Abstract
Here, we explore the application of Raman spectroscopy for the assessment of plant biodiversity. Raman spectra from 11 vascular plant species commonly found in forest ecosystems, specifically angiosperms (both monocots and eudicots) and pteridophytes (ferns), were acquired in vivo and in situ using a Raman leaf-clip. We achieved an overall accuracy of 91% for correct classification of a species within a plant group and identified lignin Raman spectral features as a useful discriminator for classification. The results demonstrate the potential of Raman spectroscopy in contributing to plant biodiversity assessment.
Similar content being viewed by others
Introduction
Diverse plant ecosystems tend to capture resources more efficiently, cycle nutrients more quickly and are more stable over time1. Techniques to rapidly assess plant biodiversity are important as we study and respond to rapid changes in ecosystem biodiversity caused by human development and climate change. Plant spectral diversity has recently emerged as a biodiversity metric that exploits the dissimilarity of leaf-level spectra which capture functional and phylogenetic differences among plant species and can be used as a metric for biodiversity and a measure for critical ecosystem function1. To date, spectral diversity measures have focused on variations in hyperspectral (400–2200 nm) reflectance acquired from plant leaves through remote sensing. Advances in imaging systems have led to the development of field-based phenotyping platforms to collect high-throughput trait measurements over a large field of view. With these systems, plants’ physical and physiological features can be interrogated non-destructively. Hyperspectral reflectance on unmanned aerial platforms and by satellite remote sensing allows for scalable plant phenotyping and disease monitoring. Data from these remote platforms can be paired with portable/proximal analytical devices to provide reference data sets free of atmospheric effects, solar illumination, variable orientation due to the three-dimensional structure of the plants2. Here, we propose Raman spectroscopy as a tool for proximal sensing of leaf chemistry that can be used to assess biodiversity.
Raman spectroscopy has emerged as a new tool for field-based phenotyping—particularly for early detection of plant stress3,4,5,6,7,8,9,10,11,12,13,14,15. This involves detecting bacterial infections12,16, insect infestations17, fungal infections7,18, nutrient deficiencies15,19,20, and various other pathogens17,21. These various stresses manifest through changes in the various metabolites observed in leaf Raman spectra: these include carotenoids, pectin, lignin, carbohydrates such as starch, amino acids, and nitrate.
Farber et al. have used a hand-held Raman spectrometer with partial least square discriminant analysis (PLS-DA) to distinguish poison ivy from weeds, grasses, and trees22. Here, we extend this work to a wider range of vascular plants with a specific focus on diverse classes and perform the survey of Raman spectra across multiple plant species with the goal of identifying differences between diverse plant species and classes. The primary plant groups in the plant kingdom include Bryophytes, Pteridophytes, Gymnosperms, and Angiosperms23,24,25,26. In this study, we focused on vascular plant groups commonly found in forest ecosystems, specifically angiosperms (both monocots and eudicots) and pteridophytes (ferns).
Spectral collection and analysis
In this work, we have made use of the portable, flexible, and field-friendly nature of portable Raman spectroscopy for on-site plant identification in various environments, including, urban farms/gardens or agricultural fields. The leaf clip Raman sensor used here consists of a Raman Fiber probe connected to a portable Raman instrument using an 830 nm excitation laser15. The laser power was adjusted to 130 mW to minimize sample damage and fluorescence interference. The laser system (830 nm excitation, Innovative Photonic Systems USA) and spectrometer setup (Avantes HSC Symmetrical Czerny-Turner) were combined within a compact, portable instrument enclosure, which featured software controls, as developed by TechnoSpex Pte Ltd (as depicted in Fig. 1b15). This integrated instrument covered a spectral range extending from 100 to 2000 cm−1, achieving a spectral resolution of 10 cm−1.
