Abstract
Testicular adrenal rest tumor (TART) is a prevalent complication associated with congenital adrenal hyperplasia (CAH), culminating in gonadal dysfunction and infertility. Early hormonal intervention is preventive, but excessive glucocorticoid poses risks. Developing reliable methods for early TART diagnosis and monitoring is crucial. The present study aims to formulate a scoring system to identify high-risk infertility through analysis of TART ultrasound features. Grayscale and power Doppler ultrasound were employed in this retrospective study to evaluate testicular lesions in male CAH patients. Lesion assessment encompassed parameters such as range, echogenicity, and blood flow, and these were subsequently correlated with semen parameters. Results of 49 semen analyzes from 35 patients demonstrated a notable inverse correlation between lesion scores and both sperm concentration (rs = − 0.83, P < 0.001) and progressive motility (rs = − 0.56, P < 0.001). The ROC curve areas for evaluating oligospermia and asthenozoospermia were calculated as 0.94 and 0.72, respectively. Establishing a lesion score threshold of 6 revealed a sensitivity of 75.00% and specificity of 93.94% for oligospermia and a sensitivity of 53.85% and specificity of 100.00% for asthenozoospermia. These findings underscore the potential utility of incorporating ultrasound into routine CAH patient management, facilitating timely interventions to preserve male fertility.
Similar content being viewed by others
Introduction
Congenital adrenal hyperplasia (CAH) is a rare autosomal recessive disorder that may cause testicular involvement in 40% of patients1. The presence of testicular involvement may result in the compression of the seminiferous tubules, causing irreversible damage to the surrounding testicular tissue. Eventually, it may lead to gonadal dysfunction and infertility2. While early hormonal intervention can avoid damage to gonadal function3, excessive use of glucocorticoids to inhibit ACTH production can lead to serious hormone-related side effects involving musculoskeletal, gastrointestinal, cardiovascular, endocrine, neuropsychiatric, dermatological, ocular, and immune systems4,5. Thus, it is important to develop methods for early diagnosis and monitoring of testicular adrenal rest tumors (TART).
Ultrasound (US) is a non-ionizing imaging technique that preserves the functionality of the testis. According to the clinical practice guideline of the Endocrine Society, US is recommended for assessing TART in men with CAH6. Although both US and magnetic resonance imaging (MRI) are equally effective in visualizing TART lesions, US is preferred as the initial diagnostic modality due to its lower cost, convenience, and ability to promptly identify patients at a higher risk of infertility7. This early detection enables timely referral for surgical intervention or sperm cryopreservation, thus preserving fertility by preventing irreversible damage.
In this study, we aim to develop a scoring system for high-risk infertility identification via analysis of the ultrasound features of TART ultrasound.
Methods
Participants
Medical records of CAH male patients treated in our hospital from November 2014 to March 2024, were retrospectively reviewed. Each testis was considered as an individual research object. A clinical diagnosis of TART in CAH patients with testicular involvement was established when the testicular lesions showed a decrease or disappearance following the administration of glucocorticoids. Patients whose lesions did not respond to hormone therapy were referred to urologists to rule out the presence of other testicular neoplasms, as recommended by the Endocrine Society6.
The exclusion criteria was presented as follows:
-
(1)
Incomplete clinical data including the absence of hormone tests and ultrasound images;
-
(2)
Chromosomal abnormalities;
-
(3)
Ultrasound-detected imaging findings potentially impacting semen quality, including microlithiasis8, heterogeneous echogenicity of the testicles9, significant testicular atrophy (testicular volume ≤ 5 mL), and epididymal obstruction;
-
(4)
Decreased level of inhibin B (INH B) or elevated level of follicle-stimulating hormone (FSH) in patients without testicular lesions.
For patients without testicular involvement, a decreased level of INH B or an elevated level of FSH are considered indicators of primary spermatogenic dysfunction10,11. Thus, participants with above hormonal abnormalities were excluded from this study. All the enrolled patients in this study were performed US after the administration of glucocorticoids. This practice stems from the hereditary nature of CAH, often leading to diagnoses at birth for a significant portion of patients.
Study protocol
B-mode and power Doppler (PD) images of the testes were analyzed in axial and transverse sections for this study. In addition, levels of 17-hydroxyprogesterone (17-OHP), androstenedione (AD), adreno-cortico-tropic hormone (ACTH), and testosterone (T) were collected. Semen examination results including measurements of sperm concentration and progressive motility are also collected. On the same day as the hormone level tests and semen analyses, an US examination of the testicle was performed. All the aforementioned data were recorded for subsequent analysis.
