Abstract
Antarctic biodiversity is affected by seasonal sea-ice dynamics driving basal resource availability. To (1) determine the role of intraspecific dietary variability in structuring benthic food webs sustaining Antarctic biodiversity, and (2) understand how food webs and the position of topologically central species vary with sea-ice cover, single benthic individuals’ diets were studied by isotopic analysis before sea-ice breakup and afterwards. Isotopic trophospecies (or Isotopic Trophic Units) were investigated and food webs reconstructed using Bayesian Mixing Models. As nodes, these webs used either ITUs regardless of their taxonomic membership (ITU-webs) or ITUs assigned to species (population-webs). Both were compared to taxonomic-webs based on taxa and their mean isotopic values. Higher resource availability after sea-ice breakup led to simpler community structure, with lower connectance and linkage density. Intra-population diet variability and compartmentalisation were crucial in determining community structure, showing population-webs to be more complex, stable and robust to biodiversity loss than taxonomic-webs. The core web, representing the minimal community ‘skeleton’ that expands opportunistically while maintaining web stability with changing resource availability, was also identified. Central nodes included the sea-urchin Sterechinus neumayeri and the bivalve Adamussium colbecki, whose diet is described in unprecedented detail. The core web, compartmentalisation and topologically central nodes represent crucial factors underlying Antarctica’s rich benthic food web persistence.
Similar content being viewed by others
Introduction
The ability of a biotic community to resist or adapt to external pressures is closely related to the level of web complexity, which depends on predictable energy supply1,2,3,4,5,6,7,8,9,10. Climate-induced environmental changes could lead to a mismatch between food supply and demand, thus altering the food web structure11,12,13.
In Antarctica, the rich marine communities, which are highly adapted to seasonal variations in sea ice10,14,15,16,17,18, are increasingly threatened by environmental modifications. These changes are primarily due to climate change, which is leading, in addition to increasing temperatures, to variation in sea-ice extent, light regimes and nutrient availability for primary producers15,19,20. Resource availability for Antarctic communities depends on seasonal cycles of sea ice11,12,16,21,22,23,24, to which the other factors are closely linked. Hence, studying food web structures under different sea-ice conditions increases our understanding of the key mechanisms underlying the persistence of complex communities and their response to environmental variation including climate change17,25,26.
Reconstructing the food webs of complex communities presents challenges9,27. These include high biodiversity coupled with trophic generalisation and the wide range of exploitable food resources. Further, high variability in resource use by consumers, even within populations, and uncertainty about their taxonomic identity, can make it harder to identify the trophic links between species12,13,28,29,30. In addition, species’ functional traits such as the variable feeding behaviour of individuals, which determine species’ responses to changes in environmental conditions11,31,32,33, can influence community stability9,30,31,33,34. However, their role in determining food web structure is still uncertain or completely unknown9,33,35,36,37. With these uncertainties, the persistence of complex communities when some theory suggests such systems will be unstable remains a paradox3,38,39. This challenges our ability to predict their response to current and future environmental change40.
The development of techniques and methods based on quantitative approaches has led to increased knowledge of the structure and metrics of food webs27,41. However, despite the importance of developing a detailed but generalisable food web model, serious difficulties remain in reconstructing food webs that include the main energy pathways across levels of biological organisation from individuals to communities9,11. Stable isotope analysis of carbon (δ13C) and nitrogen (δ15N) are powerful tools that make possible integration of information across multiple levels of biological organisation (i.e. from single individuals to the entire food web) and at meaningful timescales42,43. Stable isotopes are thus a fundamental tool for understanding the ecological mechanisms underlying food web reorganisation under conditions of environmental change44. δ13C values can vary among primary producers, making it possible to detect and quantify the distinct contribution of each carbon source to populations’ diets and hence food webs45,46,47,48,49. δ15N values progressively increase with trophic level, making it possible to obtain information on the trophic position of individuals or species in the web47,50. Using individual δ13C and δ15N values, Rossi et al.9 proposed a new approach to food web reconstruction based on Isotopic Trophic Units (ITUs). ITUs were defined as either single individuals or small groups of individuals having similar δ13C and δ15N values and thus occupying the same position in the δ13C–δ15N biplot29,51,52. Based on ITUs, complex food webs of medium-depth (from 21 to 240 m) Antarctic benthic communities in Terra Nova Bay (Ross Sea) were reconstructed in great detail, and their spatial variation under varying degrees of sea-ice persistence was described. When a classic approach based on average population isotope values was applied to food web reconstruction in shallower waters of the same area10, the links were less detailed and the linkage density was lower than what was observed by Rossi et al.9. The differences may be ascribed to the different approaches to web reconstruction, which could thus lead to biased conclusions regarding web structure and stability.
We investigated food-web reorganisation before and after sea-ice breakup in the shallow waters of Terra Nova Bay at different scales of biological aggregation (i.e. from individuals to taxa and communities). The goal was to gain greater insight into the combined role of individuals’ diets and changing ice-related energy supply in shaping Antarctic benthic biodiversity and determining its resilience, stability and robustness to species loss.
The principal aim of this study was to increase our understanding of the mechanisms that allow communities adapted to extremely variable environments to be highly complex yet resilient. This entailed investigating intrapopulation dietary variability as a central functional trait affecting food web structure. In agreement with Optimal foraging theory53,54, we tested the hypothesis that high food availability after sea-ice breakup leads to a simplification of food webs due to specialisation and diversification of individuals’ diets. Given the persistence of the Antarctic community, we also hypothesised that there exists a core web representing the invariant skeleton of community structure that remains impervious to changes in resource availability, thus playing a crucial role in community stability. Under this premise, the study addressed three specific goals. First, we reconstructed and compared food web structure and metrics before and immediately after sea-ice breakup (i.e. Low vs. High food availability14,17,21,24,55,56,57,58). Second, we compared webs reconstructed using three different approaches: a. ITU-webs (following9), in which the nodes were single ITUs regardless of species; b. population-webs, in which nodes were species. In this case, each species’ trophic links were derived from the trophic links of the ITUs belonging to that species, thus accounting for ‘intraspecific’ variability; and c. taxonomic-webs, reconstructed using each species’ average isotope values, without taking account of ITUs (according to10). Third, we identified the core food web whose links and nodes remain unchanged. Comparisons were extended from the whole web to species level, by focusing on key Antarctic species in terms of abundance and distribution (the scallop Adamussium colbecki, the sea-urchin Sterechinus neumayeri and the sea star Odontaster validus59,60).
Results
Biodiversity and food webs
Zoobenthic diversity was lower after sea-ice breakup: 59 versus 36 taxa and 109 versus 87 ITUs (before vs. after; Fig. 1, Tables S1, S2). Samples of zoobenthos included the zooplanktonic Limacina helicina, its predator Clione limacina antarctica and benthic calanoid copepods that were found on the bottom. Since most of the zoobenthos was identified at the species level (Table S1), for ease of reading, the terms 'species' and 'intraspecific variability' are generally used in the text to indicate 'taxon' and ‘inter-individual variability', respectively. The mean number of ITUs per species did not differ between the two periods (4.8 ± 0. 4 before and 4.8 ± 0.7 after sea-ice breakup; Mann Whitney test, U: 1838, n.s.), while the mean number of species per ITU was lower after sea-ice breakup (2.5 ± 0.2 vs. 1.8 ± 0.1 species/ITU; Mann Whitney test, U: 7227.50, p < 0.05).
Isotopic biplot of the benthic community (a) before (November–December 2016) and (b) after (January–February 2017) sea-ice breakup in Tethys Bay (Terra Nova Bay, Ross Sea). Each point represents a specimen. Full symbols represent basal food sources, empty symbols represent consumers. The isotopic space is divided into ITUs (Isotopic Trophic Units, i.e. 1 × 1‰ intervals of δ15N and δ13C) in accordance with Rossi et al.9. Dashed-red line polygons delimit the convex hull (Total Area) of consumers. For the detailed list of species contributing to each sediment fraction, refer to Tables S1 and S2.
Although the number of nodes was higher when taking account of ITUs, node number was unrelated to the proportion of basal, intermediate and top nodes in the webs, the number of trophic links, or the web metrics mentioned below (R2 < 0.55, p always n.s. N = 6).