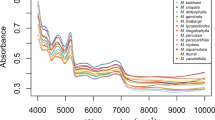
(a) The classification of vascular plants into major classes within the plant kingdom. The classification is based on various criteria such as morphological features, reproductive structures, evolutionary history, and genetic relationships. The major classes typically include Liliopsida, Magnoliopsida, and Polypodiopsida. The study focuses on the Raman analysis of ferns (polypodiopsida), along with monocots (liliopsida) and eudicots (magnoliopsida). (b) A mobile leaf clip Raman sensor used in this study for outdoor plants under full light conditions15. (c) A 9-week-old ‘lucky bamboo’ monocot showcasing its Raman spectra (black spectra). (d) ‘Kai Lan’ eudicot at 3 weeks of age and the associated spectra (blue spectra). (e) 10-week-old ‘crocodile’ fern alongside its corresponding Raman spectra (red spectra). Raman spectra were collected from five distinct leaves, with two measurements taken at separate locations on each leaf. The resulting spectra include a total of ten measurements for each type of plant, represented in light colours (red, black, blue), while the corresponding averages are depicted in darker shades (red, black, blue). All Raman measurements were conducted at a laser power of 130 mW with a 10 s acquisition time.
The Raman leaf-clip sensor was utilised for in vivo and in situ acquisition of Raman spectra under indoor growing conditions for three different types of plants: ferns, eudicots, and monocots. Raman spectra were collected on two locations (left and right side)13 for five leaves on each individual plant with an integration time of 10 s. At each location, five Raman spectra were acquired on the adaxial side of the leaf. The cosmic ray interference was detected and eliminated within the collected spectra. After the cosmic ray removal, a smoothing procedure was applied to the individual spectra across their respective wavelength domains using the Savitzky–Golay filter function27,28, a well-established method for spectral noise reduction. A representative sample spectrum was obtained by computing the mean values of the five filtered and smoothed spectra at each wavelength. To generate the Raman spectrum showcased in the results section, remaining fluorescence artifacts were effectively removed using a positive residual-style polynomial subtraction technique27. All these data processing procedures were implemented within the MATLAB and the calibration of the Raman shift was validated through polystyrene spectra (a well-known and widely documented Raman spectrum29).
For the Raman analysis, plants ranging from 3 to 15 weeks’ old grown in soil were chosen, which have different leaf shapes, sizes, and textures. Four Monocots namely, Anthurium andraeanum (flamingo lily), Dracaena sanderiana (lucky bamboo), Pandanus amaryllifolius (pandan), and Chamaedorea elegans (parlour palm); Three Eudicots: Gardenia jasminoides (cape jasmine), Brassica oleracea (Kai Lan), and Brassica rapa (Pak Choi); and Four Ferns: Asplenium nidus (bird’s nest), Microsorum musifolium (crocodile), Asplenium antiquum (Osaka) and Microsorum punctatum (fish-tail). All plants used in this study had three biological replicates and have been purchased from nursery (Candy Floriculture Pte Ltd) except the two Eudicots (Kai Lan and Pak Choi) that were grown in our Disruptive and Sustainable Technologies for Agricultural Precision (DiSTAP) Lab. The plants were not at the same stage of vegetation. Three biological replicates of cape jasmine plants were in the seed stage, while three biological replicates of flamingo lily were in the flowering stage. Overall, we have used 12 Monocots, 9 Eudicots and 12 Ferns for the Raman measurements. Figure 1a, c–e shows the plant kingdom classification and different species used in this study for Raman measurements along with their Raman spectra.
Statement for research involving plants
Plants were procured from a nursery, and few plants were cultivated in our laboratory. All experimental procedures adhered to the relevant guidelines and regulations.