Ultrasound
The US system used in this study was the Esaote Mylab 65, equipped with a superficial linear array probe LA523 (4–13 MHz), for scanning the scrotum. For each case, the B-mode settings were adjusted to the highest transmit frequency that provided a complete display of the lesion and surrounding tissue. Additionally, the power Doppler gain was increased until the appearance of artifactual Doppler signals, and then decreased to minimize spurious signals. The power Doppler parameters were as follows: frequency 6.3 MHz, pulse repetition frequency (PRF) 500 Hz, and wall filter 3. Trans-scrotal US examinations were performed by one of three experienced sonographers. Using the Prader orchidometer formula, the ultrasound measurements of testicular volume were calculated as follows: length (L) × width (W) × height (H) × 0.7112. The recorded US images were independently interpreted by two radiologists (ZQ.W and RH.W) who possessed over 8 years of experience in andrological ultrasound. In case of any discrepancies, the third radiologist (ZH.L) with 12 years of experience was consulted for resolution. All reviewers were blinded to the written reports and laboratory test results. Given that TART predominately affects the testicular interstitum13, indicators of range, echogenicity, and vascularity, reflecting the degree of interstitial fibrosis, were adopt to develop a scoring system. The scoring criteria are provided in Table 1. The total score for each examination is the average of the scores assigned to bilateral testicles, which is the sum of scores from the three parameters. It is important to note that calcifications unrelated to TART lesions, such as testicular microlithiasis, were not included in the scoring.
Semen quality evaluation
Semen analysis (SA) was performed using the Computer-Assisted Semen Analysis (CASA). In accordance with the guidelines set by the American Urological Association (AUA) and the American Society for Reproductive Medicine (ASRM)14, the lower reference limit for sperm concentration was determined to be 15 × 106/ml, and the lower reference limit for progressive motility was set at 32%.
Hormone measurement
Blood samples were procured at 08:00 A.M. ACTH levels were ascertained through the ELSA-ACTH immunoradiometric assay (Cisbio Bioassays, Codolet). Basel levels of 17-OHP and AD were determined utilizing the radioimmunoassay (Beckman Coulter Corp). INH B, FSH, and T levels were measured via the chemiluminescence immunoassay (Abbott Laboratories, Abbott Park, IL).
Sample size calculation
Due to the rarity of TART and the limited size of the population, it was not feasible to perform a sample size calculation for this study.
Statistic
Kendall’s tau-b correlation analysis between ultrasound scores and hormone levels was conducted across all patients. Additionally, for patients with semen examination results, Spearman correlation test or Kendall’s tau-b correlation analyses were carried out to explore the relationships among ultrasound scores, semen quality parameters, and hormone levels.
Receiver operating characteristic (ROC) curves were plotted to evaluate the validity of the scoring system, and the area under the curve (AUC) was calculated. The threshold that provided the best balance between sensitivity and specificity was determined using Youden’s method. The reliability of the scoring system was evaluated using the weighted Kappa statistic. The consistency of the score assigned by the two radiologists, was analyzed by Cohen κ. All statistical calculations were performed using IBM SPSS Statistics 25 software (IBM Corp.). Scatter plots with rugs were created using OriginPro 2021 software (OriginLab, Northampton), and a jitter plot was generated using the R programming language. The ROC curve analysis was conducted using MedCalc software (Version 20.014, MedCalc Software Ltd). A significance level of P < 0.05 was considered statistically significant.
Ethical approval
This retrospective study was conducted in accordance with the approval of the Ruijin Hospital Ethics Committee, Shanghai JiaoTong University School of Medicine (AF0406/14.0/2023-03-01). As an observational study, the written informed consent was waived by Ruijin Hospital Ethics Committee, Shanghai JiaoTong University School of Medicine. All experiments were performed in accordance with relevant named guidelines and regulations.
Results
Patient characteristics
Data were collected from 89 male CAH patients, 7 of 89 patients were excluded from the analysis due to insufficient clinical data. Among 82 CAH patients, 54.9% (45/82) were found to have testicular involvement, 84.4% (38/45) exhibited bilateral testis involvement, and 15.6% (7/45) had unilateral involvement. The median age of the patients at the time of their first ultrasonography was 22 years, with a range of 8 to 61 years (interquartile range 18.0, 35.0) (Table 2).
