PC (40:6)B but a weaker correlation in women and men with high PCL scores (Fig. 8b). Men with moderate and high PCL scores shared many of the changed metabolites. Ceramide (d40:0), Ceramide (d36:1), SM (d40:0), and TG (48:0), were amongst the most increased metabolites in men with high PCL scores (Fig. 7e,f). All the metabolites correlated with some form of cholesterol (Fig. 8c,d) and TG (48:0) also correlated robustly in all patients with TST and systolic blood pressure (Fig. 8d, r = 0.624, p = 10−7, and r = 0.42, p = 10−3.1, respectively). However, the correlation mostly held in men with high PCL scores and not in women (Fig. 8d).
Scatter plots showing correlations between top 2 most significantly changed lipid metabolites in (a) females with moderate PCL score (TG (44:1)), (b) females with high PCL score (TG (56:8)), (c) males with moderate PCL score (Ceramide (d36:1) and SM (d40:0)), (d) males with high PCL score (Ceramide (d42:0) and TG (48:0)). Trendlines, or an nth-degree polynomial trendline if its goodness-of-fit is either 50% greater than, or if it explains at least half of the variance not explained by the (n − 1)th-degree polynomial, were fit to the data. R2, r and p denote goodness-of-fit, Pearson’s correlation coefficient and significance of the correlation, respectively. Several changed metabolites correlated with clinical measures of (V)LDL cholesterol. Measures also correlated with triglycerides, systolic blood pressure, and TST. Most significant correlations with other lipid metabolites are also shown. Adjusted for BMI and PSQI.
Discussion
Epidemiological studies indicate that the prevalence of PTSD is higher in women. Not all traumas are equal; exposure to and experiencing certain traumas, such as rape, childhood abuse, and combat results in higher conversion to PTSD (~ 30–45%) than some other traumas (witnessing trauma, accident etc.), despite the prevalence of these traumas affecting less than 10% of the population29. Females report a higher prevalence of adverse childhood events than males30 and this could in part explain higher prevalence of PTSD in females. The role of gender has not been categorically addressed in any studies; biological sex and gender are distinct and should not be conflated31,32. Minority and multiracial individuals and those within the age range of 25 to 34 years report significantly higher adverse childhood events than any other groups30, but it is unclear if the rate of PTSD is also higher in these groups with higher childhood traumas. Adverse childhood events and early-life adversity together pose a greater health risk for several health conditions that include cardiovascular and pulmonary diseases, metabolic disorders, inflammation, liver, and gastrointestinal diseases as well as mental health conditions30,33,34. Conversely, individuals with mental health disorders have many of the comorbid health conditions mentioned above. Studies have shown a strong link between adverse childhood events and mental health disorders such as PTSD, depression, anxiety, and suicide30. Structural changes in the corticolimbic brain regions and the thalamus are reported in individuals with PTSD and history of adverse childhood events34. Thus, while much is known about the epidemiology, behavioral neurobiology, and genetics of PTSD, few studies have examined lipid metabolites in PTSD. Most of the cohorts studied consisted primarily of males24,25,26,27,28,29,31,32,33,34,35,36 and sex segregated data analysis has been performed only in one study to date to our knowledge14. We have previously examined primary metabolites in male and female PTSD patients and reported sex differences in levels of several metabolites and the contribution of perceived sleep quality to many of the differences in primary amines14.
Metabolomics in conjunction with clinical measures provides a large amount of data that is often difficult to interpret, thus an integrated and systems analysis approach is needed. No study thus far has integrated clinical health measures with lipid metabolites or analyzed the contribution of comorbidities such as insomnia in the altered lipid profile. Here, we performed, to our knowledge, first known integrated and systems levels analysis of clinical health, sleep and lipid metabolites measured using QToF mass spectrometry in trauma-exposed individuals with and without PTSD. Many more lipid metabolites were altered in men with higher PTSD symptoms than in women with similar scores. Many lipid metabolites that were significantly increased in men with PTSD compared to men without PTSD belonged to lipid subclasses that included TG, SM, Ceramide, and PE; several of these metabolites were decreased in women with PTSD, although the decreases were not significant in women without PTSD. In our cohort, triglyceride levels correlated with plasma GGT levels, whereas serum ALT/SGPT levels were only correlated in women with high PCL scores. Both GGT and ALT functions are indicators of liver dysfunction, and GGT levels were associated with BMI, blood pressure, and triglycerides in the Framingham Heart Study37,38. Overall, the lipid alterations were greater in men with PTSD who also had the greatest BMI and disturbances in sleep (e.g., poorer sleep quality (PSQI) and sleep duration (epochs)) compared to women with PTSD in this cohort. In contrast to lipids, many more changes in primary metabolites were observed in women with PTSD in this cohort as we have reported earlier14.