To further explore the distinctions between plant species based on spectral features, we employed linear discriminant analysis (LDA), a statistical technique widely applied for pattern recognition and classification30,31. This involved identifying plant species based on their characteristic attributes. We used Scikit-learn—a machine learning module in Python for performing LDA32,33. For LDA model training, we utilized a dataset comprising 7 ferns, 7 monocots, and 6 eudicots. For the test set, we employed 5 ferns, 5 monocots, and 3 eudicots. Figure 2a shows the Raman spectra of the entire dataset i.e. 12 ferns (3 biological replicates with 4 different species), 12 monocots (3 biological replicates with 4 different species) and 9 eudicots (3 biological replicates with 3 different species). Analyzing the Raman spectra across all classes presented challenges in identifying notable variations in peak intensities. This implies that distinguishing peak variability among classes is difficult, and the collective contribution of each spectral segment contributes to the overall accuracy of the LDA model. Each plant class consists of 1 spectrum, and each spectrum presents the Raman scattering specific to the respective plant species, offering valuable insights into their molecular composition and structural features. Figure 2b displays the LDA logistic regression model for the test set across the complete spectral range (400–1750 cm−1) with respect to the two discriminant functions, LDA1 and LDA2. Notably, LDA effectively distinguishes among the three distinct plant types, achieving an accuracy of 91% in the test set and a perfect 100% accuracy in the training set. The proximity of the monocots and eudicots in Fig. 2b suggests they are part of the same group of angiosperms. Figure 2c provides an overview of the performance metrics employed to evaluate the LDA model’s effectiveness in plant identification, including accuracy, precision, recall and f1-score for the test set. Furthermore, Fig. 2d presents the confusion matrix, displaying the count of true positives, true negatives, false positives, and false negatives for each plant class, offering a comprehensive understanding of the model's performance in plant identification. The confusion matrix illustrates a minor misclassification between ferns and monocots (9 misclassifications), between monocots and eudicots (2 misclassifications), and between eudicots and ferns (0 misclassifications).
This figure shows the application of logistic regression on the test set within the context of Linear Discriminant Analysis (LDA) when entire Raman spectra range (400–1750 cm−1) is selected. (a) Raman spectra of 12 ferns, 12 monocots and 9 eudicots for both test and training sets. (b) The test set plot showcases the predictive modelling and evaluation of the test set using logistic regression, providing insights into the classification performance and accuracy of the LDA model. 5 ferns, 5 monocots, and 3 eudicots have been used for the test set. (c) The performance evaluation of the Linear Discriminant Analysis (LDA) model, showcasing accuracy, precision, recall determining its effectiveness in distinguishing different plant species. LDA successfully distinguishes among the three distinct plant types, with an accuracy of 91% in the test set and 100% accuracy in the training set. (d) The confusion matrix for the linear discriminant analysis (LDA) model provides a detailed breakdown of true positive, true negative, false positive, and false negative classifications, offering a complete assessment of the model's predictive performance for distinguishing plant species.
To identify the class of molecules that provide the clearest discrimination between plant classes, we have repeated the classifier construction using spectral ranges restricted to individual peaks. Figure 3a displays the Raman peaks within the lignin region for ferns (in red), monocots (in black), and eudicots (in blue). Within the classification of angiosperms, both eudicots and monocots exhibit similar lignin compositions, whereas ferns lack the p-hydroxyphenyl component. Thus, we observed minimal differences in lignin peak intensities between eudicots and monocots when contrasted with those observed in ferns. A discussion on the lignin composition in angiosperms (monocots and eudicots) and ferns can be found in the results section of this manuscript. Figure 3b shows plots of the LDA logistic regression model specifically to the lignin region (1580–1630 cm−1) to understand the impact of the lignin peak in distinguishing the three classes. Significantly, the LDA model effectively distinguishes among the three distinct plant types, attaining an accuracy of 81% in the test set and 81.2% accuracy in the training set (Fig. 3b). The decrease in accuracy suggests that the model performs better when considering the entire spectral range; however, the lignin region indeed plays a substantial role in distinguishing different classes over other peaks. Figure 3c provides performance metrics for the lignin region, assessing the LDA model’s efficacy in plant identification, including accuracy, precision, recall, and f1-score for the test set; and Fig. 3d presents the confusion matrix, describing the count of true positives, true negatives, false positives, and false negatives for each plant class.