Web topology and metrics before sea-ice breakup differed from those after and among food web types (ITU-webs, population-webs and taxonomic-webs; Tables 1, 2, Fig. 2). Trophic Level (TL) was always lower in taxonomic-webs than the other web types and was generally lower after sea-ice breakup (Table 1, Fig. 3). ITU-webs were less complex (i.e., less connected) and compartmentalised than the other webs, and also had the lowest Neighbourhood Connectivity (NC) and potential competition. By contrast, population-webs were the most complex and compartmentalised of all types of webs, with the highest connectance, linkage density (L/S), Neighbourhood Connectivity (NC), and potential competition (Table 1, Fig. 3). The last three metrics were lower after sea-ice breakup (Table 1, Fig. 3). Robustness to secondary loss of nodes was greatest in population-webs (ANCOVA test, F: 52.29, p < 0.001; Table 1, Fig. 4).
Benthic food web structure in Tethys Bay (Terra Nova Bay, Ross Sea). Top: before sea-ice breakup; bottom: after sea-ice breakup. Each node represents one ITU in the ITU-webs and one species (or a basal resource) in population-webs and taxonomic-webs. Each arrow points from a resource to its consumer, thickness being proportional to linkage strength. Each node is positioned with respect to its δ13C value and Trophic Level (TL). Basal resources are shown in green. Superscript asterisks (*) indicate significant differences between before and after sea-ice breakup, one, two and three asterisks indicating p < 0.05, p < 0.01 and p < 0.001 respectively. Superscript letters (a,b,c) indicate differences between food web types. For statistical tests, see “Materials and methods” section. The food web graphs were developed using Cytoscape software. For the list of species, refer to Table S1.
Box plot of the distribution of Trophic Level (TL), Linkage Density (L/S) and Neighbourhood Connectivity (NC) for each food web type (ITU, population and taxonomic) before (grey) and after (white) sea-ice breakup. For each panel, the horizontal line represents the median of the distribution, and the “x” symbol represents the mean. The box includes 50% of the data while the whiskers mark the highest and lowest values including 95% of the distribution.
Diets at different biological scales (community, population, individual)
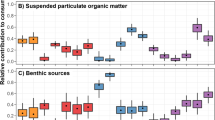
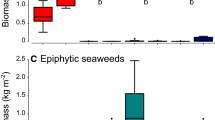
Isotopic distances between ITUs reflected differences in diet (Mantel test, before: R = 0.03, p < 0.001; after: R = 0.43, p < 0.001). At the community level, animal matter (whether living or as carrion) was a major contributor to diet, together with sympagic algae and sediments both before and after sea-ice breakup (Table 2). Its importance was highlighted by the population-webs, which took account of individual variability. In both periods the coarse sediment fraction was mainly composed of animal-derived material, which also formed a large percentage of the fine and fine-ultrafine sediment fractions (Tables 3, S3). By contrast, the ultrafine sediment fraction was exclusively composed of primary producers, mainly sympagic algae before sea-ice breakup and a combination of sympagic algae and phytoplankton afterwards. In the fine and fine-ultrafine fractions, benthic algae were the predominant primary producer after sea-ice break-up.
Focusing on three of the most abundant macroinvertebrate species in Antarctica (Fig. 5), the scallop Adamussium colbecki based its diet largely on the fine and ultra-fine organic sediment fractions. However, for some individuals sympagic algae made up a large proportion of its diet both before and after sea-ice breakup (respectively 9.2 and 10.2% in ITU-webs, 0% and 9.6% in population-webs and 7.20% and 9.8% in taxonomic-webs). The sea-urchin Sterechinus neumayeri was found to have a more omnivorous diet, with sympagic algae making up a large proportion both before and after sea-ice breakup (respectively 36.0% and 41.3% in ITU-webs, 33.4% and 31.4% in population-webs and 46.5% and 52.8% in taxonomic-webs). The sea star Odontaster validus was a top predator that preyed primarily on S. neumayeri, which formed a greater proportion of its diet before than after sea-ice breakup. This trend was highlighted by the ITU-webs and population-webs but not taxonomic-webs (respectively 25.4% and 17.5% in ITU-webs, 28.6% and 9.1% in population-webs, Kruskal–Wallis and Mann–Whitney paired comparison test, Hc: 12.9, p always < 0.001; and 20.8% and 25.0% in taxonomic-webs). The contribution of animal matter (whether living or as carrion) was remarkably high in all three species in the ITU-webs and population-webs, but comparatively low or absent in the taxonomic webs, which were based on average isotopic values and literature data (Fig. 5).
Centrality and compartmentalisation
The three common species (A. colbecki, S. neumayeri and O. validus) were also central species that played a key role in connecting other species in the food web (i.e. topologically central species; see Table S2, in which other topologically central species are reported). Specifically, A. colbecki and the predaceous large sea-star Diplasterias brucei were central species in all types of food web, the former both before and after sea-ice breakup and the latter only afterwards.
Focusing on the central nodes, 8 compartments before and 7 compartments after sea-ice breakup were found in the population-webs, which were the most complex and compartmentalised (Fig. 6). In both periods the compartments were connected by the sea-urchin S. neumayeri, the sea-star D. brucei and the fine-ultrafine fraction of sediments as a basal resource (Fig. 6).
Food web compartments (left) and respective food web structures (right) (a) before (November–December 2016) and (b) after (January–February 2017) sea-ice breakup (i.e. low and high resource availability respectively) in Tethys Bay. Hexagons represent compartments and arrows are links directed towards the target compartment (left) or consumer (right). The respective sub-network is contained within each compartment. Colours represent compartments (left) and the central nodes in the connections within and between those compartments. The filled nodes and red arrows indicate nodes and links belonging to the population-based web core.
Food web core
A web core (i.e. an unchanged group of nodes and links including central nodes) was found in each type of food web (Fig. 7). It comprised 86 nodes and 108 links in the ITU-webs, 40 nodes and 160 links in the population-webs and 45 nodes and 97 links in the taxonomic-webs.
The web core was significantly simpler in terms of connectance and linkage density than the food webs as a whole (Kruskal–Wallis and Mann–Whitney paired comparison test, Hc: 176, p always < 0.001; Fig. 7). Between 30 and 60% of the unchanged links were between basal resources and their consumers. In the ITU-based core web, links to sympagic algae, epiphytes, phytoplankton, benthic algae and fine sediments tended to be preserved. Focusing on the three common species, the sea-urchin S. neumayeri preserved links with sympagic algae, which represented its only food source in the population-web core, and with its predator O. validus. In contrast, the bivalve A. colbecki preserved links mainly with benthic algae and fine sediments, although a few individuals preserved links with resources of animal origin.
Discussion
Understanding the relationship between food web structure and community stability is still one of ecology’s greatest challenges1,2,3,25,61,62,63,64,65,66. Although the debate is ongoing67,68, analysis of random food webs shows that the more complex the community, the less resilient it is2,3,4,65,67,68,69,70. However, in nature, interactions between populations within communities are not random39,54,62,69,71 and can change with the environmental conditions6,61,72. Kondoh73 found that short-term variations in trophic links, driven by adaptive consumer food preference, are crucial for the long-term stability of complex communities. This dynamic reorganisation of the food web helps communities to buffer environmental fluctuations.
Antarctic benthic communities are complex but also appear resilient to seasonal disturbances (e.g. iceberg scouring and oscillations in light and temperature33,74,75). In the short to medium term, they exhibit the ability to recolonise disturbed areas18,74,75,76,77,78 or quickly exploit new and abundant seasonal resources9,12,17,56,57. To disentangle the factors underlying this apparent complexity-stability paradox3,38,39, in our study the food webs were reconstructed at different biodiversity scales (from individuals to communities). Some crucial points emerged from our results: (1) web structure varies with the availability of basal resources, significant differences being observed between the situation before and after sea-ice breakup, and the responses are seen at all scales; (2) complexity is higher when considering intraspecific variability than webs based on average species’ diets, although the stability can be explained by higher compartmentalisation, i.e. the degree to which food webs are organised into sub-networks79; and (3) a web core, consisting of the minimal stable structure with nodes and links persisting over time, represents a highly stable food web underlying the highly biodiverse Antarctic communities regardless of resource availability and environmental conditions.