One of the most common complaints among individuals with PTSD is sleep disturbance. Lower slow wave sleep duration and delta-band spectral power are more pronounced in men than women and correlate with PTSD status3. In contrast, greater rapid eye movement sleep is found in women with PTSD compared to healthy controls, a difference not seen in men3. In this study, we found that WASO, minutes of wake that occurs between sleep onset and waking was over ~ 2.5-fold greater in women with severe PTSD symptoms compared to women without PTSD or to men with or without PTSD. Nighttime awakening was also greater in women with PTSD, whereas men with PTSD did not report more night awakening compared to controls. Several lipid subclasses were correlated with sleep quality and TST time in men and women with PTSD, with many more differences in lipid subclasses found in men compared to women. Sleep disturbance is a key risk factor for health consequences as it alters hypothalamic–pituitary–adrenal axis function, resulting in impaired glucose and lipid metabolism. The hypothalamic–pituitary–adrenal and somatotropic axes activities are temporally associated with delta power sleep and promote insulin sensitivity and metabolic syndrome. Additionally, sleep duration correlates with metabolic risk in PTSD but does not fully account for the association between PTSD with known metabolic disturbances such as in blood insulin or glucose levels39.
We have previously shown opposite correlations between tryptophan and insulin levels in men and women with PTSD14. Moreover, while estrogen, progesterone, and testosterone and their metabolite levels did not differ between individuals with and without PTSD in this cohort, delta power sleep correlated with testosterone levels in men14. In this cohort, among men with severe PTSD, one triglyceride metabolite (TG (48:0)) was negatively correlated with TST and systolic blood pressure. Among women with no PTSD, TG (48:0) was negatively correlated with TST. Men in this cohort with high PTSD severity also had higher BMI levels and the correlation between BMI and systolic blood pressure were inversely correlated in men but not women. BMI was correlated with triglycerides in men and women with no PTSD, whereas BMI showed a weaker correlation with triglycerides in men and women with PTSD. Thus, higher weight might be contributing to differences in metabolic health in men with PTSD, whereas as women with PTSD in this cohort did not have significant differences in metabolic health and had BMI levels within normal range. Others have reported associations between PTSD symptoms and three specific glycerophospholipids in Veterans with PTSD25; these specific metabolites were not ascertained in our cohort. However, no information on BMI and other clinical measures was reported or is available for comparison.
Sphingomyelins regulate endocytic function as well as receptor-mediated ligand uptake of ion channels and G protein-coupled receptors40. SMs also regulate cardiovascular function and their distribution within cellular compartments often correlates with cholesterol. We found that nearly 65% of SM (24/37) were increased in men with moderate and severe PTSD compared with low/no PTSD. While the same SM was decreased in women with moderate PTSD and either increased or decreased in women with severe PTSD compared with women with no PTSD, the differences in women did not attain statistical significance. Sphingomyelins (> 40 carbon length) were reportedly increased in a cohort of World Trade Center responders with PTSD compared with non-PTSD individuals who were also exposed to the 9/11/2001 attacks in New York City36. In the World Trade Center cohort, after adjusting for several medical conditions, 9 different SMs correlated with BMI, whereas hexosylceramide (HCER (26:1) did not. Kuan et al. also performed an integrated analysis of lipid metabolites with proteomics and found several protein modules that contained IL-6 and ATP6V1F proteins were associated with fatty and bile acid metabolites36. However, Kuan et al. could not replicate findings from other 3 studies24,25,35 for specific lipid metabolites and their correlations with PTSD status, likely due to several variables, including clinical profiles and PTSD measures used36. Our aim was not to replicate findings but perform an unbiased analysis. Adjusting for BMI, PSQI or other clinical measures (such as total protein, cholesterol etc.) did not alter our lipid metabolite findings.
Many essential fatty acids are derived from diet. Diet is one variable that is difficult to control for and is a limitation for metabolomics analysis. Individuals in our cohort were in a 3 day/night sleep clinical inpatient study and had access to limited dietary choices. Thus, while our cohort is not diet-controlled, per se, dietary variability is far less than those that might be present in other studies that have interrogated the lipidome of individuals with PTSD. In this regard, we identified over 1500 unknown lipid metabolites. Many of these were highly altered in individuals with PTSD, more than any of the known lipids. In the future, identification of these unknown metabolites can lead to discovery of PTSD-specific lipid markers. While medical conditions may contribute to alterations in lipidomics, this sample included young and healthy participants and there were no associations between PTSD and chronic disease states in this sample. It is possible that alterations in metabolites may reflect earlier stages of future disease. While several lipid subclasses correlated with PSQI and TST in both men and women with PTSD, contrary to our expectation that individual lipid metabolites that were different in men and women with moderate and high PCL scores should correlate to some degree with PCL scores and sleep quality; we did not find any. While prior studies have found alterations in neuroendocrine, immune, and aging processes in PTSD, our knowledge of the role of metabolite disturbances is PTSD is limited but has the potential to elucidate new discovery of yet unknown biological mechanisms of disease.