(a) Enlarged lignin peak region (1580–1630 cm−1) for all plant species: red for ferns, black for monocots and blue for eudicots. (b) The logistic regression plot of the test set providing insights into the classification performance and accuracy of the LDA model. (c) The performance evaluation of the LDA model. LDA distinguishes among the three distinct plant types, with an accuracy of 81% in the test set when only the lignin region is considered. (d) The confusion matrix for the LDA model for the lignin region.
Statement of consent
The informed consent was obtained from the participant researcher for publication of photograph in the supplementary figure (Fig. S2).
Results and discussion
The Raman leaf clip sensor was able to acquire Raman spectra from all the different types of plant leaves. Figure 2a shows the Raman spectra that resulted from different plant classes and the Raman spectra shows several common Raman peaks. Based on Raman spectrum of carotenoid chemical standards, we identified the 1525 cm−1, 1155 cm−1 and 1000 cm−1 peaks to be present in all plant species samples (Table 1). The observed Raman peak at 1525 cm−1 is due to a C=C stretching vibration (ν1), the 1155 cm−1 peak is a C–C stretching vibration (ν2), and the weak peak at 1000 cm−1 is due to C–CH3 stretching (ν3)34. The observed Raman peak at 1604 cm−1 is associated with the stretching vibrations of the aromatic rings in lignin, which is a characteristic feature of lignin’s structure35,36,37,38. The other peaks associated with lignin in Raman spectroscopy are typically found in the following regions: 1265–1326 cm−1 (guaiacyl ring breathing and C–C stretching); Peaks in this region are related to the guaiacyl ring structure present in lignin39,40,41. The region between 1440 and 1488 cm−1 is associated with bending and deformation vibrations of C–H bonds in lignin39,40.
Lignin is a complex and rigid polymer that plays a critical role in the structural support and rigidity of plant cell walls36,51. It is a major component of the secondary cell walls in vascular plants, including both ferns (non-flowering plants) and angiosperms (flowering plants). The composition and structure of lignin can vary between different plant species and even within different parts of the same plant. The lignin composition in ferns mainly consists of guaiacyl (G) and syringyl (S) monolignols, with guaiacyl being the dominant type52,53,54. Angiosperms have a more complex lignin composition compared to ferns. Lignin in angiosperms contains three main types of monolignols: guaiacyl (G), syringyl (S), and p-hydroxyphenyl (H)51,55. The proportion and arrangement of these monolignols can vary between different plant tissues and species51,55. For example, hardwoods primarily contain G and S lignin, while softwoods mainly consist of G lignin52. The composition of lignin in angiosperms can also vary depending on the developmental stage of the plant, the specific tissue (e.g., xylem, phloem), and environmental factors38,52.
Conclusion
This study demonstrates the classification of plant species using Raman spectroscopy. It showcases the potential of Raman spectroscopy, when coupled with appropriate data analysis and classification techniques such as LDA, can be a powerful species-independent analytical tool for accurate and rapid identification of plant species based on their unique spectral fingerprint obtained from Raman scattering. The utilization of Linear Discriminant Analysis (LDA) in combination with a comprehensive dataset of Raman spectra enabled a detailed exploration of the unique features characterizing different plant species. The LDA plot presented in Fig. 2b, depicts a clear separation among ferns, monocots, and eudicots based on their spectral attributes. This visual representation highlights the potential of LDA as a powerful tool for precise plant species identification, essential for a wide array of applications in the realm of botanical research and beyond. Moreover, the LDA model achieved a high accuracy of 91%, indicating its effectiveness in distinguishing plant species based on the lignin peak. It shows a well-defined separation of the plant species, suggesting the efficacy of the selected features (particularly lignin) for plant identification using LDA.
As demonstrated here, Raman spectroscopy is a truly species agnostic tool for assessment of leaf chemical composition. The extension of this initial work to stand-off Raman spectroscopy with a large field-of-view would allow for the rapid classification of plant species and the assessment of plant biodiversity in a variety of ecosystems. Such a chemically-specific, optical instrument would complement existing tools and protocols for the assessment of ecosystem biodiversity.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author upon reasonable request.