82 CAH patients having 168 ultrasound and hormone examinations were included for the correlation analyses with scores and hormone levels. 46/82 patients underwent a single ultrasound examination, involving a total of 92 testicles, while 35/82 patients underwent two or more ultrasound examinations, resulting in a cumulative count of 110 examinations involving 220 testicles. Additionally, 1/82 patient, who underwent 12 follow-up visits, underwent a right-sided orchiectomy to determine the pathology of the lesion prior to presentation at our center. Consequently, a total of 324 testicles were enrolled in the study.
Among the cohort of 82 patients examined, 24 individuals lacked available semen test results, 16 patients with ultrasound-detected imaging findings potentially impacting semen quality (9 testicular atrophy, 4 testicular microlithiasis, 2 heterogeneous testicular echogenicity, and 1 epididymal obstruction), 1 manifested a chromosomal abnormality, and 6 patients devoid of discernible testicular lesions with decreased INH B levels or elevated FSH levels were excluded. Finally, 35 patients with semen test results were included in the correlation analysis, with a total of 49 tests. Of these, 25 patients underwent a single examination, while 7 patients underwent two examinations, 2 patients underwent three examinations, and 1 patient underwent four examinations.
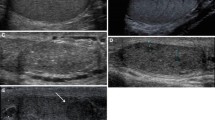
The flowchart of study population was presented in Fig. 1. Figures 2 and Fig. 3 present representative images of B-mode and Doppler ultrasound with varying scores.
Typical images of testicular adrenal rest tumor (TART) involvement range scores. (a–e) show testicular B-mode ultrasound images of five male patients with congenital adrenal hyperplasia (CAH), aged 28, 41, 13, 11 and 17, respectively. (a) Score 1: Not detectable by scrotal ultrasound; (b) Score 2: Aggregated oval lesions in the mediastinum, involving no more than 1/3 of the sagittal section of the testis; (c) Score 3: The lesion fills the mediastinum and does not involve more than 1/3 of the transverse and sagittal of the testis; (d) Score 4: Under the circumstance of all the mediastinal involvement, the lesion on the transverse involves more than 1/3 of the testis with residence of normal testicular tissue; (e) Score 5: Diffusely growing lesion, no normal testicular tissue visible.
Typical images of testicular adrenal rest tumor (TART) vascularity patterns scores. (a–c) depict testicular power Doppler images of male CAH patients aged 13, 41, and 38, respectively. (a) Score1: Abundant linear blood flow signals filling the entire lesion; (b) Score 2: Moderate linear blood flow signals occupying part of the lesion; (c) Score 3: No blood flow or very limited blood flow around lesion.
Structure of scoring system
A significant correlation has been identified between the range, echogenicity, and vascularity scores, and the parameters pertaining to semen analysis. The Spearman correlation coefficients (rs) for sperm concentration were recorded as − 0.72 (P < 0.01), − 0.74 (P < 0.01), and − 0.74 (P < 0.01), respectively. Furthermore, the Spearman correlation coefficients for motility were observed to be − 0.35 (P < 0.05), − 0.37 (P < 0.05), and − 0.38 (P < 0.05) correspondingly. Thus, the assignment of the scoring system included the three categories of range, echogenicity, and vascularity scores.
Diagnostic performance of US scoring system
Jitter plots demonstrated a negative correlation between sperm concentration and progressive motility with increasing TART lesion scores (Fig. 4). The Spearman correlation coefficients (rs) for sperm concentration and progressive motility were − 0.83 (P < 0.001) and − 0.56 (P < 0.001), respectively. The ROC analysis revealed that the AUC for the assessment of oligospermia based on the TART lesion score was 0.94 (95% CI 0.83, 0.99; P < 0.001), while the AUC for the assessment of asthenozoospermia was 0.72 (95% CI 0.57, 0.84; P = 0.003). A threshold value of 6 provided the best balance between sensitivity and specificity for both oligospermia and asthenozoospermia, yielding a sensitivity of 75.00% (95% CI 0.48, 0.93) and a specificity of 93.94% (95% CI 0.80, 0.99) for diagnosing oligospermia. For diagnosing asthenozoospermia, the threshold value of 6 resulted in a sensitivity of 53.85% (95% CI 0.33, 0.73) and a specificity of 100% (95% CI 0.85, 1.00) (Fig. 4). The relevant data of patients with multiple semen examinations have been listed in Supplemental data S.Table 2. In patients with scores less than 6, semen quality improved as the score decreased. However, in patients with scores greater than 6, semen quality did not improve significantly.
