Abstract
Filamentous fungi are eukaryotic microorganisms that differentiate into diverse cellular forms. Recent research demonstrated that phospholipid homeostasis is crucial for the morphogenesis of filamentous fungi. However, phospholipids involved in the morphological regulation are yet to be systematically analyzed. In this study, we artificially controlled the amount of phosphatidylcholine (PC), a primary membrane lipid in many eukaryotes, in a filamentous fungus Aspergillus oryzae, by deleting the genes involved in PC synthesis or by repressing their expression. Under the condition where only a small amount of PC was synthesized, A. oryzae hardly formed aerial hyphae, the basic structures for asexual development. In contrast, hyphae were formed on the surface or in the interior of agar media (we collectively called substrate hyphae) under the same conditions. Furthermore, we demonstrated that supplying sufficient choline to the media led to the formation of aerial hyphae from the substrate hyphae. We suggested that acyl chains in PC were shorter in the substrate hyphae than in the aerial hyphae by utilizing the strain in which intracellular PC levels were controlled. Our findings suggested that the PC levels regulate hyphal elongation and differentiation processes in A. oryzae and that phospholipid composition varied depending on the hyphal types.
Similar content being viewed by others
Introduction
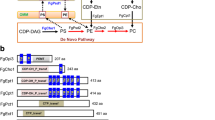
Biological membranes, a major constituent of cells, consist primarily of phospholipid bilayers, and the membranes compartmentalize the cells and organelles by forming external and internal boundaries1,2. Also, they play pivotal roles in various cellular processes driven by the formation of membrane domains, and the interactions between proteins and lipids1. Phospholipids are composed of hydrophilic head groups and hydrophobic tails, which are structurally diverse. Based on their head groups, phospholipids are classified into various types, such as phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylserine (PS), and phosphatidylinositol (PI). The phospholipid synthesis pathways have been extensively studied in the yeast Saccharomyces cerevisiae. The phospholipids are synthesized via the cytidine diphosphate-diacylglycerol (CDP-DAG) and the Kennedy pathways (Fig. S1)3,4,5. In the CDP-DAG pathway, PI is synthesized by transferring inositol to the CDP-DAG by Pis1, and PS is synthesized by displacing CMP from CDP-DAG with serine by Pss1 (also known as Cho1). PE is subsequently synthesized from PS via decarboxylation by Psd1 or Psd2. After that, PE is methylated by Pem1 (also known as Cho2) and converted to phosphatidyl-N-monomethylethanolamine (PMME), and PMME is sequentially methylated to produce phosphatidyl-N-dimethylethanolamine (PDME) and PC by Pem2 (also known as Opi3). In this article, we refer to the process synthesizing PC from PE as “the N-methylation pathway”. PE and PC are also synthesized from ethanolamine (Etn) and choline (Cho) via the Kennedy pathway, respectively. Etn and Cho are sequentially converted to phosphoethanolamine (P-Etn) and phosphocholine (P-Cho) by Eki1 and Cki1, cytidine diphosphate ethanolamine (CDP-Etn) and cytidine diphosphate choline (CDP-Cho) by Ect1 and Pct1 (also known as Cct1), and PE and PC by Ept1 and Cpt1, respectively.
Filamentous fungi are eukaryotic microorganisms and grow in a filamentous form called hyphae. The hyphae elongate their tips by establishing and maintaining hyphal polarities6. Some filamentous fungi are widely used to produce various enzymes, organic acids, and fermented foods, while others infect plants and animals7,8,9. Their morphological differentiation is a critical factor in determining their beneficial or detrimental properties of filamentous fungi. Therefore, it is essential to clarify the mechanisms of their morphogenesis. The phospholipid synthesis pathways have been studied in several species of filamentous fungi belonging to Ascomycota. In Aspergillus nidulans, the PEM2 ortholog choC is indispensable for normal growth10. A PSS1 ortholog is proposed to be crucial for its growth11. We previously reported that the deletion mutant of a PSD2 ortholog psdB showed reduced intracellular PE content and formed hyphae and conidiophores with abnormal morphologies12. In Pestalotiopsis microspora, deletion of choC or PEM1 ortholog choA resulted in defects in vegetative growth and cell wall integrity13. In Aspergillus niger, PEM1 and PEM2 orthologs were characterized14. In Fusarium graminearum, the functions of ten orthologs of the genes involved in the phospholipid synthesis pathways in S. cerevisiae were analyzed, and it was indicated that the biosynthesis of PE and PC is critical for fungal vegetative growth and virulence in plants15. Herein, the strains defective in phospholipid synthesis often exhibited altered morphologies. Therefore, regulating phospholipid synthesis is suggested to be critical for hyphal morphogenesis in filamentous fungi. However, the physiological significance of phospholipid composition in the biological membranes of filamentous fungi remains to be determined.
During the asexual life cycle of filamentous fungi, the asexual spores in Ascomycota, conidia, undergo isotropic swelling, followed by establishing polarity6. Subsequently, germ tubes are extended, and hyphae are formed. Finally, mycelia are formed by hyphal tip elongation and branching6. In the case of mycelia grown on a solid medium, hyphae extend their tips on the surface and into the interior of the solid medium16. In this study, they are referred to as surface and penetrative hyphae, respectively, and collectively termed substrate hyphae. After a certain period since substrate hyphal formation, the hyphae begin to extend into the air, resulting in aerial hyphal formation17. Numerous conidiophores that produce conidia are formed on the footcells in the substrate hyphae16,17. Our previous study demonstrated a significant decrease in PC, and a marked increase in PE and highly unsaturated phospholipids during germination in A. nidulans and A. oryzae18. Additionally, we showed that PC was enriched in the central region of the colony. Another study reported that the deletion of choC retarded hyphal growth and formed swollen hyphal tips in A. nidulans10. These findings suggested that the levels of PC and PE play significant roles in the hyphal morphogenesis of filamentous fungi.
In this study, we examined the effects of varying intracellular PC levels on A. oryzae, an industrially important filamentous fungus. Our findings revealed that the absence of PC synthesis leads to severe growth defects, and the intracellular PC levels regulate the hyphal elongation and differentiation processes in A. oryzae. Further investigation suggested that the profiles of the acyl chain constituents of the phospholipids were altered depending on the type of hyphae.
Results
PC, PI, and PS increased as the differentiation process progressed in A. oryzae
In our previous study, we observed changes in lipid composition during germination of A. oryzae in a minimal medium, Czapek-Dox (CD) liquid medium18. However, A. oryzae does not differentiate aerial hyphae and conidia in the liquid medium. Thus, to investigate the changes of lipid composition during these differentiation processes, we cultivated A. oryzae on the CD agar medium.