References
Schweiger, A. K. et al. Plant spectral diversity integrates functional and phylogenetic components of biodiversity and predicts ecosystem function. Nat. Ecol. Evol. 2, 976–982 (2018).
Cavender-Bares, J. et al. Associations of leaf spectra with genetic and phylogenetic variation in oaks: Prospects for remote detection of biodiversity. Remote Sens. 8, 221 (2016).
Lew, T. T. S. et al. Species-independent analytical tools for next-generation agriculture. Nat. Plants 6, 1408–1417. https://doi.org/10.1038/s41477-020-00808-7 (2020).
Heredia-Guerrero, J. A. et al. Infrared and Raman spectroscopic features of plant cuticles: A review. Front. Plant Sci. 5, 305 (2014).
Saletnik, A., Saletnik, B. & Puchalski, C. Overview of popular techniques of Raman spectroscopy and their potential in the study of plant tissues. Molecules 26, 1537 (2021).
Schulz, H. & Baranska, M. Identification and quantification of valuable plant substances by IR and Raman spectroscopy. Vib. Spectrosc. 43, 13–25 (2007).
Weng, S. et al. Advanced application of Raman spectroscopy and surface-enhanced Raman spectroscopy in plant disease diagnostics: A review. J. Agric. Food Chem. 69, 2950–2964 (2021).
Farber, C. et al. Raman spectroscopy enables non-invasive identification of peanut genotypes and value-added traits. Sci. Rep. 10, 7730 (2020).
Payne, W. Z. & Kurouski, D. Raman spectroscopy enables phenotyping and assessment of nutrition values of plants: a review. Plant Methods 17, 1–20 (2021).
Conrad, A. O. & Bonello, P. Application of infrared and Raman spectroscopy for the identification of disease resistant trees. Front. Plant Sci. 6, 1152 (2016).
Higgins, S., Serada, V., Herron, B., Gadhave, K. R. & Kurouski, D. Confirmatory detection and identification of biotic and abiotic stresses in wheat using Raman spectroscopy. Front. Plant Sci. 13, 1035522 (2022).
Wang, L. et al. Applications of Raman spectroscopy in bacterial infections: principles, advantages, and shortcomings. Front. Microbiol. 12, 683580 (2021).
Huang, C. H. et al. Early diagnosis and management of nitrogen deficiency in plants utilizing Raman spectroscopy. Front. Plant Sci. 11, 663 (2020).
Altangerel, N. et al. In vivo diagnostics of early abiotic plant stress response via Raman spectroscopy. Proc. Natl. Acad. Sci. 114, 3393–3396 (2017).
Gupta, S. et al. Portable Raman leaf-clip sensor for rapid detection of plant stress. Sci. Rep. 10, 20206. https://doi.org/10.1038/s41598-020-76485-5 (2020).
Farber, C., Shires, M., Ong, K., Byrne, D. & Kurouski, D. Raman spectroscopy as an early detection tool for rose rosette infection. Planta 250, 1247–1254 (2019).
Mandrile, L. et al. Nondestructive Raman spectroscopy as a tool for early detection and discrimination of the infection of tomato plants by two economically important viruses. Anal. Chem. 91, 9025–9031 (2019).
Farber, C., Mahnke, M., Sanchez, L. & Kurouski, D. Advanced spectroscopic techniques for plant disease diagnostics. A review. TrAC Trends Anal. Chem. 118, 43–49 (2019).
Krimmer, M., Farber, C. & Kurouski, D. Rapid and noninvasive typing and assessment of nutrient content of maize kernels using a handheld Raman spectrometer. ACS Omega 4, 16330–16335 (2019).
Payne, W. Z. & Kurouski, D. Raman-based diagnostics of biotic and abiotic stresses in plants. A review. Front. Plant Sci. 11, 616672 (2021).
Farber, C. & Kurouski, D. Detection and identification of plant pathogens on maize kernels with a hand-held Raman spectrometer. Anal. Chem. 90, 3009–3012 (2018).