Phospholipid subclasses regulate critical physiological activities such as cell signaling, membrane structure, fluidity, permeability, organelle, and immune functions. Membrane fluidity, especially for neuronal cells is key for their structure and function and is determined by the presence phospholipid subclasses and their topological distribution within the cell and organelle membranes41. The most abundant phospholipid subclass in cell membranes is phosphatidylcholine. PCs serve two key functions-determine membrane fluidity and storage of neurotransmitter choline. Membranes with PC as predominant composition are devoid of any curvature due to unique molecular geometry, and are typically fluid at room temperature42. Since the ratio of PC to other phospholipids determine membrane shape and permeability, altered ratios can lead to neuronal, cellular and organelle signaling dysfunction. PC also serves as an essential reservoir for storing choline, a precursor for the neurotransmitter acetylcholine and is essential for proper brain/neuronal function43. While altered levels of PC are seen in individuals with traumatic brain injury and is associated with impaired cholinergic neurotransmission and impaired neurogenesis44, it is unclear if the same class of lipids are also altered in individuals with PTSD. In this study, PC was significantly altered in men with PTSD, but not women. Although we did not measure any brain function in this cohort, plasma and cerebrospinal fluid lipidomics are used as potential biomarkers for several neurodegenerative diseases, such as the Alzheimer’s disease, Parkinson’s disease, and schizophrenia45. However, lipidomics information is limited and insufficient to understand lipid-associated changes in the brain or other organs to shed light on the association between circulating and local lipid dysregulation in health or in pathology.
Increased levels of lysophosphatidylcholine disrupt mitochondrial membrane integrity and dysregulate cytochrome C release in hepatocytes to modulate cholesterol biosynthesis. High LPCs levels are associated with several pathologies such as cardiovascular diseases and diabetes. LPCs may serve as a group of proinflammatory lipids that are involved in the pathogenesis of central nervous system-associated disorders41. Phosphatidylethanolamine together with phosphatidylcholine, phosphatidylserine and phosphatidylinositol form the backbone of most biological membranes. The relative proportions of lipid subclasses are maintained at a steady state under homeostasis. Ceramides are members of the sphingolipid family, that play critical roles in diverse cellular processes such as cell death, autophagy, inflammation, fatty acid oxidation, and ER stress46. PEs have an essential role in chaperoning membrane proteins to their folded state; PEs catalyze the conversion of prions from the nontoxic to the toxic conformation. PEs initiate autophagosome formation and are associated with ER stress associated with diabetes and neurodegeneration. Neuronal activity and immune function are two key physiological processes that are altered in individuals with PTSD. The subclasses of phospholipids that are altered in men and women with PTSD is largely not known. In our cohort, PE alterations were associated with age, BMI, PCL, PSQI and total sleep time in men and with PCL, PSQI, and TST in women with PTSD. Ceramides were increased in men with severe PTSD symptoms in our cohort, but whether men had greater differences in immune function associated with ceramides is unclear.
While highly promising, there are several limitations to the metabolomics research to date: (1) While these studies point to several plausible biological pathways with the potential to explain alterations in PTSD and common comorbidities, a major limitation is that these studies were conducted almost entirely in males or had insufficient numbers of female participants to examine sex differences. Indeed, some of the differences in findings may be attributed to differences in sex as there were differences in findings in the studies that included women, but this has not been tested. (2) The studies that included women did not control for menstrual cycle day. Since sex steroids may interact with metabolites in other biological pathways and may fluctuate over the menstrual cycle, determining menstrual cycle day and examining sex steroids along with other metabolites of interest is critical. (3) All studies used cross-sectional designs with single time point blood draws. Control groups in some studies were not trauma exposed. Thus, it is unclear if these findings reflect PTSD symptom state, trait, or a response to trauma exposure regardless of the presence of PTSD symptoms. To establish biomarkers of disease, it will be important to determine whether these alterations are stable over repeated measures. (4) A major challenge to finding a reliable biological signal in PTSD lies in the heterogeneity of the disorder. There is a great deal of variability in the symptom profile and in combinations of symptoms in PTSD, as described by Galatzer–Levy20. PTSD symptoms are typically assessed by self-report or diagnostic interview, resulting in subjective perceptions of severity which can be unreliable. Given that PTSD is a heterogenous disorder, analysis by PCL score, rather than grouping all people with a PTSD diagnosis with large variation in scores, yields more robust findings. There is also substantial comorbidity between PTSD and other mental health conditions such as depression, traumatic brain injury or substance use, among others. Overlapping symptoms may obscure biological findings.
Our study has several limitations and strengths. Caveats include a cross-sectional study design. A longitudinal study design would be needed to demonstrate stability of metabolite changes over time over the course of the disease process. In our ongoing studies, longitudinal sample collection from PTSD patients is ongoing and data from that study should address some of these limitations in the future. Limitations also include a small sample size for sex segregated analysis (n = 20/sex/group). Men with PTSD were significantly more obese and consumed more alcohol than any other group and these variables could account for many of the differences in lipid subclasses. A single timepoint measure and adjusting for BMI, sleep quality, smoking status are not sufficient to fully explain changes in lipidomics. Our study also has several strengths- first, we performed an analysis of lipids using validated QToF mass spectrometry; TGs identified with mass spectrometry correlated highly with those measured using standard laboratory techniques (e.g, cholesterol), thereby further validating our lipidomics findings. Second, we performed an integrated and systems level analysis taking several clinical and anthropomorphic measures into account. Third, in-depth sleep measures were obtained in addition to self-report of sleep measures. Fourth, the non-PTSD population was age-matched and trauma-exposed.