We observed that consumer trophic generalisation decreased with increasing per capita availability of basal resources due to sea-ice breakup. Further, in accordance with optimal foraging theory53,80, this inverse trend occurred at all scales (individual, population and community). This change was reflected in a reduction in linkage density and the other main food web metrics. Indeed, the foraging choice of individuals has important effects on the entire web structure and stability by determining the level of web complexity, as already predicted by diet breadth models81. Connectance, linkage density, direct and indirect interconnection (Neighbourhood Connectivity) and food chain length, all measures of web complexity, as well as robustness to diversity loss, were greater when taking account of individual feeding choices within species (population-webs) than when considering only the average stable isotope signatures for each species (taxonomic-webs; see also10). The differences were not due to the number of nodes and thus trophic aggregation (sensu82,83).
In contrast, we observed a lower linkage density in the ITU-webs than in the other two web types. This was related to the rather homogeneous distribution of individuals in the isotopic space and reflected a differential use of basal resources. We also observed that after sea-ice breakup intraspecific variability in terms of the number of ITUs per species remained unchanged while the average number of species per ITU was lower. This supports the reduction of interspecific overlap due to diet specialisation when, according to the literature10,74,84, the per capita availability of resources increases and the number of species decreases.
The contribution of individual specialisation to foraging diversity, favouring niche differentiation between consumers within a given trophic level, has already been observed9,85,86,87. This is relevant, since despite not being a rare trophic strategy, the specialisation of individuals on different resources in a generalist population (sensu88) is rarely considered in food web studies, which do not generally take account of intra-population phenotypic variability9,89,90,91.
Low direct and indirect interconnection in the ITU-webs (NC) was also observed, being even lower after sea-ice breakup, and this may reduce the food web’s vulnerability to the propagation of disturbances10,92,93,94. In accordance with Stewart et al.95, the intraspecific specialisation of individuals on distinct food sources can be considered an analogue of compartmentalisation and an emergent property of food webs. Many studies based on empirical models have observed that in nature, the organisation into ‘blocks’ of highly complex communities reduces the instability of the food web3,4,39,70,96. The compartmentalisation promoted by intraspecific trophic diversity, combined with the presence of nodes as connectors between compartments observed in this study, could explain the stability of complex Antarctic benthic communities, in which high trophic generalisation, omnivory and intraguild predation are all common9,14,32,70,79,94,97,98.
In each type of food web we observed the presence of an invariant subset of nodes and links, or web core, which persisted regardless of resource availability. In recent decades, researchers have hypothesised the existence of minimal structures that allow the community to maximise energy transfer at ‘minimal cost’ through key pathways within the food web99,100,101. Food web complexity, in terms of linkage density and connectance, was lower in the web core than in the food web as a whole. This suggests that the web core actually represents the minimal structure, highly stable over time, of a complex community that opportunistically adds new links based on resource availability and consumers’ adaptive foraging behaviours73,99,102,103. In these sub-webs, the trophic variability of the central species is maintained. Together with compartmentalisation, the presence of a trophic-functional web core may provide the key to understanding the extraordinary persistence of complex Antarctic communities.
Sympagic algae, sediment, and animal matter were the most important carbon sources in the food webs. This is consistent with several studies showing that sympagic algae and organic matter storage in the sediment are among the resources that most support Antarctic benthic communities in both medium-shallow and deep waters9,17,104,105. The former is an energy-rich resource whose availability is limited to a short period of the year, i.e. the end of spring and the beginning of summer23,55,56,106. During this period, sea-ice thinning due to increased temperature increases light radiation, favouring the growth of sympagic algae that are released when the sea ice breaks up and melts21,23,55,56. In contrast, sediment represents an important food bank mostly during the unproductive season, powered by seasonal depositions of particulate organic matter from various sources55,107,108. Our study shows that the composition of organic sediment in Tethys Bay varied from almost exclusively animal-derived matter in the coarse fraction, both before and after sea-ice breakup, to solely sympagic algae before sea-ice breakup and both sympagic algae and phytoplankton afterwards in the ultra-fine fraction. After sea-ice breakup, in the intermediate fractions of the sediment, i.e. fine and fine-ultrafine, the previously large contribution of animal-derived matter decreased, while that of benthic algae increased. The fine-ultrafine sediment fraction was an important connector of food web compartments in both periods, confirming its important role as a persistent food source for benthic organisms108.
Variation in the contribution of carbon sources to diets was observed in the two omnivorous key species, the scallop A. colbecki and the sea-urchin S. neumayeri. Sediments and animal matter were the main carbon sources for the scallop, whose consumption of sympagic algae was higher after sea-ice breakup, while the sea-urchin was able to consume sympagic algae both before and after. By clapping, the bivalve A. colbecki is able to resuspend and filter the organic matter deposited in the sediment109,110. It can thus exploit the sympagic algae in the sediments in periods of low availability and those released by melting ice in periods of high availability. The sea star O. validus exhibited highly predatory behaviour. Its diet relied mostly on the sympagic chain, consuming mainly the sea urchin in both periods. These three common central species are also important to the web core, playing a key role in carbon processing and transfer, as well as in benthic-pelagic-sympagic coupling over time97,98,109.
The substantial contribution of animal matter to the diets of consumers in the food webs, including scallops, was revealed by the ITU-based analyses. Conventional diet reconstruction methods, based on species’ average trophic preferences, require a priori awareness of possible resources to include in the models63. Pre-assigning a restricted set of possible resources based on limited or inadequate scientific information on the diet of target species can represent a further impediment to the accurate reconstruction of food webs9,27,63. The ITU-based approach helps overcome this limitation, allowing a more accurate estimate of consumers’ diets and thus yielding useful information for effective ecosystem management measures in both the short and long term.
In this kind of study, tissue turnover may constitute a limitation on the interpretation of mixing model results104. However, determining turnover rates is particularly complicated in food webs with many species, since it is costly and time-consuming, and the relevance of the estimates to other species is not clear111. It is particularly challenging in Antarctica due to logistics. Although we know of no experiments determining isotopic fractionation in Antarctic benthic invertebrate organisms, some studies of their metabolism support the hypothesis that fractionation increases in periods of greater food availability112,113. Indeed the metabolism and growth rates of Antarctic marine species are significantly affected by seasonal cycles and the availability of food sources113,114,115,116,117. While they display slow metabolism for most of the year76,112,113, in the most productive period, during sea-ice breakup, metabolic and growth rates increase112,113,115,118,119. Increasing metabolic rate is associated with increasing tissue turnover and changes in isotopic ratios46,50,120. Kaufman et al.120 reported a tissue turnover rate of about 3 weeks in an Arctic amphipod in high-productivity periods, and Weems et al.121 observed similar results in two Arctic bivalves. Consistent with these observations, the tissues collected from Antarctic invertebrates might indicate recent dietary shifts. Calizza et al.17 showed the consumption of sympagic algae even before sea-ice breakup by isotopic analysis of muscles, and consumption of sympagic algae and phytoplankton only 3 days after complete sea-ice disappearance by gut content analysis.
Laboratory measurements of Antarctic invertebrate tissue turnover rates would improve the accuracy of attempts to quantify temporal changes in their diets on an isotopic basis. The incorporation of additional biochemical tracers, such as fatty acids122, and isotopic analysis of specific amino acids123 could further enhance our ability to discriminate between the different sources of carbon that fuel complex food webs such as those found in Antarctica.
In conclusion, the persistence of high levels of biodiversity can be explained by the ability of organisms to adapt their diet to different conditions of food availability12,27,28, seasonally rewiring the food webs. This ability varies even within the same population of a given species27,28,30. Neglecting intrapopulation variability in food choice could result in underestimation of species’ diets and thus oversimplification of food web structure and complexity, leading to possible misinterpretation of the mechanisms underlying community stability9,27,34,63,89,124.
Understanding the actual structure and functioning of food webs is critical to making predictions about the increasingly widespread impacts of climate and other environmental change on communities and ecosystems6,11,69,104,125. However, the reconstruction of food webs is still affected by several limitations, mainly due to the difficulty of analysing communities with high trophic and taxonomic diversity9,13,27,29,30,35,126. In addition, taxonomic resolution is not always homogeneous, and generalisation of observations is often impeded by the different approaches and non-comparable datasets adopted across sites and periods127. By grouping individuals with similar tropho-functional traits, the ITU-based approach provides an effective method for describing in detail and comparing food web architecture even when communities are extremely complex and biodiverse in variable environments such as Antarctica.