Inter-observer consistency assessment
The two radiologists who assigned lesion scores demonstrated excellent agreement, as evidenced by a weighted kappa value (κw) of 0.97(95%CI 0.96–0.99).
Hormone evaluation
Among all the analyses of hormonal levels, only testosterone (T) exhibited a weak negative correlation with increasing lesion scores (τb = − 0.19; P = 0.001), and a weak positive correlation with sperm concentration (τb = 0.24; P = 0.002) and progressive motility (τb = 0.18; P = 0.02). No statistically significant correlations were found between the lesion scores and the other hormone levels (Fig. 5). Additionally, there were no significant correlations observed between semen parameters and the hormone levels except T. The Kendall tau-b correlation coefficients and corresponding p-values are presented in Table 3.
Discussion
TART is a commonly observed condition in patients with CAH, and its occurrence cannot be prevented due to the negative feedback from impaired cortisol production, which allows hypersecreted ACTH to stimulate the proliferation of testicular adrenal remnant cells15. Claahsen-van der Grinten et al. described five distinct stages of the TART process based on histological features2: Stage (1) presence of adrenal rest cells in the rete testis; Stage (2) proliferation of adrenal rest cells; Stage (3) further growth of adrenal rest cells compressing the rete testis; Stage (4) Further hypertrophy and hyperplasia of adrenal rest cells leading to progressive obstruction of the rete testis may induce the initiation of fibrotic changes within the tumor and the occurrence of focal lymphocytic infiltration; Stage (5) finally, chronic obstruction resulting in destruction of surrounding testicular parenchyma and irreversible testicular damage.
In our current study, we found a similar range of US features among the included patients, which served as the basis for developing our scoring system. Our scoring system revealed a negative correlation between lesion score and sperm concentration (rs = − 0.83, P < 0.001) as well as progressive motility (rs = − 0.56, P < 0.001). Currently, the association between the size of TART lesion and ACTH levels remains controversial16. Our results showed that ACTH levels did not significantly correlate with the lesion scores (rs = − 0.003, P = 0.95). We hypothesize that prolonged mechanical obstruction of the seminiferous tubules may lead to dysfunction of spermatogenic function and peritubular fibrosis13. Consequently, adrenal rest cells may dedifferentiate over time and lose their dependence on ACTH2, explaining the lack of correlation between ACTH and semen quality in TART patients.
Effective management of TART requires long-term approaches. Hormone therapy and surgical treatment are currently the main methods for preserving fertility in TART patients. Early-stage high-dose glucocorticoid treatment can reduce tumor size, prevent compression of the testicular rete, slow down tumor growth, and inhibit the development of testicular fibrosis, thereby preventing infertility17. However, in cases of longstanding TART, high-dose glucocorticoids may not always restore testicular spermatogenesis and can have side effects18,19. Testis-sparing surgery has been suggested to maximize fertility preservation in TART patients19,20. However, this procedure does not improve gonadal function in patients with longstanding TART18,21,22. Therefore, timely treatment before the irreversible loss of gonadal function is crucial. Our scoring system, based on readily available and easy-to-operate B-mode and power Doppler ultrasound, may serve as a basis for making surgical decisions before the onset of gonadal failure in TART patients. For oligospermia, a threshold of 6 yielded a sensitivity of 75.00% (95% CI 0.48, 0.93) and specificity of 93.94% (95% CI 0.80, 0.99) while for asthenospermia, a sensitivity of 53.85% (95% CI 0.33, 0.73) and specificity of 100% (95% CI 0.85, 1.00) were obtained. This study found that the semen quality of patients with scores greater than 6 did not change significantly over time, likely due to irreversible damage to testicular spermatogenic function. These findings demonstrate that the scoring system can serve as a valuable reference for decisions related to fertility preservation. Previous studies have indicated the desirability but costliness of sperm cryopreservation during the early stages of TART, necessitating a careful assessment of the benefit-risk balance23. Therefore, TART patients with scores exceeding 6 may have the risk of irreversible gonadal failure, attributed to a combination of obstructive and non-obstructive factors, whereby neither hormonal therapy nor surgery can enhance spermatogenic function. A timely sperm cryopreservation should be recommended.