Materials and methods
Human subjects
This study was a 2 × 2 cross-sectional design with 4 groups (PTSD/control × women/men) of 44 individuals with current chronic PTSD (22 women and 22 men) and 46 control subjects (24 women and 22 men). Blood samples for lipidomics analysis was available from 20 participants per group. Participant ages ranged from 19 to 39 years. Sleep measures were recorded in an inpatient sleep laboratory at the General Clinical Research Center (GCRC) at the University of California, San Francisco. The Committee on Human Research at the University of California, San Francisco approved this study. Written informed consent was provided from all participants before enrollment and start of any study procedures.
PTSD subjects met DSM-IV diagnostic criteria as ascertained using the Clinician-Administered PTSD Scale (CAPS) or score > 40 and as previously described3. Control subjects had no lifetime or current history of a PTSD diagnosis. Women participants were premenopausal and were scheduled during the follicular phase of the menstrual cycle. Participants were unmedicated during the study, except for birth control in women (n = 9), diovan for hypertension (n = 1) topical steroids (n = 2), Izoniozid (n = 1), thyroid medication (n = 1).
All study procedures were timed according to habitual sleep onset, determined by actigraphy and sleep diary in the week prior to the GCRC study. Study participants were limited to one cup of caffeine daily, maintained regular bed and waking times, and did not consume alcohol or illicit substances. Exclusion criteria for PTSD and control subjects was previously described3 and included: history of traumatic brain injury, presence of psychosis, neurologic disorders or systemic illness; use of psychiatric, anticonvulsant, antihypertensive, sympathomimetic, steroidal, statin or other prescription medications; obesity (defined as body mass index (BMI) > 30); alcohol abuse or dependence in the prior two years; substance abuse or dependence in the previous year; any psychiatric disorder with psychotic features; bipolar disorder or obsessive–compulsive disorder; and pregnancy. Exclusion criteria for control subjects also included a lifetime history of major depressive disorder or panic disorder.
Psychiatric diagnoses and trauma history
The Life Stressor Checklist-Revised interview was used to determine trauma exposure and age of occurrence47. The PTSD Checklist (civilian version) for DSM-IV (PCL)48 is a self-report measure used to assess the severity of chronic PTSD symptoms49, with higher scores indicating greater severity. The PCL consists of 17 items that correspond to the DSM-IV criteria and include intrusive thoughts and re-experiencing symptoms (cluster B), avoidance (cluster C), and hyperarousal (cluster D). All other psychiatric disorders, including major depressive disorder were diagnosed using the structured clinical interview for DSM-IV, non-patient edition (SCID-NP)50.
Sleep measures
The Pittsburgh Sleep Quality Index51 (PSQI) is a 19-item self-report questionnaire which assesses sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbances (including nightmares) over a 1 month period, with higher scores indicating poorer sleep quality. The use of sleep medication, and daytime dysfunction over the previous month has been described elsewhere3.
Polysomnographic measurements and power spectral analysis for polysomnographic measures
Electroencephalogram (EEG) from leads C3, C4, O1 and O2, left and right electrooculograms (EOG), submental electromyogram, bilateral anterior tibialis EMGs, and electrocardiogram were recorded in accordance with standardized guidelines by Rechtschaffen52 using the Ambulatory polysomnography (Nihon Kohden Trackit Ambulatory Recording System) as described previously3. Sleep activity in all frequency bands delta through gamma from the C3 electrode was measured by power spectral analysis using the Pass Plus (Delta Software) analytic software. Delta sleep spectral power density (μV2) was natural log transformed to normalize its distributions.
Blood collection and clinical laboratory measures
Blood was collected at habitual wake-up time on the morning after the second night on the GCRC, while the subject was fasting, from an indwelling catheter inserted the night before. Blood (10 mL) was drawn into a chilled EDTA tube and processed for plasma separation for mass spectrometry analysis.