Farber, C., Sanchez, L. & Kurouski, D. Confirmatory non-invasive and non-destructive identification of poison ivy using a hand-held Raman spectrometer. RSC Adv. 10, 21530–21534. https://doi.org/10.1039/D0RA03697H (2020).
Reece, J. B. et al. Campbell Biology 9th edn. (Pearson, 2011).
Raven, P. H., Evert, R. F. & Eichhorn, S. E. Biology of Plants (Freeman, 2005).
Bramwell, D. How many plant species are there. Plant Talk 28, 32–34 (2002).
Pimm, S. & Joppa, L. How many plant species are there, where are they, and at what rate are they going extinct?. Ann. Mo. Bot. Gard. 100, 170–176. https://doi.org/10.3417/2012018 (2015).
Lieber, C. A. & Mahadevan-Jansen, A. Automated method for subtraction of fluorescence from biological Raman spectra. Appl. Spectrosc. 57, 1363–1367 (2003).
Barton, S. J., Ward, T. E. & Hennelly, B. M. Algorithm for optimal denoising of Raman spectra. Anal. Methods 10, 3759–3769 (2018).
Creely, C., Singh, G. & Petrov, D. Dual wavelength optical tweezers for confocal Raman spectroscopy. Opt. Commun. 245, 465–470 (2005).
Balakrishnama, S. & Ganapathiraju, A. Linear discriminant analysis-a brief tutorial. Inst. Signal Inf. Process. 18, 1–8 (1998).
Tharwat, A., Gaber, T., Ibrahim, A. & Hassanien, A. E. Linear discriminant analysis: A detailed tutorial. AI Commun. 30, 169–190 (2017).
Kluyver, T. et al. Jupyter notebooks-a publishing format for reproducible computational workflows. Elpub 2016, 87–90 (2016).
Pedregosa, F. et al. Scikit-learn: Machine learning in Python. J. Mach. Learn. Res. 12, 2825–2830 (2011).
De Gelder, J., De Gussem, K., Vandenabeele, P. & Moens, L. Reference database of Raman spectra of biological molecules. J. Raman Spectrosc. 38, 1133–1147 (2007).
Gierlinger, N. & Schwanninger, M. Chemical imaging of poplar wood cell walls by confocal Raman microscopy. Plant Physiol. 140, 1246–1254 (2006).
Gierlinger, N., Keplinger, T. & Harrington, M. Imaging of plant cell walls by confocal Raman microscopy. Nat. Protoc. 7, 1694–1708 (2012).
Zeng, Y. et al. In situ label-free imaging of hemicellulose in plant cell walls using stimulated Raman scattering microscopy. Biotechnol. Biofuels 9, 1–16 (2016).
Sanaeifar, A. et al. A spatial-temporal analysis of cellular biopolymers on leaf blight-infected tea plants using confocal Raman microspectroscopy. Front. Plant Sci. 13, 846484 (2022).
Rashid, T., Kait, C. F. & Murugesan, T. A “Fourier transformed infrared” compound study of lignin recovered from a formic acid process. Procedia Eng. 148, 1312–1319 (2016).
Bock, P. et al. Infrared and Raman spectra of lignin substructures: Dibenzodioxocin. J. Raman Spectrosc. 51, 422–431 (2020).
Perera, P. N., Schmidt, M., Chiang, V. L., Schuck, P. J. & Adams, P. D. Raman-spectroscopy-based noninvasive microanalysis of native lignin structure. Anal. Bioanal. Chem. 402, 983–987 (2012).
Edwards, H. G. M., Farwell, D. W. & Webster, D. FT Raman microscopy of untreated natural plant fibres. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 53, 2383–2392. https://doi.org/10.1016/S1386-1425(97)00178-9 (1997).
Synytsya, A., Čopı́ková, J., Matějka, P. & Machovič, V. Fourier transform Raman and infrared spectroscopy of pectins. Carbohydr. Polym. 54, 97–106. https://doi.org/10.1016/S0144-8617(03)00158-9 (2003).