Ultrasound, as a tool for morphological attribution in the assessment of male infertility, is widely known to be independent of environmental factors, which may be able to make up for the shortcomings of semen as a tool for testicular functional assessment, which is affected by the environment. In this study, the developed scoring system may serve as an alternative tool for screening high-risk male infertility in CAH. Additionally, it can guide patients in taking full advantage of relevant assisted reproductive methods to preserve their fertility. This is particularly important as TART can occur in children, and most fertility problems in CAH arise during childhood24. Although semen analysis is an important non-invasive method of monitoring reproductive function, it is not easy to obtain semen in prepubertal patients. During follow-up, US serves as an excellent imaging modality for evaluating TART, especially when intratesticular masses cannot be palpated. US enables the early detection of these masses, facilitating timely hormone replacement therapy and the prevention of potential infertility. Thus, ultrasonography fills the gap in evaluating gonadal function in children.
While CAH is rare, it typically manifests in early childhood and may lead to a shortened life expectancy, necessitating ongoing healthcare services. This condition often impacts the entire family, creating ongoing stress. Although TART is relatively uncommon, it is prevalent in CAH patients and can strain healthcare and social services disproportionately. Excessive hormone therapy solely to mitigate testicular lesions incurs unnecessary costs and risks significant side effects for patients. With timely diagnosis and proper treatment, these individuals can lead fulfilling lives and contribute positively to society.
Limitations
TART, as a rare autosomal recessive disorder, the research sample size is limited. Meanwhile, as a retrospective study, this study absent the regular assessments conducted at consistent time intervals. To address these limitations and enhance the validity of the findings, further multicenter prospective studies involving larger sample sizes and regular follow-ups are warranted. Additionally, prospective studies are necessary to establish appropriate patient follow-up intervals over time in the future.
Conclusions
To the best of our knowledge, this scoring system is first developed by our group, and there are no previous studies have reported on it. In conclusion, US can be used as the preferred imaging modality for the evaluation of TART. US should be included as a routine examination in the follow-up of patients with CAH to ensure early intervention before gonadal failure to preserve male fertility.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Engels, M. et al. Testicular adrenal rest tumors: Current insights on prevalence, characteristics, origin, and treatment. Endocr. Rev. 40, 973–987 (2019).
Claahsen-Van der Grinten, H. et al. Testicular adrenal rest tumours in congenital adrenal hyperplasia. Best Pract. Res. Clin. Endocrinol. Metab. 23, 209–220 (2009).
Mendes-Dos-Santos, C. T. et al. Prevalence of testicular adrenal rest tumor and factors associated with its development in congenital adrenal hyperplasia. Horm. Res. Paediatr. 90, 161–168. https://doi.org/10.1159/000492082 (2018).
Mallappa, A. & Merke, D. P. Management challenges and therapeutic advances in congenital adrenal hyperplasia. Nat. Rev. Endocrinol. 18, 337–352. https://doi.org/10.1038/s41574-022-00655-w (2022).
Oray, M., Abu Samra, K., Ebrahimiadib, N., Meese, H. & Foster, C. S. Long-term side effects of glucocorticoids. Expert Opin. Drug Saf. 15, 457–465. https://doi.org/10.1517/14740338.2016.1140743 (2016).
Speiser, P. W. et al. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 103, 4043–4088 (2018).
Stikkelbroeck, N. M. et al. Testicular adrenal rest tumours in postpubertal males with congenital adrenal hyperplasia: Sonographic and MR features. Eur. Radiol. 13, 1597–1603. https://doi.org/10.1007/s00330-002-1786-3 (2003).
Xu, C. et al. The association between testicular microlithiasis and semen parameters in Chinese adult men with fertility intention: Experience of 226 cases. Urology 84, 815–820 (2014).
Spaggiari, G., Granata, A. R. & Santi, D. Testicular ultrasound inhomogeneity is an informative parameter for fertility evaluation. Asian J. Androl. 22, 302–308 (2020).
Pierik, F. H., Vreeburg, J. T., Stijnen, T., de Jong, F. H. & Weber, R. F. Serum inhibin B as a marker of spermatogenesis. J. Clin. Endocrinol. Metab. 83, 3110–3114 (1998).