The wild-type strain Δku5-2 was inoculated on the CD agar medium, and colony formation was monitored in detail (Fig. 1A). From 16 to 24 h after inoculation, only substrate hyphae grew, and no aerial hyphae were observed. Aerial hyphae emerged at 28 h after inoculation, and conidiophore formation was evident by 48 h after inoculation. To assess lipid composition changes during aerial hyphal formation, we quantified the amounts of PC, PE, PI, and PS, the major phospholipids in eukaryotes, at 24, 32, 40, and 48 h after inoculation (Fig. 1B). The amounts of PC, PI, and PS increased as the differentiation process progressed while those of PE did not change significantly.
Changes in the phospholipid levels during the formation of aerial hyphae and conidiophores. (A) The growth of A. oryzae on the CD agar medium. The conidia of the wild-type strain were inoculated on the CD agar medium and incubated at 30 °C. The aerial hyphae and substrate hyphae were observed. (B) Changes in the phospholipid levels. Lipids were extracted from the harvested mycelia and subjected to the targeted lipidome analysis. Z-scores were calculated within each head group by utilizing the peak areas associated with each group (see “Experimental procedures” for details). The dots and error bars indicate individual data (n = 4), and standard errors, respectively.
Defects in PC synthesis resulted in a severe growth retardation
For regulating PC levels in A. oryzae, we inhibited PC synthesis via the N-methylation pathway and bypassed PC supply via the Kennedy pathway. Although genes involved in PC synthesis in the N-methylation pathway have not yet been reported in A. oryzae, we identified genes encoding homologous proteins of Pem1 (SGDID: S000003389) and Pem2 (SGDID: S000003834) in Saccharomyces Genome Database (SGD, https://www.yeastgenome.org): XP_001727259.1 (BLASTP E-value: 2e-129) and XP_001818525.1 (BLASTP E-value: 4e-76), respectively, in the A. oryzae RIB40 genome database. These protein-encoding genes are AO090012000204 and AO090005001620, and are designated as pemA and pemB, respectively. Using 5′-RACE analysis, we identified transcription initiation sites of pemA and pemB (Fig. S2A) and confirmed that pemA and pemB encode proteins comprising 971 and 202 amino acid residues, respectively. PemA is predicted to possess two phospholipid methyltransferase and ten transmembrane domains. Those features are conserved in S. cerevisiae Pem1. In contrast, predicted numbers of transmembrane domains differed between PemB, having five, and S. cerevisiae Pem2, having three, while both have one phospholipid methyltransferase domain (Fig. S2B).
We constructed deletion mutants of pemA and pemB (named the ΔpemA and ΔpemB strains, respectively) and cultivated them on the CD medium. These strains exhibited significant growth defects and produced numerous balloon-like structures near the hyphal tips (Fig. 2A and B). When we supplemented Cho in the medium, both strains regained their growth and hyphal morphology similar to the wild-type strain (Fig. 2A and B), while supplementation of Etn did not revert these phenotypes (Fig. 2A and B). These results suggested that Cho is necessary for hyphal elongation in the absence of PC synthesis via the N-methylation pathway and that PC can be supplied via an alternative route using Cho. There are proteins closely resembling Cki1, Pct1, and Cpt1 in the A. oryzae database strongly suggesting that the Kennedy pathway supplied PC in A. oryzae (Table S1). The ΔpemA and ΔpemB strains both exhibited similar dry cell weight (DCW) compared to the wild-type strain when cultured in the CD liquid medium containing Cho. However, the growth of these deletion mutants was severely retarded in the CD liquid medium lacking Cho (Fig. S3).
Morphologies of colonies and hyphal tips of the deletion mutant of pemA or pemB. (A) The conidia of the wild-type, ΔpemA, or ΔpemB strain were inoculated on a CD medium (-) or a CD medium containing 1 mM Etn (+ Etn) or 1 mM Cho (+ Cho) and incubated at 30 °C for 96 h. (B) The hyphae at the edge of the colonies in (A) were observed. (C) The conidia of the ΔpemA strain were inoculated on a CD medium containing 1 mM Etn and Cho at different concentrations: 5, 20, 100, or 1000 μM. Then, the conidia were incubated at 30 °C for 96 h. Top and side views were shown on each concentration of Cho. (D) The wild-type, ΔpemA, or ΔpemB strain was grown on a CD medium containing 1 mM Etn and 5 μM Cho, and their aerial hyphae and substrate hyphae were observed. The way that aerial and substrate hyphae were observed is shown in the box. (E) Colony areas were quantified at each concentration of Cho supplementation shown in Fig. S5. The conditions used in (C) are indicated by arrowheads.
Next, we investigated the expression levels of genes involved in PC synthesis of the wild-type strain using RT-qPCR. Although genes involved in the Kennedy pathway have not yet been reported in A. oryzae, we identified genes encoding homologous proteins of Eki1, Ect1, Ept1, Cki1, Pct1, and Cpt1 in SGD, and designated these genes as follows; ekiA, ectA, eptA, ckiA, cctA, and cptA (Table S1). Relative to the transcript levels at 24 h, those of genes involved in the Kennedy pathway, except for ckiA, increased, particularly at 40 and 48 h (Fig. S4), while those of pemA and pemB decreased. These findings raise the possibility that the Kennedy pathway may be more active than the N-methylation pathway in the PC synthesis during aerial hyphal formation.
PC levels regulate the formation of substrate and aerial hyphae
Subsequently, we added Cho to the CD medium at different concentrations to control PC supply in the ΔpemA and ΔpemB strains (Figs. 2C and S5). Ranging from 1000 to 10 μM, the density of aerial hyphae gradually decreased, and the height of aerial hyphae became shorter. In the presence of 5 μM supplementation, aerial hyphae were hardly formed (Fig. 2D). In contrast, the extension rate of substrate hyphae was almost the same as that of the wild-type strain under the same conditions (Fig. 2C). There was little difference in colony area between 1000 and 5 μM supplementations (Fig. 2E). Ranging from 5 to 0.5 μM, the area of substrate hyphae gradually decreased (Figs. 2E and S5). Finally, 0.2 μM Cho hardly recovered their growth defects on the CD medium (Fig. S5). These results suggested that the intracellular PC levels affected the hyphal elongation and differentiation process in A. oryzae. No morphological changes of the substrate hyphae were observed in either the ΔpemA nor ΔpemB strains compared to the wild-type strain when the 5–50 μM Cho was added to the medium (Fig. S6A). On the other hand, the density of substrate hyphae near the agar surface increased and aerial hyphae differentiation occurred as the concentration of Cho increased (Fig. S6B).