Analysis of lipid metabolites in human plasma using liquid chromatography-quadrupole Time-of-Flight mass spectrometry (LC–MS/MS)
Lipid metabolites that included diverse classes of phospholipids, ceramides, sphingomyelins, free fatty acids, acylcarnitines, triacylglycerides, cholesterol and cholesterol esters were measured using validated LC–MS/MS procedures53. Briefly, lipids from 40 µL plasma were extracted using 300 µL degassed, − 20 ℃ cold methanol 300 μL containing a mixture of lipid internal standards, specifically LPE(17:1), LPC(17:0), PC(12:0/13:0), PE(17:0/17:0), PG(17:0/17:0), d7-cholesterol, SM(d18:1/17:0), Cer(d18:1/17:0), sphingosine(d17:1), DG(12:0/12:0), DG(18:1/2:0), and d5-TG-(17:0/17:1/17:0). After adding 1 mL of cold methyl tertbutyl ether (MTBE) was added containing CE(22:1) as additional internal standard, lipids were separated from hydrophilic metabolites by adding 250 μL LC–MS grade water. Dried lipid extracts were resuspended in 110 µL methanol/toluene (9:1, v/v) containing CUDA as system suitability internal standard prior to LC–MS/MS analysis. 1.7 µL were injected onto an Acquity UPLC CSH C18 column (100 × 2.1 mm; 1.7 μm) coupled to an Acquity UPLC CSH C18 VanGuard precolumn (5 × 2.1 mm; 1.7 μm) (Waters, Milford, MA). The column was maintained at 65 ℃ at a flow-rate of 0.6 mL/min with a water/acetonitrile/isopropanol gradient using mobile phases (A) 60:40 (v/v) acetonitrile:water and (B) 90:10 (v/v) isopropanol:acetonitrile54. Both mobile phases were buffered with ammonium formate (10 mM) and formic acid (0.1%). Lipid separation was performed with the following gradient: 0 min 15% (B); 0–2 min 30% (B); 2–2.5 min 48% (B); 2.5–11 min 82% (B); 11–11.5 min 99% (B); 11.5–12 min 99% (B); 12–12.1 min 15% (B); and 12.1–15 min 15% (B). Mass spectrometry was performed on an Agilent 6530 quadrupole/time-of-flight mass spectrometer (QTOF MS) with a Dual Spray ESI ion source (Agilent Technologies, Santa Clara, CA). Simultaneous MS1 and data dependent MS/MS acquisition was used. Electrospray (ESI) parameters were set as: capillary voltage, 3.5 kV; nozzle voltage, 1 kV; gas temperature, 325 ℃; drying gas (nitrogen), 8 L/min; nebulizer gas (nitrogen), 35 psi; sheath gas temperature, 350 ℃; sheath gas flow (nitrogen), 11 L/min; MS1 acquisition speed, 2 spectra/s; MS1 mass range, m/z 60–1700; MS/MS acquisition speed, 2 spectra/s; MS/MS mass range, m/z 60–1700; collision energy, 25 eV. The instrument was tuned using an Agilent tune mix. A reference solution (m/z 121.0509, m/z 922.0098) was used to correct small mass drifts during the acquisition.
For quality control (QC), we randomized injection orders, used pool QC samples to equilibrate the LC–MS system before data acquisition, injected method blank samples and a pool QC samples between each set of 10 study samples, and injected the NIST SRM 1950 community plasma QC sample before and after the study sample sequence. We also monitored peak shapes and intensities of all internal standards during data acquisition. Data were processed by MS-DIAL (v. 2.69) software program with MS1 (centroiding) tolerance 10 mDa, mass slice width 50 mDa, smoothing level 3 scans, minimum peak height 500 amplitude, and alignment at 25 mDa and 6 s retention time tolerance. Lipid were identified at 9 s retention time tolerance with accurate mass and MS/MS matching against the LipidBlast library55. Quantification was performed by combining the two most abundant adducts per lipid56, followed by normalization using the sum of all internal standards. Due to recursive backfilling during data processing, missing values were limited to 4.1% of all data. Such missing values imputed using half of the minimum detected value for each compound. All metabolomic data were investigated using pooled quality control samples and blank samples. Metabolites that had more than 30% relative standard deviation in pooled QC samples were removed, and metabolites that showed less than threefold higher intensity compared to blank samples were removed as well. Technical errors reduce biological power, so findings reported in this work were observed at statistical power despite possible technical variance.
Statistical analysis
Aseesa Stars version 0.1 (www.aseesa.com) analysis tool was used for generation of heat maps, bar charts, principal component analysis, correlation scatter plots, volcano plots and Venn diagrams as described previously57 and below. For our initial analysis, we did not adjust for BMI or PSQI (Fig. 2a bar charts and Tables 2, 3), but for all subsequent analyses for data shown in Figs. 2b, 3, 4, 5, 6, 7 and 8, BMI and PSQI were adjusted.
Heat maps
Heat maps were generated to depict several symbols in a single chart instead of multiple bar charts as described in detail elsewhere57. Value labels show the group average or average log2 fold change vs the respective control group. Value labels are drawn for every symbol in absolute heat maps, and for values greater than 33% of the heat map’s maximum value in relative heat maps. Labels for values less than 16.67% of the maximum are drawn in black for legibility as reported earlier57. Labels next to gene symbols denote the percentage of participants across all test groups in which metabolite of the symbol were detected. Numbers above test group labels denote the number of samples that were included (n); if not all metabolites were present, then the minimum sample size is shown.
Bar charts and volcano plots
Values in bar charts are calculated in the same way as those in heat maps. Bar chart legends includes the top four correlated measures sorted by significance value. Error bars represent the standard deviation. The comparison mode Value-to-Average was used as described previously57. Measures with p < 0.1 are included in volcano plots for clarity. Welch’s t-test was performed as in heat maps, with †, †† and ††† denoting p < 0.05, 0.01 and 0.001 versus the previous test group (one bar above). Labels next to symbols denote the number of samples that were included (n). The filled fraction of a bar represents the percentage of cells in which transcripts of the symbol were detected; in charts showing changes in cell types, it represents the percentage of samples in which cells of that type were detected.