The detailed description of populations’ diets based on the diet of each individual made it possible to identify the role of each population in the food web and the set of resources actually assimilated by individuals. This enabled us to identify not just a core web persisting independently of resource availability and environmental conditions, but also ‘community hubs’. These are sets of key species with high trophic plasticity, which play a key role in maintaining web structure and therefore ecosystem functions128,129 and energy flows in Antarctic benthic ecosystems9,17,98,104.
Comparing food web structure before and after sea-ice breakup has proven to be a powerful tool for studying the mechanisms underlying the architecture of biodiversity maintained by predictable energy supply. This yielded knowledge needed to predict community response to environmental change. For the purposes of managing and conserving complex communities and key species threatened by environmental change, it is therefore appropriate to first consider and describe in detail the trophic-functional traits and diet of community members. This will provide an accurate representation of the architecture of biodiversity and predict future scenarios.
Climate projections suggest a significant reduction in sea ice cover across Antarctica, with the exception of the Ross Sea130,131. Changes in sea ice cover, including alterations in seasonal dynamics, ice thickness, annual ice loss, and variations in sunlight diffusion15,84 can alter availability of energy-efficient resources (e.g., sympagic algae), which are crucial for the survival and reproduction of benthic organisms21,55,56.
Our findings suggest that taxa in these communities, typically adapted to an extremely stable environment18,20,74,77, could adapt their dietary habits further by increasing trophic generalisation during prolonged periods of limited resources. The results are consistent with other studies conducted both in the same area and elsewhere, at a range of depths9,14,17,104,132. These studies indicate that variations in sea ice coverage could affect the structure of the Antarctic benthic food web on various spatio-temporal scales. In addition to reduced ice algae production, the reduction of seasonal sea ice coverage could raise the frequency of catastrophic events such as iceberg drift, especially in shallow coastal waters33,74,75,133. This would increase the availability of animal carcasses, either fresh or stored in sediments, favouring necrophagy among Antarctic organisms77,133. It has also been demonstrated that conditions of extreme sea ice reduction, or absence, result in decreased Antarctic benthic biodiversity15,84. Such conditions are already evident in some areas of the Antarctic Peninsula and sub-Antarctic regions, which exhibit growing vulnerability to the local loss of the most connected species65,94. In contrast, multiyear sea ice is common in McMurdo Sound and New Harbor134. Norkko et al.14 found that compared to fresh algal rich areas near ice-free waters, in conditions of high sea-ice persistence and thickness, taxa shift towards omnivory, primarily based on sediments and detritus. Michel et al.104 showed that the multiyear absence of sea-ice breakup but low sea-ice thickness (< 200 cm), favours algal growth. Under these conditions, benthic consumers base their diet mostly on the sympagic chain, reducing omnivory and lowering trophic positions. These changes in the food web structure at the benthic level could have effects on higher trophic levels in the Antarctic ecosystem20,135.
Materials and methods
Study area and sampling
The study area is Tethys Bay (74° 41′ 8″ S, 164° 04′ 8″ E), a 3 km-long inlet of Terra Nova Bay in the Ross Sea MPA (Antarctica) characterised by high biodiversity9,136. It hosts several species of gastropods, echinoderms (asteroids, ophiuroids, holothuroids, echinoids and crinoids), bivalves, pycnogonids, sponges, polychaetes and many other macroinvertebrates9,136,137.
The marked seasonality in the dynamics of sea-ice coverage, breakup and melting in the bay regulates the availability of food for benthic communities10. Sampling was carried out both in the period of maximum ice thickness (from mid-November to mid-December) and at least 31 days after the start of sea-ice breakup, which causes massive release of sympagic algae. Sea-ice breakup began on 18 December 2016, leaving the sea completely ice-free by around 10 January 2017. The sampling periods reflect the timing of tissue turnover in polar benthic marine invertebrates during the most productive season120, before the shift to a phytoplankton-based diet, typical of ice-free conditions, can take place17,21,57,97,109,110.
Samples were collected by SCUBA divers at depths of 10–30 m. Macroinvertebrates and basal resources including micro and macroalgae (Iridaea cordata and Phyllophora antarctica), together with associated epiphytes, Weddell seal faeces and sediments were sampled. Sympagic algae were collected by hand by scraping ice cores. Sediments were fractionated as Coarse (> 1.00 mm), Fine (1.00–0.75 mm), Fine-Ultra-Fine (0.75–0.56 mm) and Ultra-Fine (< 0.56 mm). A total of 1732 specimens belonging to 61 different animal species were sampled and processed. Approximately half were common before and after sea-ice breakup. For the isotopic analysis, tissues of large invertebrates were used, while small individuals were analysed whole or pooled to reach sufficient biomass for the analysis. Samples were then freeze-dried, ground into a fine powder and analysed. Where necessary, samples were pre-acidified using drop-by-drop 1 M HCl138 to remove inorganic carbon, which can interfere with the δ13C signature, while δ15N was measured in non-acidified powders9,17,46. Samples were analysed in two replicates with a mass spectrometer (IsoPrime100, Isoprime Ltd., Cheadle Hulme, United Kingdom) coupled with an elemental analyser (Elementar Vario Micro-Cube, Elementar Analysensysteme GmbH, Germany). The results (δ13C and δ15N) are expressed in delta units per thousand (‰) relative to international standards and indicate the presence of heavier isotopes. Further details on the study area and sampling and lab activities can be found in Sporta Caputi et al.10.
Food web reconstruction
Isotopic Trophic Units (ITUs) based on the isotopic data of individual organisms were used to reconstruct the communities’ trophic-functional web structure. The Isotopic Trophic Units were defined as single individuals or groups of individuals having similar carbon and nitrogen isotopic signatures9, i.e. occupying the same position within the isotopic space, considered as trophospecies (sensu139). ITUs were identified by subdividing the bi-dimensional isotopic space into squares corresponding to 1‰ on the δ15N and δ13C axes, starting from the lowest δ13C value in the dataset and a δ15N value of zero (Fig. 1). Each ITU was labelled with a number and a letter corresponding to its isotopic values on the δ13C and δ15N axes respectively.
The 1‰ intervals defining the ITUs (corresponding to 1‰ × 1‰ squares in the biplot) represent the minimum units for comparing communities with different isotopic signatures in time and space9. These dimensions also allow us to reconstruct food webs with nodes occupying equal portions of the community isotopic space, reducing the potentially large and variable numbers of individuals and species included in each ITU. This, in turn, allows for better and more accurate extrapolation of trophic links between species. Since the maximum isotopic carbon difference between a consumer and its resource is 1‰ among aquatic animals50, differences greater than 1‰ on the δ13C axis may be indicative of dietary differences between individuals. To confirm this, as in Rossi et al.9, the correlation between the similarity in trophic links and the isotopic distance between ITUs was verified by means of a Mantel test. While the isotopic discrimination factor for nitrogen is generally broader (~ 2.3‰ for invertebrates9,50), the 1‰ resolution on the δ15N axis allows for the categorisation of individuals belonging to omnivorous populations into different trophic units. This preserves information on the vertical distribution of individuals along the food chains and the complexity of the food web.
The diet of each ITU was estimated by means of Bayesian Mixing Models (SIMMr package, R-software ver. 4.1.1140,141), considering Trophic Enrichment Factors of 0.4 ± 0.2‰ for δ13C and 2.3 ± 0.4‰ for δ15N9,10,17. The model required three inputs: (1) the isotopic signatures of the ITU consumer, (2) the isotopic signatures (mean and standard deviation) of the ITU consumer’s food sources and (3) the ITU consumer’s Trophic Enrichment Factor (TEF). The uncertainties considered in the TEF values make it possible to take into account any variability due to differing assimilation of the various food source items by each consumer30,44,50,142. The model output provides probability distributions of the proportional contribution of each food source to the diet of each consumer expressed as mean ± standard deviation and respective upper and lower limits of credibility ranges (CI 50%, 75%, 95%).