To ascertain the quantity of PC in the ΔpemA and ΔpemB strains in the abovementioned experiments, we harvested mycelia cultivated on the CD solid medium containing 5, 20, 100, or 1000 μM Cho covered with a cellophane sheet. We quantified the amounts of PC, PE, PI, and PS (Fig. 3A). We added 1 mM Etn to the medium to supply sufficient PE levels via the Kennedy pathway in this analysis. As Cho concentration was reduced from 1000 to 5 µM, the amounts of PC gradually decreased in the ΔpemA and ΔpemB strains. The amounts of PE increased significantly as the concentrations of Cho decreased in the ΔpemA strain, while those of PE gradually decreased under the same conditions in the wild-type and ΔpemB strains. Since PemA, a predicted PE methyltransferase, is present in the ΔpemB strain, it is considered that an increase in PE was undetectable due to the conversion of PE to PMME. It is possible that the conversion of PE to PC is suppressed when enough PC is synthesized from exogenous Cho, and that reducing levels of Cho concentration decrease the PE level in the wild-type strain by facilitating the conversion of PE to PC. A correlation analysis was performed to determine how the phospholipid balance was affected by the deletion of pemA or pemB. The correlation analysis between PC and PE showed a negative correlation for the ΔpemA strain and positive correlations for the wild-type and ΔpemB strains (Fig. 3B). The decrease in Cho concentration gradually increased the amounts of PI in the ΔpemA and ΔpemB strains; thus, the PI levels negatively correlated with the PC levels in both strains (Fig. 3B). In addition, the relationships between PI and PE were positive in the ΔpemA strain and negative in the ΔpemB strain (Fig. 3B). These results suggested that phospholipid balance may be adjusted by the PC, PE, and PI amounts in both strains. On the other hand, the amount of PS was not significantly different in the ΔpemA and ΔpemB strains (Fig. 3A). Considering that supplementation with 5 μM Cho resulted in drastically low levels of aerial hyphal formation (Fig. 2C–E), our findings suggested that a sufficient amount of PC and/or a low-level amount of PI is required to produce aerial hyphae in A. oryzae. As described below in Fig. 4, we proposed that the PC level is a significant factor in inducing the formation of aerial hyphae. In the following experiments, we focused on the characteristics of the ΔpemA and ΔpemB strains grown on the CD medium with 1000 μM Cho (High-Cho medium) and 5 μM Cho (Low-Cho medium) as aerial hyphae-formed and -barely formed conditions, respectively.
Phospholipid level of each head group at various concentrations of Cho. (A) The conidia of the wild-type, ΔpemA, or ΔpemB strain were inoculated on a CD medium containing 1 mM Etn and 5, 20, 100, or 1000 μM Cho covered with a cellophane sheet and incubated at 30 °C for 36 h. Phospholipids were extracted from the harvested mycelia and subjected to lipidome analysis. The mean values are depicted as bars (n = 4), while dots indicate individual data points. The error bars denote the standard error. Statistically significant differences are indicated by asterisks (***P < 0.001, **P < 0.01, *P < 0.05; one-way analysis of variance (one-way ANOVA)). (B) For each strain, the correlations between the amounts of phospholipids were examined. WT; red, ΔpemA; green, ΔpemB; blue. The values shown indicate the correlation coefficient for each combination. Statistically significant differences are indicated by asterisks (***P < 0.001, **P < 0.01, *P < 0.05; test of no correlation).
Phospholipid composition in substrate and aerial hyphae. (A) The conidia of the wild-type, ΔpemA, or ΔpemB strain were inoculated on a CD medium containing 1 mM Etn and 5 μM Cho (LC) covered with a cellophane sheet and incubated at 30 °C for 72 h. Then, a cellophane sheet on which mycelia were grown was transferred to the CD medium containing 1 mM Etn and 5 μM Cho (LC) or 1,000 μM Cho (HC) and incubated at 30 °C for 72 h. (B) The conidia of the wild-type, ΔpemA, or ΔpemB strain were inoculated on the “HC” medium covered with a cellophane sheet and incubated at 30 °C for 72 h. Then, a cellophane sheet on which mycelia were grown was transferred to the “LC” or “HC” medium and incubated at 30 °C for 72 h. The dotted rectangles are shown as magnified views. A region of substrate hyphae without aerial hyphae is seen between the arrowheads. (C) Mycelia of the ΔpemA or ΔpemB strain were collected from the margin of the colony with a width of 5 mm after the transfer in (A) and (B). Phospholipids were extracted from these samples and subjected to lipidome analysis. The mean values are depicted as bars (n = 4), while dots indicate individual data points. The error bars denote the standard error. Statistically significant differences among phospholipids are indicated by different letters (P < 0.01; Tukey–Kramer post-hoc test). (D) The lipidomic data in (C) were classified into two groups based on those obtained from the mycelia of either substrate hyphae or both substrate and aerial hyphae. Phospholipid level of each group was shown as a box plot and a violin plot.
Substrate hyphae formed on the Low-Cho medium can form aerial hyphae by supplying a sufficient amount of Cho
We investigated whether the substrate hyphae formed under PC-limited conditions still could form aerial hyphae. We incubated the wild-type, ΔpemA, or ΔpemB strain for three days on a Low-Cho medium covered with a cellophane sheet and transferred the sheet to a Low-Cho or High-Cho medium. Then, it was incubated for additional three days (Fig. 4A). When transferred to the High-Cho medium, aerial hyphae were differentiated from a region where substrate hyphae predominantly formed in the ΔpemA and ΔpemB strains. Furthermore, judging from the coloration of the colonies, these aerial hyphae produced conidia. The formation of aerial hyphae, conidiophores, and conidia was not observed when transferred from a Low-Cho medium to the same medium in both strains. In contrast, when the ΔpemA or ΔpemB strain was transferred from a High-Cho medium to a Low-Cho medium, aerial hyphae were hardly observed, and only substrate hyphae extended after the transfer (Fig. 4B, between arrowheads). Subsequently, we harvested mycelia from the margin of the colony on the plate medium with a width of 5 mm to analyze their phospholipid compositions before and after the transfer. There were differences between the hyphae harvested for lipidomic analyses in Figs. 3 and 4; the entire colony formed after 36 h incubation (Fig. 3), and the region excluding the colony's center formed after 72 h incubation before the medium was changed (Fig. 4). Although slight differences existed, we confirmed that the overall phospholipid compositions were correlated between these conditions (Fig. S7A). In the wild-type strain, slight fluctuations were observed before and after the medium was changed, but only few significant changes were observed (Fig. S7B). In the ΔpemA or ΔpemB strain, the PC levels significantly increased when transferred to the High-Cho medium compared to the Low-Cho medium, regardless of the type of medium prior to the transfer (Fig. 4C). These PC levels correlated with the formation of aerial hyphae (Fig. 4A–C).