Kurouski, D., Van Duyne, R. P. & Lednev, I. K. Exploring the structure and formation mechanism of amyloid fibrils by Raman spectroscopy: A review. Analyst 140, 4967–4980. https://doi.org/10.1039/C5AN00342C (2015).
Tschirner, N. et al. The anomaly of the ν1-resonance Raman band of bβ-carotene in solution and in photosystem I and II. Physica Status Solidi (b) 246, 2790–2793. https://doi.org/10.1002/pssb.200982299 (2009).
Schulz, H., Baranska, M. & Baranski, R. Potential of NIR-FT-Raman spectroscopy in natural carotenoid analysis. Biopolymers 77, 212–221. https://doi.org/10.1002/bip.20215 (2005).
Wiercigroch, E. et al. Raman and infrared spectroscopy of carbohydrates: A review. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 185, 317–335. https://doi.org/10.1016/j.saa.2017.05.045 (2017).
Cao, Y., Shen, D., Lu, Y. & Huang, Y. A Raman-scattering study on the net orientation of biomacromolecules in the outer epidermal walls of mature wheat stems (Triticum aestivum). Ann. Bot. 97, 1091–1094. https://doi.org/10.1093/aob/mcl059 (2006).
Yu, M. M. L., Schulze, H. G., Jetter, R., Blades, M. W. & Turner, R. F. B. Raman microspectroscopic analysis of triterpenoids found in plant cuticles. Appl. Spectrosc. 61, 32–37. https://doi.org/10.1366/000370207779701352 (2007).
Devitt, G., Howard, K., Mudher, A. & Mahajan, S. Raman spectroscopy: An emerging tool in neurodegenerative disease research and diagnosis. ACS Chem. Neurosci. 9, 404–420. https://doi.org/10.1021/acschemneuro.7b00413 (2018).
Ralph, J., Lapierre, C. & Boerjan, W. Lignin structure and its engineering. Curr. Opin. Biotechnol. 56, 240–249 (2019).
Katahira, R., Elder, T. J. & Beckham, G. T. A brief introduction to lignin structure. In Lignin Valorization: Emerging Approaches (ed. Beckham, G. T.) (Royal Society of Chemistry, 2018).
Weng, J.-K., Li, X., Stout, J. & Chapple, C. Independent origins of syringyl lignin in vascular plants. Proc. Natl. Acad. Sci. 105, 7887–7892 (2008).
Agarwal, U. P. An overview of Raman spectroscopy as applied to lignocellulosic materials. In Advances in Lignocellulosics Characterization, 201–225 (1999).
Sun, R. C. Lignin source and structural characterization. ChemSusChem 13, 4385–4393 (2020).
Acknowledgements
This research was supported by the National Research Foundation (NRF), Prime Minister’s Office, Singapore under its Campus for Research Excellence and Technological Enterprise (CREATE) program. The Disruptive and Sustainable Technology for Agricultural Precision (DiSTAP) is an interdisciplinary research group (IRG) of the Singapore MIT Alliance for Research and Technology (SMART) Centre supported by the National Research Foundation (NRF), Prime Minister’s Office, Singapore under its Campus for Research Excellence and Technological Enterprise (CREATE) program.
Author information
Authors and Affiliations
Contributions
E.J. wrote the main manuscript text, designed experiments, prepared figures, obtained plant samples, conducted data generation, and analysed data. M.R. assisted in experiment design and data generation for the initial set of plants. P.K.J. contributed to scientific discussions, and prepared Fig. 1c–e. G.P.S. participated in scientific discussions and created the MATLAB script for spectral analysis. R.J.R. revised the manuscript, conceptualized the study, supervised the project, and secured funding. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jain, E., Rose, M., Jayapal, P.K. et al. Harnessing Raman spectroscopy for the analysis of plant diversity. Sci Rep 14, 12692 (2024). https://doi.org/10.1038/s41598-024-62932-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-62932-0
- Springer Nature Limited