Principal component analyses (PCA)
For Symbol PCAs, only symbols with p < 0.05 were included, and samples with zero values were excluded. Covariance matrixes were created by standardizing all values for each symbol using \(\text{z}=\frac{\text{v}-\upmu }{\upsigma }\) for a sample value \(\text{v}\), group average \(\upmu\) and standard deviation \(\upsigma\), and calculating the covariance between two symbols57. Donut charts show the primary components necessary to explain at least 90% of the dataset’s variance. PCA biplots show the correlation of each included symbol/sample with Component 1 (x axis) and Component 2 (y axis) as given by the components’ eigenvectors. The color of points in biplots for Symbol PCAs denotes log2 fold change versus control, calculated in the same way as in bar charts, with increased symbols shown in red and decreased symbols in blue.
Study approval
The study was approved by UCSF’s Institutional Review Board and all research was performed in accordance with relevant guidelines/regulations in accordance with the Declaration of Helsinki. All participants signed an informed consent form approved by the IRB. All HIPAA identifiers were removed and only deidentified data was used for analysis in all sections of the manuscript, including supplementary information. This study does not include any human images or involves any organ/tissue procedures.
Data availability
All data are contained within this manuscript or included in the supplemental data. Patient information was deidentified before analysis.
References
Association, A. P. Diagnostic and Statistical Manual of Mental Disorders 5th edn. (American Psychiatric Association, 2013).
Neylan, T. C. et al. Sleep disturbances in the Vietnam generation: Findings from a nationally representative sample of male Vietnam veterans. Am. J. Psychiatry 155, 929–933 (1998).
Richards, A. et al. Sex differences in objective measures of sleep in post-traumatic stress disorder and healthy control subjects. J. Sleep Res. 22, 679–687. https://doi.org/10.1111/jsr.12064 (2013).
Ford, D. E. & Kamerow, D. B. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention?. J. Am. Med. Assoc. 262, 1479–1484 (1989).
Breslau, N., Roth, T., Rosenthal, L. & Andreski, P. Sleep disturbance and psychiatric disorders: A longitudinal epidemiological study of young adults. Biol. Psychiatry 39, 411–418 (1996).
Livingston, G., Blizard, B. & Mann, A. Does sleep disturbance predict depression in elderly people? A study in inner London. Br. J. Gen. Pract. 43, 445–448 (1993).
Chang, P. P., Ford, D. E., Mead, L. A., Cooper-Patrick, L. & Klag, M. J. Insomnia in young men and subsequent depression. The Johns Hopkins Precursors Study. Am. J. Epidemiol. 146, 105–114 (1997).
Agargun, M. Y. et al. Repetitive and frightening dreams and suicidal behavior in patients with major depression. Compr. Psychiatry 39, 198–202 (1998).
Agargun, M. Y. et al. Nightmares, suicide attempts, and melancholic features in patients with unipolar major depression. J. Affect Disord. 98, 267–270 (2007).
Sjostrom, N., Waern, M. & Hetta, J. Nightmares and sleep disturbances in relation to suicidality in suicide attempters. Sleep 30, 91–95 (2007).
Jakupcak, M. et al. Posttraumatic stress disorder as a risk factor for suicidal ideation in Iraq and Afghanistan War veterans. J. Trauma Stress 22, 303–306. https://doi.org/10.1002/jts.20423 (2009).
Lee, D. H. et al. Neuroinflammation in post-traumatic stress disorder. Biomedicines. https://doi.org/10.3390/biomedicines10050953 (2022).
Nilaweera, D. et al. Lifetime posttraumatic stress disorder as a predictor of mortality: A systematic review and meta-analysis. BMC Psychiatry 23, 229. https://doi.org/10.1186/s12888-023-04716-w (2023).
Bhargava, A. et al. Chemical set enrichment analysis: Novel insights into sex-specific alterations in primary metabolites in posttraumatic stress and disturbed sleep. Clin. Transl. Med. 11, e511. https://doi.org/10.1002/ctm2.511 (2021).
Breslau, N. et al. Trauma and posttraumatic stress disorder in the community: The 1996 Detroit area survey of trauma. Arch. Gen. Psychiatry 55, 626–632 (1998).
Tanielian, T. & Jaycox, L. RAND Center for Military Health Policy Research (RandCorporation, 2008).
Henstridge, D. C., Abildgaard, J., Lindegaard, B. & Febbraio, M. A. Metabolic control and sex: A focus on inflammatory-linked mediators. Br. J. Pharmacol. 176, 4193–4207. https://doi.org/10.1111/bph.14642 (2019).
Narayanan, S. P., Anderson, B. & Bharucha, A. E. Sex- and gender-related differences in common functional gastroenterologic disorders. Mayo Clin. Proc. 96, 1071–1089. https://doi.org/10.1016/j.mayocp.2020.10.004 (2021).
Klein, S. L. & Flanagan, K. L. Sex differences in immune responses. Nat. Rev. Immunol. 16, 626–638. https://doi.org/10.1038/nri.2016.90 (2016).
Galatzer-Levy, I. R. & Bryant, R. A. 636,120 Ways to have posttraumatic stress disorder. Perspect. Psychol. Sci. 8, 651–662. https://doi.org/10.1177/1745691613504115 (2013).
Konjevod, M. et al. Metabolomic and glycomic findings in posttraumatic stress disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 88, 181–193. https://doi.org/10.1016/j.pnpbp.2018.07.014 (2019).