The lipidomic analysis of the mycelia collected from the entire colonies showed the possibility that PI also correlated with aerial hyphal formation (Figs. 2C and 3A). However, those collected from the margins of the colonies showed no such correlation (Fig. 4C). To clarify the relationship between the aerial hyphal formation and phospholipid levels in hyphae, the data in Fig. 4C were classified into two groups based on those obtained from the mycelia of either only substrate hyphae or both substrate and aerial hyphae regardless of the concentration of Cho in the medium. Analysis of the phospholipid composition exhibited that the distribution of PC level is completely bisected between these two groups (Fig. 4D). In contrast, the distributions of levels of other phospholipids showed overlap between the two groups (Fig. 4D). These findings suggested that PC strongly influences inducing aerial hyphal formation.
PC synthesized in aerial hyphae is not efficiently utilized near the hyphal tip of substrate hyphae
Aerial hyphae were not formed after transferring from a High-Cho medium to a Low-Cho medium and the amount of PC was low in the substrate hyphae in the Low-Cho medium after the transfer, implying that PC in the posterior part of the hyphae was not efficiently transported to the hyphal tips. Therefore, PC must be synthesized near the hyphal tips for their elongation and aerial hyphal formation. In Fig. 4, PC is supplied via the Kennedy pathway in the ΔpemA or ΔpemB strain since PC synthesis via the N-methylation pathway is completely blocked. To investigate the effects of PC synthesis on the N-methylation pathway near the hyphal tips, we constructed a strain in which the synthesis of PemA or PemB is regulated via the thiA promoter19. In this strain, the translation of PemA or PemB is repressed in the presence of thiamine. We named the strain tA-pemA or tA-pemB, respectively. It was demonstrated that these strains showed a similar growth as the wild-type strain when they were grown on the CD medium without thiamine. In contrast, the strains exhibited severe growth retardation in the presence of thiamine (Fig. 5A). When these strains were transferred from a thiamine-free to a thiamine-containing medium, hyphal elongation stopped after the transfer (Fig. 5B), even though PC had been synthesized in a thiamine-free medium before the transfer to the same extent as the wild-type strain (Fig. 5C). However, the aerial hyphae elongated upward (Fig. 5B), possibly due to the de-repression of PemA or PemB synthesis within the upper part of aerial hyphae because of the relatively large distance from a thiamine-containing medium. In fact, in the colony margins containing aerial hyphae after transferred to a thiamine-containing medium, the amount of PC was significantly lower than that in a medium without thiamine (Fig. 5D) but higher than in the area where only substrate hyphae were formed (Figs. 3A and 4C).
Roles of PC synthesis at the tip of substrate hyphae. (A) The conidia of the wild-type, tA-pemA, or tA-pemB strain were inoculated on a CD medium with or without 10 μM thiamine and incubated at 30 °C for 96 h. (B) The conidia of the wild-type, tA-pemA, or tA-pemB strain were inoculated on a CD medium covered with a cellophane sheet and incubated at 30 °C for 72 h. Then, a cellophane sheet on which mycelia were grown was transferred to a CD medium with or without 10 μM thiamine (“ + thi”, “-thi”, respectively). (C, D) Mycelia of the strains were collected from the margin of the colony with a width of 5 mm before the transfer (C) or after the transfer (D) in (B). Phospholipids were extracted from these samples and subjected to lipidome analysis. The mean values are depicted as bars (n = 4), while dots indicate individual data points. The error bars denote the standard error. (C) Statistically significant differences among phospholipids are indicated by asterisks (**P < 0.01; Tukey–Kramer post-hoc test). (D) Statistically significant differences among phospholipids are indicated by asterisks (***P < 0.001, *P < 0.05; Welch’s t-test). (E) The conidia of the wild-type or tA-pemA strain were inoculated on a CD medium containing 0.01, 0.1, or 1 μM thiamine and incubated at 30 °C for 96 h.
The above results suggested that PC is not transported from the aerial hyphae to the hyphal tips of substrate hyphae and that only PC synthesized near the tips of the substrate hyphae could be utilized to elongate hyphal tips. To explore the significance of PC synthesis at the hyphal tips of substrate hyphae, the tA-pemA and tA-pemB strains were grown on the CD medium containing various thiamine concentrations (Figs. 5E and S8). At all concentrations, the phenotype that only substrate hyphae were formed was not observed. Notably, even at very low thiamine concentrations (0.002–0.1 μM), colony area gradually decreased as thiamine concentration increased, while the degree of aerial hyphal formation remained the same. This phenotype was markedly different from that of the ΔpemA or ΔpemB strain, where the colony area remained unchanged, and only aerial hyphal formation was repressed when the concentration of Cho was decreased. These results suggested that a slight decrease in PemA or PemB reduced the rate of hyphal extension and that PC synthesis at the tips of substrate hyphae is considered an essential factor for tip elongation.
Substrate hyphae have shorter acyl chains in PC and PE and longer chains in PS
In a previous study using A. nidulans, we demonstrated that the PC levels decreased and at the same time, highly unsaturated phospholipids increased in the hyphae at the colony margins compared to those at the relative center of the colony18. Since the colony margin is predominantly composed of substrate hyphae, the low levels of PC in this area align with the findings of low levels of PC in substrate hyphae (Fig. 4D). Hence, we addressed whether acyl-chain profiles change between substrate and aerial hyphae. We used lipidomic data derived from mycelia grown on the Low-Cho or High-Cho medium to calculate the mol% of detailed phospholipid moiety in each head group. We performed a principal component analysis (PCA) using these values (Fig. 6A). The PCA results showed that the ΔpemA or ΔpemB strains grown on the Low-Cho medium were distinct from the other samples, indicating differences in acyl-chain profiles between the substrate and aerial hyphae.