In order to obtain the actual resource pool for each consumer, several steps based on the mixing model outputs were performed in accordance with Rossi et al.9. The contribution of the various organic carbon sources to each sediment fraction was estimated both about 4 weeks before and 4 weeks after sea-ice breakup, without incorporating trophic enrichment into the model.
At population level, in both sea-ice periods, the diet of each species was determined by analysing the diets of the ITUs including specimens of that species9,30. Similar to Rossi et al.9, we assumed that all food sources consumed by specimens within ITUs were equally likely to be included in the overall diet of the consumer population. Consequently, the overall contribution of a specific food source to each consumer's diet will be important at the individual level (> 5%) but less (< 5%) at the population level30. To obtain detailed information on the diet of each population, and therefore on the structure of the food web, we considered only food sources with an overall contribution to a population’s diet of > 2%. This was to exclude resources whose standard deviation could result in values close to zero. These resources were basal and animal items that (1) were generally poorly explored by population individuals, or (2) were consumed by only one or a few individuals, contributing less on average to the diet of the population as a whole than other items.
Both ITU-webs and population-webs were compared with the ones published in Sporta Caputi et al.10. In the latter (taxonomic-webs) the potential food sources for each species were selected based on the literature14,17,97,104,110,124 and/or their position in the bi-dimensional isotopic space.
The web core was considered as the minimum existing structure, considering nodes and links common to both pre- and post-sea-ice breakup conditions.
Food web metrics and data analysis
After assigning trophic links, web metrics were obtained using Cytoscape 3.9.1 and the Cheddar and NetworkExtinction packages in R-software ver. 4.1.1143,144.
The nodes (S) in the webs were either ITUs or species, while the links (L) were the feeding links from resources to consumers. The linkage density (L/S) is the average number of feeding links per node while connectance (C) is:
Potential competition for resources was measured both as the average number of nodes sharing at least one resource and as resource use overlap (α) in accordance with Levins’ (1968) equation:
where i and j form a pair of overlapping nodes, and pik and pjk are the proportional contributions of resource k to the diets of i and j respectively. For each node, the Trophic Level (TL) was also calculated in accordance with the formula:
where i is the target consumer node, k the resource item and pik the proportional contribution of resource k to the diet of i145. The TL of basal resources was 1.
Neighbourhood Connectivity (NC), Betweenness Centrality (BC) and web robustness to node loss were also estimated. NC measures the degree of interconnection (direct and indirect) between nodes in the food web, while BC is a topological measure of the centrality of a node in a food web10,146,147. The top ten nodes (ITUs or species) in terms of their role in connecting other nodes or compartments in the food web were identified with reference to their BC values. The ModuLand plug-in for Cytoscape148 was used to identify the compartments of the food web and the role of nodes in connecting them. Web robustness was quantified by simulating primary extinctions (sensu71) from the most to the least connected node within the food web9,10. This represents the worst possible extinction scenario, giving us useful information on the maximum vulnerability of communities to diversity loss. Secondary extinction occurs when a consumer node loses all its resource items, and the robustness index was expressed as the proportion of primary losses leading to the primary or secondary loss of 50% of all community nodes71.
The metrics of the food webs before and after sea-ice breakup, hence under conditions of low and high resource availability, and of the three types of food web (ITU-webs, population-webs and taxonomic-webs) were compared. Due to computational limits, which do not allow chains of > 105 to be accurately estimated143, quantifying the exact mean chain length in the population-web was not possible. Comparisons were also made at population level by focusing on key species in terms of their centrality in the food web (sensu Martín González et al.147).
Before performing statistical comparisons, the Shapiro–Wilk normality test was applied to each dataset to verify the Gaussian distribution and then use the most appropriate statistical tests. A Mann–Whitney test was performed to determine the difference in the mean number of species per ITU before and after sea-ice breakup. A Mantel test was then applied in order to determine whether the isotopic signatures of ITUs were predictive of the feeding links they established in the food web9,10,149. The correlation test was performed between the matrices of isotopic Euclidean distances and the Bray–Curtis diet similarity9,10,149.
Kruskal–Wallis and Mann–Whitney pairwise tests were used to compare linkage density, Neighbourhood Connectivity, trophic level and the number of competitors, as well as resource consumption before and after sea-ice breakup in the three food web types. Finally, ANCOVA with primary extinction as a covariate factor was used to test the difference between web types in terms of web robustness to node loss.
Unless otherwise specified, the results are reported as mean ± standard error (SE). In order to exclude differences arising from the scale of trophic aggregation (i.e. ITU-webs, population-webs and taxonomic-webs), the relationships between (1) the number of nodes in the webs and the proportion of basal, intermediate and top nodes and (2) the number of nodes and the web metrics were tested in accordance with Martinez and Lawton83.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Elton, C. Animal Ecology (Macmillan Co, New York, NY, 1927).
MacArthur, R. Fluctuations of animal populations and a measure of community stability. Ecology 36, 533–536 (1955).
May, R. M. Will a large complex system be stable?. Nature 238, 413–414 (1972).
May, R. M. Stability and Complexity in Model Ecosystems (Princeton University Press, 1974).
Paine, R. T. Food webs: Linkage, interaction strength and community infrastructure. J. Anim. Ecol. 49, 667–685 (1980).
Chapin Iii, F. S. et al. Consequences of changing biodiversity. Nature 405, 234–242 (2000).
Thompson, R. M. et al. Food webs: Reconciling the structure and function of biodiversity. Trends Ecol. Evol. 27, 689–697 (2012).
Gibert, J. P. Temperature directly and indirectly influences food web structure. Sci. Rep. 9, 5312 (2019).
Rossi, L. et al. Antarctic food web architecture under varying dynamics of sea ice cover. Sci. Rep. 9, 1–13 (2019).
Sporta Caputi, S. et al. Seasonal food web dynamics in the Antarctic Benthos of Tethys Bay (Ross Sea): Implications for biodiversity persistence under different seasonal sea-ice coverage. Front. Mar. Sci. 7, 594454 (2020).
McCormack, S. A. et al. Southern ocean food web modelling: Progress, prognoses, and future priorities for research and policy makers. Front. Ecol. Evol. 9, 624763 (2021).
McMeans, B. C., McCann, K. S., Humphries, M., Rooney, N. & Fisk, A. T. Food web structure in temporally-forced ecosystems. Trends Ecol. Evol. 30, 662–672 (2015).
Polis, G. A. & Strong, D. R. Food web complexity and community dynamics. Am. Nat. 147, 813–846 (1996).
Norkko, A. et al. Trophic structure of coastal Antarctic food webs associated with changes in sea ice and food supply. Ecology 88, 2810–2820 (2007).
Clark, G. F. et al. Light-driven tipping points in polar ecosystems. Glob. Change Biol. 19, 3749–3761 (2013).
Constable, A. J. et al. Climate change and Southern Ocean ecosystems I: How changes in physical habitats directly affect marine biota. Glob. Change Biol. 20, 3004–3025 (2014).
Calizza, E., Careddu, G., Sporta Caputi, S., Rossi, L. & Costantini, M. L. Time- and depth-wise trophic niche shifts in Antarctic benthos. PLoS ONE 13, e0194796 (2018).
Gutt, J. Some, “driving forces” structuring communities of the sublittoral Antarctic macrobenthos. Antarct. Sci. 12, 297–313 (2000).
Chown, S. L. et al. The changing form of Antarctic biodiversity. Nature 522, 431–438 (2015).
Rogers, A. D. et al. Antarctic futures: An assessment of climate-driven changes in ecosystem structure, function, and service provisioning in the Southern Ocean. Annu. Rev. Mar. Sci. 12, 87–120 (2020).
Pusceddu, A., Cattaneo-Vietti, R., Albertelli, G. & Fabiano, M. Origin, biochemical composition and vertical flux of particulate organic matter under the pack ice in Terra Nova Bay (Ross Sea, Antarctica) during late summer 1995. Polar Biol. 22, 124–132 (1999).
Wing, S. R. et al. Contribution of sea ice microbial production to Antarctic benthic communities is driven by sea ice dynamics and composition of functional guilds. Glob. Change Biol. 24, 3642–3653 (2018).
Lizotte, M. P. The contributions of sea ice algae to Antarctic marine primary production. Am. Zool. 41, 57–73 (2001).