Baenke, F., Peck, B., Miess, H. & Schulze, A. Hooked on fat: The role of lipid synthesis in cancer metabolism and tumour development. Dis. Model Mech. 6, 1353–1363. https://doi.org/10.1242/dmm.011338 (2013).
Dean, K. R. et al. Multi-omic biomarker identification and validation for diagnosing warzone-related post-traumatic stress disorder. Mol. Psychiatry 25, 3337–3349. https://doi.org/10.1038/s41380-019-0496-z (2020).
Mellon, S. H. et al. Metabolomic analysis of male combat veterans with post traumatic stress disorder. PLoS ONE 14, e0213839. https://doi.org/10.1371/journal.pone.0213839 (2019).
Karabatsiakis, A. et al. Metabolite profiling in posttraumatic stress disorder. J. Mol. Psychiatry 3, 2. https://doi.org/10.1186/s40303-015-0007-3 (2015).
Konjevod, M. et al. Metabolomics in posttraumatic stress disorder: Untargeted metabolomic analysis of plasma samples from Croatian war veterans. Free Radic. Biol. Med. 162, 636–641. https://doi.org/10.1016/j.freeradbiomed.2020.11.024 (2021).
Kuan, P.-F. et al. Metabolomics analysis of post-traumatic stress disorder symptoms in World Trade Center responders. Transl. Psychiatry 12, 174. https://doi.org/10.1038/s41398-022-01940-y (2022).
Muhie, S. et al. Integrated analysis of proteomics, epigenomics and metabolomics data revealed divergent pathway activation patterns in the recent versus chronic post-traumatic stress disorder. Brain Behav. Immun. 113, 303–316. https://doi.org/10.1016/j.bbi.2023.07.015 (2023).
Yehuda, R. et al. Post-traumatic stress disorder. Nat. Rev. Dis. Primers 1, 15057. https://doi.org/10.1038/nrdp.2015.57 (2015).
Giano, Z., Wheeler, D. L. & Hubach, R. D. The frequencies and disparities of adverse childhood experiences in the U.S.. BMC Public Health 20, 1327. https://doi.org/10.1186/s12889-020-09411-z (2020).
Bhargava, A. et al. Considering sex as a biological variable in basic and clinical studies: An endocrine society scientific statement. Endocr. Rev. 42, 219–258. https://doi.org/10.1210/endrev/bnaa034 (2021).
Miller, V. M. Why are sex and gender important to basic physiology and translational and individualized medicine?. Am. J. Physiol. Heart Circ. Physiol. 306, H781–H788. https://doi.org/10.1152/ajpheart.00994.2013 (2014).
Liu, S., Hagiwara, S. I. & Bhargava, A. Early-life adversity, epigenetics, and visceral hypersensitivity. Neurogastroenterol. Motil. https://doi.org/10.1111/nmo.13170 (2017).
Xie, H. et al. Adverse childhood experiences associate with early post-trauma thalamus and thalamic nuclei volumes and PTSD development in adulthood. Psychiatry Res. Neuroimaging 319, 111421. https://doi.org/10.1016/j.pscychresns.2021.111421 (2022).
Konjevod, M. et al. Plasma lipidomics in subjects with combat posttraumatic stress disorder. Free Radic. Biol. Med. 189, 169–177. https://doi.org/10.1016/j.freeradbiomed.2022.07.012 (2022).
Kuan, P. F. et al. Metabolomics analysis of post-traumatic stress disorder symptoms in World Trade Center responders. Transl. Psychiatry 12, 174. https://doi.org/10.1038/s41398-022-01940-y (2022).
Franzini, M. et al. Association between plasma gamma-glutamyltransferase fractions and metabolic syndrome among hypertensive patients. Sci. Rep. 7, 12003. https://doi.org/10.1038/s41598-017-12356-w (2017).
Lee, D. S. et al. Gamma glutamyl transferase and metabolic syndrome, cardiovascular disease, and mortality risk: The Framingham heart study. Arterioscler. Thromb. Vasc. Biol. 27, 127–133. https://doi.org/10.1161/01.ATV.0000251993.20372.40 (2007).
Rao, M. N. et al. Hyperinsulinemic response to oral glucose challenge in individuals with posttraumatic stress disorder. Psychoneuroendocrinology 49, 171–181. https://doi.org/10.1016/j.psyneuen.2014.07.006 (2014).
Slotte, J. P. Biological functions of sphingomyelins. Prog. Lipid Res. 52, 424–437. https://doi.org/10.1016/j.plipres.2013.05.001 (2013).
Tracey, T. J., Kirk, S. E., Steyn, F. J. & Ngo, S. T. The role of lipids in the central nervous system and their pathological implications in amyotrophic lateral sclerosis. Semin. Cell Dev. Biol. 112, 69–81. https://doi.org/10.1016/j.semcdb.2020.08.012 (2021).
Thiam, A. R., Farese, R. V. Jr. & Walther, T. C. The biophysics and cell biology of lipid droplets. Nat. Rev. Mol. Cell Biol. 14, 775–786. https://doi.org/10.1038/nrm3699 (2013).