Analysis of phospholipid’s acyl chain composition of substrate and aerial hyphae. (A) The data obtained in Fig. 4 were used to calculate the mol% of each acyl chain length. A principal component analysis was performed using the mol% value. (B) The ratios of ΔpemA/wild-type or ΔpemB/wild-type on each acyl-chain moiety in each head group from the data obtained from the mycelia grown on the Low-Cho medium were plotted. For phospholipid species that both log2(ΔpemA/wild-type) and log2(ΔpemB/wild-type) values are greater than 2 (blue) or less than -1 (red), the names are given. (C) Using the lipidomic data obtained from the mycelia grown on the Low-Cho medium, the acyl chain length composition of each phospholipid head group was summed. The mean values are depicted as bars (n = 4), while dots indicate individual data points. The error bars denote the standard error. Statistically significant differences compared to the wild-type are indicated by asterisks (***P < 0.001, **P < 0.01, *P < 0.05; Dunnett's test). (D, E) The data obtained in Fig. 4 were used to calculate the weighted average length of phospholipid acyl chains. Blue dots indicate the mean values (n = 4), while gray dots indicate individual data points. The error bars denote the standard error. HC; High-Cho condition, LC; Low-Cho condition. (D) Asterisks indicate statistically significant differences (***P < 0.001, **P < 0.01; Welch’s two-sample t-test). (E) Statistically significant differences among phospholipids are indicated by different letters (P < 0.01; Tukey–Kramer post-hoc test).
The PCA demonstrated discernible differences in the acyl-chain profiles between the ΔpemA and ΔpemB strains despite their comparable substrate hyphal formation in the Low-Cho medium. We plotted the ΔpemA/wild-type or ΔpemB/wild-type ratios for each phospholipid moiety under the Low-Cho condition to explore the common phospholipid changes between these strains (Fig. 6B). These deletion mutants both exhibited high enrichment of C32:0-PC, C32:1-PC, C32:3-PC, and C32:1-PE; conversely, C36:0-PC, C38:2-PC, C38:6-PC, C36:6-PE, and C34:3-PS were reduced. These findings suggested that substrate hyphae have shorter acyl chains while aerial hyphae have longer ones. Thus, we focused on the phospholipid acyl-chain length by calculating the respective ratios (Fig. 6C). Compared to the wild-type strain, C32-PC, C34-PC, and C32-PE were significantly increased in the ΔpemA strain, and C34-PC, C32-PE, and C34-PE were increased in the ΔpemB strain. On the other hand, C38-PC and C36-PE exhibited a significant decrease in both strains, and C38-PE drastically decreased in the ΔpemB strain. Conversely, PS showed the opposite trend; C34-PS decreased, while C36-PS increased in both strains (Fig. 6C). There was a slight difference in PI, where only C32-PI increased in the ΔpemA strain (Fig. 6C). We calculated the weighted average of the lengths in each head group to compare the phospholipid acyl-chain lengths under various conditions. This value decreased in PC and PE but increased in PS under the Low-Cho condition, where only substrate hyphae were formed (Fig. 6D). For the results obtained in the experiments where the strains were transferred from a Low-Cho to a High-Cho medium and vice versa (Fig. 4), we computed the weighted average length of acyl chains on phospholipids extracted from the mycelia. Consequently, we observed that the type of medium after the transfer affected these values, albeit to varying degrees (Fig. 6E). These results suggested that acyl chains in PC and PE were shorter in substrate hyphae than in aerial hyphae, while those in PS were longer. Considering the relatively high concentrations of PC and PE among the main phospholipids in mycelia, A. oryzae may shorten the acyl chains, thereby increasing membrane fluidity in substrate hyphae.
Discussion
Many eukaryotes have PC as a significant component of their membrane lipids, consisting of around 40–60% of total phospholipids1,20. In S. cerevisiae, the mechanisms and regulations involved in phospholipid synthesis have been studied extensively20,21. Although some progress has been made in understanding the PC synthesis pathways and the phenotypic changes caused by perturbations in filamentous fungi, the physiological role of PC is yet to be elucidated. In this study, the amount of PC was artificially altered in the filamentous fungus A. oryzae. The study revealed that the intracellular content of PC is crucial for the differentiation process from substrate to aerial hyphae. Although several studies measured PC concentrations in A. nidulans and F. graminearum when different concentrations of Cho were added to the media22,23, this is the first report to demonstrate that aerial hyphae differentiation is regulated by the amount of PC in filamentous fungi.
In A. oryzae, ΔpemA and ΔpemB strains showed severe growth retardation in a minimal medium (Fig. 2A). In A. niger, the PEM1 ortholog, cho2, deletion strain did not grow on a minimal medium, while the PEM2 ortholog, opi3, deletion strain grew poorly on the same medium14. These results suggested that Cho2 of A. niger could perform the Opi3 function, and that there are some functional differences between Cho2 of A. niger and PemA of A. oryzae. Cho2 of A. niger is a 1077-amino acid protein, while PemA of A. oryzae is a 971-amino acid protein. Comparing these two proteins, PemA has a large deletion in its C-terminal region (Fig. S9A, Region 3). Additionally, two regions of A. oryzae PemA were deleted in A. niger Cho2 (Fig. S9A, Regions 1 and 2). These regions are thought to be located at the surfaces of the enzymes, according to the predicted structures by AlphaFold224 (Fig. S9B), and their deduced domain organizations are quite similar (Figs. S2B and S9C). Since the catalytic mechanism of Cho2 is still unknown even in S. cerevisiae, it is currently unclear whether these differences in amino acid sequences affect their catalytic activities.
Balloon-like structures were formed from the hyphal tips to the subapical regions when culturing the ΔpemA or ΔpemB strain on a medium without Cho (Fig. 2B). Previous studies in A. nidulans showed a correlation between PC levels and a chitin synthase activity was observed23. A deficiency in PC synthesis led to a shift from polarized to depolarized growth in A. nidulans25. The balloon formation phenotypes were also observed in the ΔchoC strains of A. nidulans and P. microspore10,13. These results strongly supported that a higher PC level is required for the chitin synthesis and the polarity establishment in filamentous fungi. Furthermore, since some deletion mutants of chitin synthase-encoding genes in A. nidulans and A. oryzae formed balloon-like structures26,27, it is possible that their formation in the ΔpemA and ΔpemB strains is caused by malfunctions of these chitin synthases.
Previous studies have shown that the deletion of specific genes involved in autophagy leads to the failure to form aerial hyphae in A. oryzae28,29. The defects in aerial hyphal formations with the deletion of autophagy-associated genes were also observed in other filamentous ascomycete fungi, such as Arthrobotrys oligospora30, Bipolaris maydis31, Botrytis cinerea32,33,34, Cochliobolus heterostrophus35, F. graminearum36,37,38, Fusarium verticillioides39, Magnaporthe oryzae40, and Podospora anserina41. It is currently unknown how these genes contribute to aerial hyphal formation, and the precise mechanisms that induce these phenotypic changes have yet to be determined. Recently, studies in S. cerevisiae suggested that de novo-synthesized PC is incorporated into the autophagosomes42, and that de novo PC is required for autophagosome formation43. Hence, when the ΔpemA and ΔpemB strains are cultured in a Low-Cho medium, it is likely that their autophagy does not function properly due to the lack of PC. However, it is possible that autophagy supplies the substrates of the enzymes involved in the Kennedy pathway during the formation of aerial hyphae in the wild-type strain (Fig. 1), and that the autophagy deficiency may cause depletion of PC, resulting in the absence of aerial hyphal formation in the autophagy-deficient strains. The fact that most of genes involved in the Kennedy pathway were upregulated during aerial hyphae and conidiophore formation in the wild-type strain supports this idea (Fig. S4). A closer examination of the relationships between PC and autophagy in filamentous fungi could unravel the intricate mechanism behind aerial hyphal formation.