Dayton, P. K., Mordida, B. J. & Bacon, F. Polar marine communities. Integr. Comp. Biol. 34, 90–99 (1994).
Rooney, N. & McCann, K. S. Integrating food web diversity, structure and stability. Trends Ecol. Evol. 27, 40–46 (2012).
Rosenblatt, A. E. & Schmitz, O. J. Climate change, nutrition, and bottom-up and top-down food web processes. Trends Ecol. Evol. 31, 965–975 (2016).
Petchey, O. L., Beckerman, A. P., Riede, J. O. & Warren, P. H. Size, foraging, and food web structure. Proc. Natl. Acad. Sci. 105, 4191–4196 (2008).
Feiner, Z. S. et al. Individual and spatial variation are as important as species-level variation to the trophic complexity of a lentic food web. Ecol. Freshw. Fish 28, 516–532 (2019).
Layman, C. A. et al. Applying stable isotopes to examine food-web structure: An overview of analytical tools. Biol. Rev. 87, 545–562 (2012).
Sporta Caputi, S. et al. Changing isotopic food webs of two economically important fish in Mediterranean coastal lakes with different trophic status. Appl. Sci. 10, 2756 (2020).
Akçakaya, H. R. et al. Assessing ecological function in the context of species recovery. Conserv. Biol. 34, 561–571 (2020).
Mérillet, L. et al. Effects of life-history traits and network topological characteristics on the robustness of marine food webs. Glob. Ecol. Conserv. 34, e02048 (2022).
Robinson, B. J. O., Barnes, D. K. A., Grange, L. J. & Morley, S. A. The extremes of disturbance reduce functional redundancy: Functional trait assessment of the shallow Antarctic benthos. Front. Mar. Sci. 8, 797 (2022).
Ings, T. C. et al. Ecological networks–beyond food webs. J. Anim. Ecol. 78, 253–269 (2009).
Martinez, N. D. Effects of resolution on food web structure. Oikos 66, 403–412 (1993).
Fulton, E. A., Smith, A. D. M. & Johnson, C. R. Effect of complexity on marine ecosystem models. Mar. Ecol. Prog. Ser. 253, 1–16 (2003).
Bates, M. L. et al. Construction of a trophically complex near-shore Antarctic food web model using the Conservative Normal framework with structural coexistence. J. Mar. Syst. 145, 1–14 (2015).
Moran, N. P., Wong, B. B. M. & Thompson, R. M. Weaving animal temperament into food webs: Implications for biodiversity. Oikos 126, 917–930 (2017).
Johnson, S., Domínguez-García, V., Donetti, L. & Muñoz, M. A. Trophic coherence determines food-web stability. Proc. Natl. Acad. Sci. 111, 17923–17928 (2014).
Rubenstein, M. A. et al. Do empirical observations support commonly-held climate change range shift hypotheses? A systematic review protocol. Environ. Evid. 9, 10 (2020).
Steele, J. H. Assessment of some linear food web methods. J. Mar. Syst. 76, 186–194 (2009).
Thomaz, S. M. et al. Using space-for-time substitution and time sequence approaches in invasion ecology. Freshw. Biol. 57, 2401–2410 (2012).
Cucherousset, J. & Olden, J. D. Ecological impacts of nonnative freshwater fishes. Fisheries 36, 215–230 (2011).
Costantini, M. L. et al. The role of alien fish (the centrarchid Micropterus salmoides) in lake food webs highlighted by stable isotope analysis. Freshw. Biol. 63, 1130–1142 (2018).
DeNiro, M. J. & Epstein, S. Influence of diet on the distribution of carbon isotopes in animals. Geochim. Cosmochim. Acta 42, 495–506 (1978).
Peterson, B. J. & Fry, B. Stable isotopes in ecosystem studies. Annu. Rev. Ecol. Syst. 18, 293–320 (1987).
Careddu, G. et al. Effects of terrestrial input on macrobenthic food webs of coastal sea are detected by stable isotope analysis in Gaeta Gulf. Estuar. Coast. Shelf Sci. 154, 158–168 (2015).
Sporta Caputi, S. et al. Trophic attractiveness for soil fauna of residues of Bt and near-isogenic maize: A C and N stable isotope-based study. Agric. Ecosyst. Environ. 329, 107868 (2022).
Calizza, E. et al. Climate-related drivers of nutrient inputs and food web structure in shallow Arctic lake ecosystems. Sci. Rep. 12, 2125 (2022).
McCutchan, J. H., Lewis, W. M., Kendall, C. & McGrath, C. C. Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur. Oikos 102, 378–390 (2003).
Layman, C. A., Arrington, D. A., Montaña, C. G. & Post, D. M. Can stable isotope ratios provide for community-wide measures of trophic structure?. Ecology 88, 42–48 (2007).
Newsome, S. D., Martinez del Rio, C., Bearhop, S. & Phillips, D. L. A niche for isotopic ecology. Front. Ecol. Environ. 5, 429–436 (2007).
Pyke, G. H., Pulliam, H. R. & Charnov, E. L. Optimal foraging: A selective review of theory and tests. Q. Rev. Biol. 52, 137–154 (1977).
Rossi, L., di Lascio, A., Carlino, P., Calizza, E. & Costantini, M. L. Predator and detritivore niche width helps to explain biocomplexity of experimental detritus-based food webs in four aquatic and terrestrial ecosystems. Ecol. Complex. 23, 14–24 (2015).
Cau, A. et al. Particulate organic matter release below melting sea ice (Terra Nova Bay, Ross Sea, Antarctica): Possible relationships with zooplankton. J. Mar. Syst. 217, 103510 (2021).
Pusceddu, A. et al. Microbial loop malfunctioning in the annual sea ice at Terra Nova Bay (Antarctica). Polar Biol. 32, 337–346 (2009).
Peck, L. S., Barnes, D. K. A. & Willmott, J. Responses to extreme seasonality in food supply: Diet plasticity in Antarctic brachiopods. Mar. Biol. 147, 453–463 (2005).
Arrigo, K. R. Sea ice ecosystems. Annu. Rev. Mar. Sci. 6, 439–467 (2014).
Guidetti, M. et al. Exchange between populations of Adamussium colbecki (Mollusca: Bivalvia) in the Ross Sea. Antarct. Sci. 18, 645–653 (2006).
Peirano, A., Bordone, A., Corgnati, L. P. & Marini, S. Time-lapse recording of yearly activity of the sea star Odontaster validus and the sea urchin Sterechinus neumayeri in Tethys Bay (Ross Sea, Antarctica). Antarct. Sci. 35, 4–14 (2023).
Yodzis, P. The stability of real ecosystems. Nature 289, 674–676 (1981).
Allesina, S. et al. Predicting the stability of large structured food webs. Nat. Commun. 6, 7842 (2015).
Pringle, R. M. & Hutchinson, M. C. Resolving food-web structure. Annu. Rev. Ecol. Evol. Syst. 51, 55–80 (2020).
Galiana, N. et al. Ecological network complexity scales with area. Nat. Ecol. Evol. 6, 307–314 (2022).
Rodriguez, I. D., Marina, T. I., Schloss, I. R. & Saravia, L. A. Marine food webs are more complex but less stable in sub-Antarctic (Beagle Channel, Argentina) than in Antarctic (Potter Cove, Antarctic Peninsula) regions. Mar. Environ. Res. 174, 105561 (2022).
Lever, J. J., Van Nes, E. H., Scheffer, M. & Bascompte, J. Five fundamental ways in which complex food webs may spiral out of control. Ecol. Lett. 26, 1765–1779 (2023).
McCann, K. S. The diversity–stability debate. Nature 405, 228–233 (2000).
Landi, P., Minoarivelo, H. O., Brännström, Å., Hui, C. & Dieckmann, U. Complexity and stability of ecological networks: A review of the theory. Popul. Ecol. 60, 319–345 (2018).
Calizza, E., Rossi, L., Careddu, G., Sporta Caputi, S. & Costantini, M. L. Species richness and vulnerability to disturbance propagation in real food webs. Sci. Rep. 9, 19331 (2019).
Stouffer, D. B. & Bascompte, J. Compartmentalization increases food-web persistence. Proc. Natl. Acad. Sci. 108, 3648–3652 (2011).