Blusztajn, J. K., Liscovitch, M. & Richardson, U. I. Synthesis of acetylcholine from choline derived from phosphatidylcholine in a human neuronal cell line. Proc. Natl. Acad. Sci. U.S.A. 84, 5474–5477. https://doi.org/10.1073/pnas.84.15.5474 (1987).
Javaid, S. et al. Dynamics of choline-containing phospholipids in traumatic brain injury and associated comorbidities. Int. J. Mol. Sci. https://doi.org/10.3390/ijms222111313 (2021).
Yoon, J. H. et al. Brain lipidomics: From functional landscape to clinical significance. Sci. Adv. 8, eadc9317. https://doi.org/10.1126/sciadv.adc9317 (2022).
Stith, J. L., Velazquez, F. N. & Obeid, L. M. Advances in determining signaling mechanisms of ceramide and role in disease. J. Lipid Res. 60, 913–918. https://doi.org/10.1194/jlr.S092874 (2019).
Wolfe, J., Kimerling, R., Brown, P. J., Chresman, K. R. & Levin, K. Psychometric Review of the Life Stressor Checklist-Revised Vol. 149 (Sidran Press, 1996).
Blanchard, E. B., Jones-Alexander, J., Buckley, T. C. & Forneris, C. A. Psychometric properties of the PTSD checklist (PCL). Behav. Res. Ther. https://doi.org/10.1016/0005-7967(96)00033-2 (1996).
Blake, D. D. et al. The development of a clinician-administered PTSD scale. J. Trauma Stress 8, 75–90. https://doi.org/10.1007/bf02105408 (1995).
Spitzer, R. L., Williams, J. B., Gibbon, M. & First, M. B. The structured clinical interview for DSM-III–R (SCID): I. History, rationale, and description. Arch. Gen. Psychiatry 49, 624–629 (1992).
Buysse, D. J., ReynoldsMonk, C. F. T. H., Berman, S. R. & Kupfer, D. J. The pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 28, 193–213. https://doi.org/10.1016/0165-1781(89)90047-4 (1989).
Kales, A. & Rechtschaffen, A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects (United States Government Printing Office, 1968).
Cajka, T., Smilowitz, J. T. & Fiehn, O. Validating quantitative untargeted lipidomics across nine liquid chromatography-high-resolution mass spectrometry platforms. Anal. Chem. 89, 12360–12368. https://doi.org/10.1021/acs.analchem.7b03404 (2017).
Cajka, T. & Fiehn, O. Increasing lipidomic coverage by selecting optimal mobile-phase modifiers in LC–MS of blood plasma. Metabolomics. https://doi.org/10.1007/s11306-015-0929-x (2016).
Kind, T. et al. LipidBlast in silico tandem mass spectrometry database for lipid identification. Nat. Methods 10, 755–758. https://doi.org/10.1038/nmeth.2551 (2013).
Bishop, L. M., Shen, T. & Fiehn, O. Improving quantitative accuracy in nontargeted lipidomics by evaluating adduct formation. Anal. Chem. 95, 12683–12690. https://doi.org/10.1021/acs.analchem.3c01221 (2023).
Bhargava, A. & Knapp, J. D. Immunological misfiring and sex differences/similarities in early COVID-19 studies: Missed opportunities of making a real impact. Cells. https://doi.org/10.3390/cells12222591 (2023).
Acknowledgements
The authors thank Drs. Anne Richards, Madhu Rao, Aoife O’Donovan, and Lisa Talbot for sharing data sets and Thomas Metzler and Erin Madden for help with clinical measures data. They acknowledge Maryann Lenoci and Leslie Ruoff, PGST for their logistical and technical support. This research was supported by Discovery Awards from the U.S. Army Medical Research & Materiel Command (USAMRMC), the Telemedicine & Advanced Technology Research Center (TATRC), at Fort Detrick, MD (SI: W81XWH-05-2-0094, W81XWH-15-PRMRP-DA, and SI: W81XWH-15-PRMRP-DA), and grants from the National Institute for Mental Health (TCN: 5R01MH073978-04, 5R34MH077667-03). Support was also provided by Northern California, Institute for Research and Education (NCIRE), the Mental Illness Research and Education Clinical Center of the US Veterans Health Administration, the Clinical Research Center of the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1 RR024131 and donor funds to AB.
Author information
Authors and Affiliations
Contributions
AB, TCN, and SSI contributed to the design of the study. AB wrote the first draft of the manuscript with input from SSI, JDK. AB, JDK, TCN, OF and SSI revised the final manuscript. SSI collected patient data, OF provided and curated metabolomic data. AB and JDK extracted and analyzed the data. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
All other authors declare no competing interests. AB and JDK are co-founders of Aseesa Inc. The views, opinions and/or findings contained in this research are those of the authors and do not necessarily reflect the views of the Department of Defense, Department of Veteran Affairs, or NIH and should not be construed as an official DoD/Army/VA/NIH position, policy, or decision unless so designated by official documentation. No official endorsement should be made.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bhargava, A., Knapp, J.D., Fiehn, O. et al. An exploratory study on lipidomic profiles in a cohort of individuals with posttraumatic stress disorder. Sci Rep 14, 15256 (2024). https://doi.org/10.1038/s41598-024-62971-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-62971-7
- Springer Nature Limited