The synthesis of PC is achieved via the Kennedy or CDP-DAG pathway, as shown in Fig. S1. In A. oryzae, the phenotype remained unchanged even if PC was obtained via either pathway as long as enough PC was supplied (Fig. 2A, Wild-type (−) vs. ΔpemA (+ Cho) or ΔpemB (+ Cho)). On the other hand, when the PC levels in hyphae were insufficient, the phenotypes varied based on the PC synthesis pathway. When limited amounts of PC were/was synthesized via the Kennedy pathway, only substrate hyphae were formed and elongated (Fig. 2A). In contrast, similar phenotypes were not observed when PC was supplied via the N-methylation pathway (Fig. 5E). This phenotypic difference could be caused by the difference of the pathways that synthesize PC. However, comparing the intracellular PC levels between both conditions (Fig. 3A and 4C vs. Fig. 5D), PC level of the ΔpemA or ΔpemB strain grown on the medium containing 5 μM Cho was lower than that of tA-pemA or tA-pemB strain grown on the medium containing thiamine. Thus, it is possible that aerial hyphal formation is dependent on the intracellular concentration of PC and not dependent on the PC synthesis pathway. In the tA-pemA or tA-pemB strain, where the translational repression of thiA is leaky even on the medium containing sufficient amount of thiamine44, the aerial hyphae could extend slightly due to the leaky production of PemA or PemB. The development of alternative approaches that do not use the thiA promoter is required to clarify the cause of these phenotypic differences between these deletion mutants and conditional mutants using thiA promoter. In S. cerevisiae, Ept1 and Cpt1 localize to the ER (endoplasmic reticulum) and/or Golgi2,21, while Pem1 and Pem2 predominantly localize to the ER21. Recent researches suggested that active vesicle transport is essential for rapidly extending hyphal tips45,46, with the Golgi playing a crucial role in the process. This raises the possibility that PC synthesis via the Kennedy pathway may be crucial for the rapid extension of hyphae.
This study demonstrated that mycelia grown on either the Low-Cho or High-Cho medium had different trends in their phospholipid acyl chain lengths (Fig. 6B–D). There could be a requirement for longer acyl chains to facilitate aerial hyphal formation. However, the possibility that the substrate hyphae grown on the High-Cho medium would have the different acyl chain composition from those grown on the Low-Cho medium could not be excluded. To determine whether phospholipids with longer acyl chains are found only in aerial hyphae or in both aerial and substrate hyphae grown on the High-Cho medium, it is necessary to establish a method for measuring the acyl chain length in each of the two types of hyphae. The ΔpemA and ΔpemB strains exhibited similar phenotypes when cultured in a Low-Cho medium. However, significant differences were observed in their phospholipid composition (Fig. 6A and C). Since the transcriptome profiles are dramatically different between the Δcho2 and Δopi3 strains of A. niger14, it is possible that phospholipid compositions could become diverse due to the global changes in the transcriptome profiles in A. oryzae. A more comprehensive lipidome and transcriptomic analysis could help understand the regulation of phospholipid balance in filamentous fungi.
Experimental procedures
Strains, growth media, and transformations
Strains used in this study are listed in Table S2. The R40Δku5-247 was used as a wild-type strain, and the R40Δku5-2ΔpyrG (Katayama unpublished) was constructed by deleting the pyrG gene of R40Δku5-2 and was used as a parental strain for transformation. The A. oryzae strains were incubated at 30 °C in a modified Czapek-Dox (CD) medium (30.0 g/L glucose, 3.0 g/L sodium nitrate, 1.0 g/L dipotassium phosphate, 0.5 g/L magnesium sulfate heptahydrate, 0.5 g/L potassium chloride, 1.0 mg/L iron(II) sulfate heptahydrate, 8.8 mg/L zinc sulfate heptahydrate, 0.4 mg/L copper(II) sulfate pentahydrate, 0.15 mg/L manganese(II) sulfate tetrahydrate, 0.1 mg/L sodium tetraborate decahydrate, 0.05 mg/L ammonium molybdate tetrahydrate, and 1 μL/L sulfuric acid). 1.5%(w/v) agar was added to prepare a solid medium. Ten μM thiamine, 10 mM uridine, 10 mM uracil, 1 mM Etn, and 1 mM Cho were added to the CD medium when necessary.
To prepare a stock conidia solution, the A. oryzae strain was incubated on the polypepton dextrin (PD) solid medium48 at 30 °C for seven days, after which 8 mL of 0.01% (v/v) Tween 80 was poured into the plate and conidia were scraped off with a cell spreader. The obtained solution was filtered with Miracloth (475855; Merck Millipore, Burlington, MA).
In solid media covered with or without a cellophane sheet, 1.0 × 103 conidia were spotted and incubated at 30 °C. For solid medium transfer, a cellophane sheet on which mycelia were grown was transferred to a new medium. In liquid media, 2.0 × 107 conidia were inoculated and incubated at 30 °C and 120 rpm. To extract total RNAs for 5′-RACE, 1.0 × 107 conidia of the NSPlD1 strain49 were incubated at 30 °C overnight in 50 mL of malt medium (30 g/L malt extract, 3 g/L yeast extract, 5 g/L glucose, 2.44 g/L uridine, pH 6.0). The transformations of A. oryzae and Escherichia coli were performed as described previously50,51,52.
Construction of plasmids and A. oryzae strains
Plasmids and primers used in this study are listed in Tables S3 and S4, respectively.
We constructed the plasmid p18-ΔpemA-pG as follows: a fragment containing an upstream (−804 to −1 bp) or a downstream (+ 3170 to +3972 bp) region of pemA was amplified from the RIB40 genomic DNA by polymerase chain reaction (PCR) using primers TSAP184 and TSAP185 or TSAP187 and TSAP188, respectively. The amplified fragments were cloned into pUC18 by SLiCE53,54,55, yielding p18-ΔpemA. The fragment containing pyrG (− 1004 to + 1394 bp) was amplified from the RIB40 genomic DNA by PCR using primers TSAP1 and TSAP2, and the amplified fragment was cloned into pUC18, yielding p18-pG. A fragment containing an upstream and a downstream region of pemA was amplified from the p18-ΔpemA by PCR using primers TSAP237 and TSAP238, and a pyrG fragment was amplified from the p18-pG using primers TSAP132 and TSAP15. These fragments were ligated by SLiCE, yielding the plasmid p18-ΔpemA-pG.