Dunne, J. A., Williams, R. J. & Martinez, N. D. Food-web structure and network theory: The role of connectance and size. Proc. Natl. Acad. Sci. 99, 12917–12922 (2002).
Pimm, S. L., Lawton, J. H. & Cohen, J. E. Food web patterns and their consequences. Nat. Lond. 350, 669 (1991).
Kondoh, M. Foraging adaptation and the relationship between food-web complexity and stability. Science 299, 1388–1391 (2003).
Convey, P. et al. The spatial structure of Antarctic biodiversity. Ecol. Monogr. 84, 203–244 (2014).
Zwerschke, N., Morley, S. A., Peck, L. S. & Barnes, D. K. A. Can Antarctica’s shallow zoobenthos ‘bounce back’ from iceberg scouring impacts driven by climate change?. Glob. Change Biol. 27, 3157–3165 (2021).
Arntz, W., Brey, T. & Gallardo, V. A. Antarctic zoobenthos. Oceanogr. Mar. Biol. 32, 241–304 (1994).
Ingels, J. et al. Antarctic ecosystem responses following ice-shelf collapse and iceberg calving: Science review and future research. WIREs Clim. Change 12, e682 (2020).
Peck, L. S., Brockington, S., Vanhove, S. & Beghyn, M. Community recovery following catastrophic iceberg impacts in a soft-sediment shallow-water site at Signy Island, Antarctica. Mar. Ecol. Prog. Ser. 186, 1–8 (1999).
Pimm, S. L. & Lawton, J. H. Are food webs divided into compartments?. J. Anim. Ecol. 49, 879–898 (1980).
MacArthur, R. H. & Pianka, E. R. On optimal use of a patchy environment. Am. Nat. 100, 603–609 (1966).
Beckerman, A. P., Petchey, O. L. & Warren, P. H. Foraging biology predicts food web complexity. Proc. Natl. Acad. Sci. 103, 13745–13749 (2006).
Sugihara, G., Schoenly, K. & Trombla, A. Scale invariance in food web properties. Science 245, 48–52 (1989).
Martinez, N. D. & Lawton, J. H. Scale and food-web structure: From local to global. Oikos 73, 148–154 (1995).
Clark, G. F., Stark, J. S., Palmer, A. S., Riddle, M. J. & Johnston, E. L. The roles of sea-ice, light and sedimentation in structuring shallow Antarctic benthic communities. PLoS ONE 12, e0168391 (2017).
Duffy, J. E. et al. The functional role of biodiversity in ecosystems: Incorporating trophic complexity. Ecol. Lett. 10, 522–538 (2007).
Ingram, T., Costa-Pereira, R. & Araújo, M. S. The dimensionality of individual niche variation. Ecology 99, 536–549 (2018).
Zhao, Q. et al. Horizontal and vertical diversity jointly shape food web stability against small and large perturbations. Ecol. Lett. 22, 1152–1162 (2019).
Roughgarden, J. Evolution of niche width. Am. Nat. 106, 683–718 (1972).
Bolnick, D. I. et al. The ecology of individuals: Incidence and implications of individual specialization. Am. Nat. 161, 1–28 (2003).
Quevedo, M., Svanbäck, R. & Eklöv, P. Intrapopulation niche partitioning in a generalist predator limits food web connectivity. Ecology 90, 2263–2274 (2009).
Svanbäck, R., Quevedo, M., Olsson, J. & Eklöv, P. Individuals in food webs: the relationships between trophic position, omnivory and among-individual diet variation. Oecologia 178, 103–114 (2015).
Estrada, E. Characterization of topological keystone species: Local, global and “meso-scale” centralities in food webs. Ecol. Complex. 4, 48–57 (2007).
Lai, S.-M., Liu, W.-C. & Jordán, F. On the centrality and uniqueness of species from the network perspective. Biol. Lett. 8, 570–573 (2012).
Marina, T. I. et al. The food web of Potter Cove (Antarctica): Complexity, structure and function. Estuar. Coast. Shelf Sci. 200, 141–151 (2018).
Stewart, S. D., Kelly, D., Biessy, L., Laroche, O. & Wood, S. A. Individual diet specialization drives population trophic niche responses to environmental change in a predator fish population. Food Webs 27, e00193 (2021).
Noto, A. E. & Gouhier, T. C. The effects of intraspecific and interspecific diversity on food web stability. Theor. Ecol. 13, 399–407 (2020).
McClintock, J. B. Trophic biology of antarctic shallow-water echinoderms. Mar. Ecol. Prog. Ser. 111, 191–202 (1994).
Michel, L. N., David, B., Dubois, P., Lepoint, G. & Ridder, C. D. Trophic plasticity of Antarctic echinoids under contrasted environmental conditions. Polar Biol. 39, 913–923 (2016).
Garlaschelli, D., Caldarelli, G. & Pietronero, L. Universal scaling relations in food webs. Nature 423, 165–168 (2003).
Pascual, M. & Dunne, J. A. Ecological Networks: Linking Structure to Dynamics in Food Webs (Oxford University Press, Oxford, 2005).
Klaise, J. & Johnson, S. The origin of motif families in food webs. Sci. Rep. 7, 16197 (2017).
Pimm, S. L. Food webs. in Food Webs (ed. Pimm, S. L.) 1–11 (Springer Netherlands, Dordrecht, 1982). https://doi.org/10.1007/978-94-009-5925-5_1.
Pawar, S. Community assembly, stability and signatures of dynamical constraints on food web structure. J. Theor. Biol. 259, 601–612 (2009).
Michel, L. N. et al. Increased sea ice cover alters food web structure in East Antarctica. Sci. Rep. 9, 8062 (2019).
Yunda-Guarin, G. et al. Reliance of deep-sea benthic macrofauna on ice-derived organic matter highlighted by multiple trophic markers during spring in Baffin Bay, Canadian Arctic. Elem. Sci. Anthr. 8, 047 (2020).
Cronin, K. E. et al. Growth and longevity of the Antarctic scallop Adamussium colbecki under annual and multiannual sea ice. Antarct. Sci. 32, 466–475 (2020).
Smith, C. R., Mincks, S. & DeMaster, D. J. A synthesis of bentho-pelagic coupling on the Antarctic shelf: Food banks, ecosystem inertia and global climate change. Deep Sea Res. Part II Top. Stud. Oceanogr. 53, 875–894 (2006).
Mincks, S. L., Smith, C. R., Jeffreys, R. M. & Sumida, P. Y. G. Trophic structure on the West Antarctic Peninsula shelf: Detritivory and benthic inertia revealed by δ13C and δ15N analysis. Deep Sea Res. Part II Top. Stud. Oceanogr. 55, 2502–2514 (2008).
Cattaneo-Vietti, R., Chiantore, M., Misic, C., Povero, P. & Fabiano, M. The role of pelagic-benthic coupling in structuring littoral benthic communities at Terra Nova Bay (Ross Sea) and in the Straits of Magellan. Sci. Mar. 63, 113–121 (1999).
Chiantore, M., Cattaneo-Vietti, R., Albertelli, G., Misic, C. & Fabiano, M. Role of filtering and biodeposition by Adamussium colbecki in circulation of organic matter in Terra Nova Bay (Ross Sea, Antarctica). J. Mar. Syst. 17, 411–424 (1998).
Thomas, S. M. & Crowther, T. W. Predicting rates of isotopic turnover across the animal kingdom: A synthesis of existing data. J. Anim. Ecol. 84, 861–870 (2015).
Peck, L. S. Antarctic marine biodiversity: adaptations, environments and responses to change. Oceanogr. Mar. Biol. Annu. Rev. (2018).
Souster, T. A., Morley, S. A. & Peck, L. S. Seasonality of oxygen consumption in five common Antarctic benthic marine invertebrates. Polar Biol. 41, 897–908 (2018).
Clarke, A. Seasonality in the antarctic marine environment. Comp. Biochem. Physiol. Part B Comp. Biochem. 90, 461–473 (1988).
Brockington, S. & Clarke, A. The relative influence of temperature and food on the metabolism of a marine invertebrate. J. Exp. Mar. Biol. Ecol. 258, 87–99 (2001).