Selice, R., Ferlin, A., Garolla, A., Caretta, N. & Foresta, C. Effects of endogenous FSH on normal human spermatogenesis in adults. Int. J. Androl. 34, e511–e517 (2011).
Sakamoto, H. et al. Testicular volume measurement: comparison of ultrasonography, orchidometry, and water displacement. Urology 69, 152–157. https://doi.org/10.1016/j.urology.2006.09.012 (2007).
Claahsen-van der Grinten, H. L., Stikkelbroeck, N., Falhammar, H. & Reisch, N. Management of endocrine disease: gonadal dysfunction in congenital adrenal hyperplasia. Eur. J. Endocrinol. 184, R85–R97 (2021).
Schlegel, P. N. et al. Diagnosis and treatment of infertility in men: AUA/ASRM guideline part II. J, Urol, 115, 62–69 (2021).
Delfino, M. et al. Testicular adrenal rest tumors in patients with congenital adrenal hyperplasia: Prevalence and sonographic, hormonal, and seminal characteristics. J, Ultrasound Med, 31, 383–388 (2012).
Dumic, M. et al. Testicular adrenal rest tumors in congenital adrenal hyperplasia—Cross-sectional study of 51 Croatian male patients. Eur. J. Pediatr. 176, 1393–1404 (2017).
Aycan, Z., Bas, V. N., Cetinkaya, S., Yilmaz Agladioglu, S. & Tiryaki, T. J. C. E. Prevalence and long-term follow-up outcomes of testicular adrenal rest tumours in children and adolescent males with congenital adrenal hyperplasia. Clin. Endocrinol. 78, 667–672 (2013).
Claahsen-van der Grinten, H. L. et al. Testicular adrenal rest tumors in adult males with congenital adrenal hyperplasia: Evaluation of pituitary-gonadal function before and after successful testis-sparing surgery in eight patients. J. Clin. Endocrinol. Metab. 92, 612–615 (2007).
Walker, B. R., Skoog, S. J., Winslow, B. H., Canning, D. A. & Tank, E. S. Testis sparing surgery for steroid unresponsive testicular tumors of the adrenogenital syndrome. J. Urol. 157, 1460–1463 (1997).
Tiryaki, T., Aycan, Z., Hucumenoglu, S. & Atayurt, H. Testis sparing surgery for steroid unresponsive testicular tumors of the congenital adrenal hyperplasia. Pediatr. Surg. Int. 21, 853–855. https://doi.org/10.1007/s00383-005-1547-x (2005).
Tiryaki, T., Aycan, Z., Hücümenoğlu, S. & Atayurt, H. Testis sparing surgery for steroid unresponsive testicular tumors of the congenital adrenal hyperplasia. Pediatric. Surg. Int. 21, 853–855 (2005).
Claahsen-van der Grinten, H. L. et al. Testicular adrenal rest tumors in patients with congenital adrenal hyperplasia can cause severe testicular damage. Fertil. Steril. 89, 597–601 (2008).
Chougar, T. et al. Sperm cryopreservation in young males with congenital adrenal hyperplasia (CAH). Clin. Endocrinol. (Oxf) 97, 860–862. https://doi.org/10.1111/cen.14792 (2022).
Martinez-Aguayo, A. et al. Testicular adrenal rest tumors and Leydig and Sertoli cell function in boys with classical congenital adrenal hyperplasia. J. Clin. Endocrinol. Metab. 92, 4583–4589. https://doi.org/10.1210/jc.2007-0383 (2007).
Acknowledgements
Our gratitude goes to the patients, medical staff, and researchers involved. Special acknowledgment goes to Shanghai Clinical Center for Endocrinology and Metabolic Diseases for their accurate hormone evaluations.
Author information
Authors and Affiliations
Contributions
The author Z.Q.W. contributed to article drafting and data interpretation. R.H.W. contributed to study design and data analysis. X.W., S.C.Z., M.L. and Y.F.Y. contributed to data acquisition. Z.H.L. contributed to data analysis. S.Y.S. revised it critically for important intellectual content. W.W.Z. contributed to article revision and approval for submission of the final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, Z., Wang, R., Wang, X. et al. Infertility risk assessment with ultrasound in congenital adrenal hyperplasia male patients. Sci Rep 14, 12058 (2024). https://doi.org/10.1038/s41598-024-62954-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-62954-8
- Springer Nature Limited