We constructed the plasmid p18-ΔpemB-pG as follows: a fragment containing an upstream (− 804 to − 1 bp) or a downstream (+ 985 to + 1784 bp) region of pemB was amplified from the RIB40 genomic DNA by PCR using primers TSAP189 and TSAP190 or TSAP192 and TSAP193, respectively. The amplified fragments were cloned into pUC18 by SLiCE, yielding p18-ΔpemB. A fragment containing an upstream and a downstream region of pemB was amplified from the p18-ΔpemB by PCR using primers TSAP239 and TSAP240, and a pyrG fragment was amplified from the p18-pG using primers TSAP132 and TSAP15. These fragments were ligated by SLiCE, yielding the plasmid p18-ΔpemB-pG.
To construct a strain in which the expression of pemA or pemB is regulated by thiA promoter (thiAp), a fragment containing the promoter region of thiA (thiAp: − 1288 to − 1 bp) was amplified from the RIB40 genomic DNA by PCR using primers TSAP25 and TSAP34. A pyrG fragment was amplified from p18-pG using primers TSAP132 and TSAP15. The thiAp and pyrG fragments were cloned into pUC18 by SLiCE, yielding p18-pG-tA. The fragments containing an upstream region of pemA (− 1093 to − 1 bp), a part of pemA coding region (+ 1 to + 1012 bp), an upstream region of pemB (− 1071 to − 1 bp), or the pemB coding region and a downstream region (+ 1 to + 1049 bp) were amplified from the RIB40 genomic DNA by PCR using primers TSAP103 and TSAP104, TSAP106 and TSAP126, TSAP108 and TSAP109, or TSAP111 and TSAP112, respectively. The fragments of the upstream and the Open Reading Frame (ORF)-encoding regions of pemA or pemB and a pyrG-thiAp fragment, amplified from p18-pG-tA using primers TSAP1 and TSAP124, were cloned into pBlueScript II SK ( +) by SLiCE, yielding the plasmid pBS-pG-tA-pemA or pBS-pG-tA-pemB, respectively.
The DNA fragments were amplified by PCR from p18-ΔpemA-pG or p18-ΔpemB-pG using primers CGUP1_UF1 and CGUP2_UR1, or from pBS-pG-tA-pemA or pBS-pG-tA-pemB using primers CGUP3_UF2 and CGUP4_UR2. Each fragment was transformed into the R40Δ ku5-2ΔpyrG strain. Several transformants were selected and confirmed by genomic PCR. The strains were designated as ΔpemA, ΔpemB, tA-pemA, or tA-pemB, respectively.
5′-Rapid amplification of cDNA ends (RACE)
The harvested mycelia were frozen in liquid nitrogen and ground using a mortar and pestle. The broken mycelia and 0.5 mm zirconia balls were added to a microtube. The mycelia were completely broken using a Multi-Beads Shocker (Yasui Kikai, Osaka, Japan) at 2700 rpm, 60 s, and repeated three times. According to the manufacturer’s instructions, the total RNA was extracted from the broken mycelia using ISOGEN II (317-07363; NIPPON GENE, Tokyo, Japan). 5'-Rapid amplification of cDNA ends (5′-RACE) was performed using the extracted RNAs and the SMARTer RACE 5′/3′ Kit (Z4858N, TaKaRa Bio, Shiga, Japan), according to the manufacturer’s instructions, with primers pemA_GSP_R and pemA_NGSP_R for 5′-RACE of pemA, pemB_GSP_R and pemB_NGSP_R for 5′-RACE of pemB (Table S4).
Microscopic observations
The hyphae were observed using a BX52 microscope (Evident, Tokyo, Japan) equipped with a CCD camera DP80 (Evident). The images captured by DP80 were acquired and processed using the cellSens software (Evident). Substrate hyphae growing on and in the solid medium were observed using a video microscope JVA-0756 (Wraymer, Osaka, Japan), equipped with a CMOS camera WRAYCAM-NOA2000 (Wraymer). The images captured by WRAYCAM-NOA2000 were acquired and processed using the Spectman software (Wraymer).
Lipid extraction and the targeted lipidomic analysis
The mycelia formed on a solid medium covered with a cellophane sheet were collected using a spatula. The harvested mycelia were lyophilized using FLEXI DRY (Kinetics, Stone Ridge, NY), and their dry cell weight was measured. This was followed by lipid extraction and the targeted lipidomic analysis of extracted lipids, as described previously18. Briefly, the lyophilized mycelia were broken using a Micro Smash MS-100R (Tomy Seiko, Tokyo, Japan), and lipids were extracted from the broken mycelia using the BUME method56. As internal standards, 1 nmol of 15:0–18:1-d7-PC, 1 nmol of 15:0–18:1-d7-PE, 1 nmol of 15:0–18:1-d7-PS, and 1 nmol of 15:0–18:1-d7-PI (Avanti Polar Lipids, Alabaster, AL) were added during the extraction. The extracted lipids were dissolved in equal volumes of methanol and acetonitrile and analyzed according to Nakao et al.57. The individual lipid contents were calculated by relating the peak area of the analyte to the peak area of the corresponding internal standard and these values were referred to as the "ratio to internal standard". To clarify sample-to-sample variation in each head group, Z scores were calculated within the experimental group using the sum of the "ratio to internal standard" in each head group.
Quantitative reverse transcription PCR (RT-qPCR)
Harvested mycelia were frozen in liquid nitrogen in a microtube and disrupted using a Freeze Crusher μT-48 (Taitec) with a 48pcs-Holder for μT-48 (TH-0248 T, Taitec) at 1000 rpm, 15 s 3 times. The metal crusher was removed, and total RNAs were extracted using a Maxwell RSC simplyRNA Tissue Kit (AS1340; Promega K.K., Tokyo, Japan). Reverse transcription of RNA was performed using a ReverTra Ace qPCR RT Master Mix with gDNA Remover (FSQ-301; Toyobo, Osaka, Japan). Quantitative PCR was performed using the 7300 Real-Time PCR System (Applied Biosystems, Waltham, MA) and THUNDERBIRD Next SYBR™ qPCR Mix (QPX-201; Toyobo, Osaka, Japan). The ΔΔCt method was utilized to compare the expression of each gene, with primers TSAP230 and TSAP231 for pemA, TSAP212 and TSAP213 for pemB, TSAP708 and TSAP709 for ekiA, TSAP208 and TSAP209 for ectA, TSAP712 and TSAP713 for eptA, TSAP705 and TSAP706 for ckiA, TSAP204 and TSAP205 for cctA, and TSAP702 and TSAP703 for cptA, respectively (Table S4). A gene encoding histone H2B was utilized as a house-keeping gene with primers QP037 and QP038.