Norkko, J., Norkko, A., Thrush, S. F. & Cummings, V. J. Detecting growth under environmental extremes: Spatial and temporal patterns in nucleic acid ratios in two Antarctic bivalves. J. Exp. Mar. Biol. Ecol. 326, 144–156 (2005).
Doyle, S. R., Momo, F. R., Brêthes, J.-C. & Ferreyra, G. A. Metabolic rate and food availability of the Antarctic amphipod Gondogeneia antarctica (Chevreux 1906): Seasonal variation in allometric scaling and temperature dependence. Polar Biol. 35, 413–424 (2012).
Brockington, S., Clarke, A. & Chapman, A. Seasonality of feeding and nutritional status during the austral winter in the Antarctic sea urchin Sterechinus neumayeri. Mar. Biol. 139, 127–138 (2001).
Heilmayer, O. et al. Temperature effects on summer growth rates in the Antarctic scallop, Adamussium colbecki. Polar Biol. 28, 523–527 (2005).
Kaufman, M. R., Gradinger, R. R., Bluhm, B. A. & O’Brien, D. M. Using stable isotopes to assess carbon and nitrogen turnover in the Arctic sympagic amphipod Onisimus litoralis. Oecologia 158, 11–22 (2008).
Weems, J., Iken, K., Gradinger, R. & Wooller, M. J. Carbon and nitrogen assimilation in the Bering Sea clams Nuculana radiata and Macoma moesta. J. Exp. Mar. Biol. Ecol. 430–431, 32–42 (2012).
North, C. A., Lovvorn, J. R., Kolts, J. M., Cooper, L. W. & Grebmeier, J. M. Discriminating trophic niches of carnivorous benthic macroinvertebrates with gut contents, stable isotopes, and fatty acids. Mar. Ecol. Prog. Ser. 631, 49–66 (2019).
Larsen, T. et al. Tracing carbon sources through aquatic and terrestrial food webs using amino acid stable isotope fingerprinting. PLoS ONE 8, e73441 (2013).
Dayton, P. K., Robilliard, G. A., Paine, R. T. & Dayton, L. B. Biological accommodation in the benthic community at McMurdo Sound, Antarctica. Ecol. Monogr. 44, 105–128 (1974).
Thompson, R. M. & Williams, R. Unpacking resilience in food webs: An emergent property or a sum of the parts?. Adapt. Food Webs Stab. Transit. Real Model Ecosyst. https://doi.org/10.1017/9781316871867.009 (2017).
Monckton, S. K., Johal, S. & Packer, L. Inadequate treatment of taxonomic information prevents replicability of most zoological research. Can. J. Zool. https://doi.org/10.1139/cjz-2020-0027 (2020).
Mestre, F., Rozenfeld, A. & Araújo, M. B. Human disturbances affect the topology of food webs. Ecol. Lett. 25, 2476–2488 (2022).
Jordán, F. Keystone species and food webs. Philos. Trans. R. Soc. B Biol. Sci. 364, 1733–1741 (2009).
Kortsch, S., Primicerio, R., Fossheim, M., Dolgov, A. V. & Aschan, M. Climate change alters the structure of arctic marine food webs due to poleward shifts of boreal generalists. Proc. R. Soc. B 282, 20151546 (2015).
Swathi, M., Kumar, A. & Mohan, R. Spatiotemporal evolution of sea ice and its teleconnections with large-scale climate indices over Antarctica. Mar. Pollut. Bull. 188, 114634 (2023).
Schofield, O. et al. How do polar marine ecosystems respond to rapid climate change?. Science 328, 1520–1523 (2010).
Iken, K. et al. Macroalgal input into the coastal food web along a gradient of seasonal sea ice cover along the Western Antarctic Peninsula. Mar. Ecol. Prog. Ser. 718, 1–22 (2023).
Dunlop, K. M., Barnes, D. K. A. & Bailey, D. M. Variation of scavenger richness and abundance between sites of high and low iceberg scour frequency in Ryder Bay, west Antarctic Peninsula. Polar Biol. 37, 1741–1754 (2014).
Currie, A. A. et al. Sea ice dynamics drive benthic microbial communities in McMurdo Sound, Antarctica. Front. Microbiol. 12, 745915 (2021).
Maccapan, D. et al. Effects of sea-ice persistence on the diet of Adélie penguin (Pygoscelis adeliae) chicks and the trophic differences between chicks and adults in the Ross Sea, Antarctica. Biology 12, 708 (2023).
Cattaneo-Vietti, R., Chiantore, M., Schiaparelli, S. & Albertelli, G. Shallow- and deep-water mollusc distribution at Terra Nova Bay (Ross Sea, Antarctica). Polar Biol. 23, 173–182 (2000).
Cattaneo-Vietti, R. et al. Spatial and vertical distribution of benthic littoral communities in Terra Nova Bay. in Ross Sea Ecology: Italiantartide Expeditions (1987–1995) (eds. Faranda, F. M., Guglielmo, L. & Ianora, A.) 503–514 (Springer, Berlin, Heidelberg, 2000). https://doi.org/10.1007/978-3-642-59607-0_36.
Hobson, K. A., Ambrose, W. G. & Renaud, P. E. Sources of primary production, benthic-pelagic coupling, and trophic relationships within the Northeast Water Polynya: insights from δ13C and δ15N analysis. Mar. Ecol. Prog. Ser. 128, 1–10 (1995).
Cohen, J. E. & Briand, F. Trophic links of community food webs. Proc. Natl. Acad. Sci. 81, 4105–4109 (1984).
Parnell, A. C. et al. Bayesian stable isotope mixing models. Environmetrics 24, 387–399 (2013).
Parnell, A. Simmr: A Stable Isotope Mixing Model (2021).
Post, D. M. Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology 83, 703–718 (2002).
Hudson, L. N. et al. Cheddar: Analysis and visualisation of ecological communities in R. Methods Ecol. Evol. 4, 99–104 (2013).
Corcoran, D. et al. NetworkExtinction: Extinction Simulation in Food Webs (2022).
Odum, W. E. & Heald, E. J. The detritus-based food web of an estuarine mangrove community. in Estuarine Research: Chemistry, Biology, and the Estuarine System vol. 1 265–286 (Academic Press, New York, 1975).
Newman, M. E. J. Detecting community structure in networks. Eur. Phys. J. B 38, 321–330 (2004).
Martín González, A. M., Dalsgaard, B. & Olesen, J. M. Centrality measures and the importance of generalist species in pollination networks. Ecol. Complex. 7, 36–43 (2010).
Szalay-Bekő, M. et al. ModuLand plug-in for Cytoscape: Determination of hierarchical layers of overlapping network modules and community centrality. Bioinformatics 28, 2202–2204 (2012).
Signa, G. et al. Horizontal and vertical food web structure drives trace element trophic transfer in Terra Nova Bay, Antarctica. Environ. Pollut. 246, 772–781 (2019).
Acknowledgements
We thank Mr. George Metcalf for revising the English text and the Editor and two anonymous Reviewers for their comments, which considerably improved the manuscript. We also thank Simone D’Alessandro, Lorenzo Maria Iozia and Flaminia Tito. This work was supported by Italian Antarctic Research Programme grants PNRA-2015/AZ1.01 (MLC) and PNRA16_00291 (LR) and Italian Arctic Programme grants PRA2021 CHANGE (MLC), issued by MIUR-PNRA.
Author information
Authors and Affiliations
Contributions
Conceptualisation, L.R., M.L.C; methodology, L.R., M.L.C, S.S.C.; software, G.C., S.S.C., J.P.K., M.V.; validation, J.P.K., S.S.C, L.R., M.L.C.; formal analysis, J.P.K., S.S.C; investigation, J.P.K., S.S.C, L.R., M.L.C.; resources, L.R., M.L.C.; data curation, S.S.C, J.P.K., G.C., E.C., M.V.; writing—original draft preparation, S.S.C., L.R., M.L.C.; writing—review and editing, All Authors; supervision, L.R., M.L.C.; funding acquisition, L.R., M.L.C. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sporta Caputi, S., Kabala, J.P., Rossi, L. et al. Individual diet variability shapes the architecture of Antarctic benthic food webs. Sci Rep 14, 12333 (2024). https://doi.org/10.1038/s41598-024-62644-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-62644-5
- Springer Nature Limited