Data analysis, statistical analysis, and visualizations of numerical data
All analyses were performed using R Statistical Software (v4.2.3; R Core Team, 2023) and RStudio (v2023.03.0 + 386; Posit team, 2023), with R packages: tidyverse (v1.3.2; Wickham H, 2019)58, multcomp (v1.4.23; Hothorn T et al., 2008), and readxl (v1.4.0; Wickham H, Bryan J, 2022). The visualization of numerical data was performed using R packages; ggplot2 (v3.4.1; Wickham H, 2016), factoextra (v1.0.7; Kassambara A, Mundt F, 2020), GGally (v2.1.2; Schloerke B et al., 2021), ggrepel (v0.9.3; Slowikowski K, 2023), and lemon (v0.4.6; Edwards S, 2022).
Data availability
All data generated or analyzed during this study are included in this published article (and a Supplementary Information file).
References
van Meer, G., Voelker, D. R. & Feigenson, G. W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 9, 112–124 (2008).
Renne, M. F. & de Kroon, A. I. P. M. The role of phospholipid molecular species in determining the physical properties of yeast membranes. FEBS Lett. 592, 1330–1345 (2018).
Henry, S. A., Kohlwein, S. D. & Carman, G. M. Metabolism and regulation of glycerolipids in the yeast Saccharomyces cerevisiae. Genetics 190, 317–349 (2012).
Carman, G. M. & Han, G.-S. Regulation of phospholipid synthesis in the yeast Saccharomyces cerevisiae. Annu. Rev. Biochem. 80, 859–883 (2011).
Daum, G., Lees, N. D., Bard, M. & Dickson, R. Biochemistry, cell biology and molecular biology of lipids of Saccharomyces cerevisiae. Yeast 14, 1471–1510 (1998).
Riquelme, M. et al. Fungal morphogenesis, from the polarized growth of hyphae to complex reproduction and infection structures. Microbiol. Mol. Biol. Rev. 82, 1–47 (2018).
Riquelme, M. Tip growth in filamentous fungi: A road trip to the apex. Annu. Rev. Microbiol. 67, 587–609 (2013).
Peng, Y. et al. Research progress on phytopathogenic fungi and their role as biocontrol agents. Front. Microbiol. 12, 670135 (2021).
Arie, T. Fusarium diseases of cultivated plants, control, diagnosis, and molecular and genetic studies. J. Pestic. Sci. 44, 275–281 (2019).
Tao, L., Gao, N., Chen, S. & Yu, J.-H. The choC gene encoding a putative phospholipid methyltransferase is essential for growth and development in Aspergillus nidulans. Curr. Genet. 56, 283–296 (2010).
Schultzhaus, Z., Yan, H. & Shaw, B. D. Aspergillus nidulans flippase DnfA is cargo of the endocytic collar and plays complementary roles in growth and phosphatidylserine asymmetry with another flippase. DnfB. Mol. Microbiol. 97, 18–32 (2015).
Takagi, K., Kikkawa, A., Iwama, R., Fukuda, R. & Horiuchi, H. Type II phosphatidylserine decarboxylase is crucial for the growth and morphogenesis of the filamentous fungus Aspergillus nidulans. J. Biosci. Bioeng. 131, 139–146 (2021).
Akhberdi, O. et al. Roles of phospholipid methyltransferases in pycnidia development, stress tolerance and secondary metabolism in the taxol-producing fungus Pestalotiopsis microspore. Microbiol. Res. 210, 33–42 (2018).
Dong, H., Wang, B. & Pan, L. Study on the interaction mechanism of phospholipid imbalance and endoplasmic reticulum protein secretion imbalance in Aspergillus niger. Biochim. Biophys. Acta - Biomembr. 1863, 183530 (2021).
Wang, J. et al. Phospholipid homeostasis plays an important role in fungal development, fungicide resistance and virulence in Fusarium graminearum. Phytopathol. Res. 1, 16 (2019).
Krijgsheld, P. et al. Development in Aspergillus. Stud. Mycol. 74, 1–29 (2013).
Adams, T. H., Wieser, J. K. & Yu, J.-H. Asexual Sporulation in Aspergillus nidulans. Microbiol. Mol. Biol. Rev. 62, 35–54 (1998).
Iwama, R. et al. Comprehensive analysis of the composition of the major phospholipids during the asexual life cycle of the filamentous fungus Aspergillus nidulans. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1868, 159379 (2023).
Shoji, J., Maruyama, J., Arioka, M. & Kitamoto, K. Development of Aspergillus oryzae thiA promoter as a tool for molecular biological studies. FEMS Microbiol. Lett. 244, 41–46 (2005).
de Kroon, A. I. P. M. Metabolism of phosphatidylcholine and its implications for lipid acyl chain composition in Saccharomyces cerevisiae. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1771, 343–352 (2007).
McMaster, C. R. From yeast to humans – roles of the Kennedy pathway for phosphatidylcholine synthesis. FEBS Lett. 592, 1256–1272 (2018).
Wiebe, M. G., Robson, G. D. & Trinci, A. P. J. Effect of choline on the morphology, growth and phospholipid composition of Fusarium graminearum. Microbiology (N Y) 135, 2155–2162 (1989).
Binks, P. R., Robson, G. D., Goosey, M. W. & Trinci, A. P. J. Relationships between phosphatidylcholine content, chitin synthesis, growth, and morphology of Aspergillus nidulans choC. FEMS Microbiol. Lett. 83, 159–164 (1991).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Markham, P. & Bainbridge, B. W. Effect of choline deprivation on the growth, morphology and ultrastructure of a choline-requiring mutant of Aspergillus nidulans. FEMS Microbiol. Lett. 90, 217–222 (1992).
Horiuchi, H., Fujiwara, M., Yamashita, S., Ohta, A. & Takagi, M. Proliferation of intrahyphal hyphae caused by disruption of csmA, which encodes a Class V chitin synthase with a myosin motor-like domain in Aspergillus nidulans. J. Bacteriol. 181, 3721–3729 (1999).
Müller, C., Hjort, C. M., Hansen, K. & Nielsen, J. Altering the expression of two chitin synthase genes differentially affects the growth and morphology of Aspergillus oryzae. Microbiology (N Y) 148, 4025–4033 (2002).
Kikuma, T., Ohneda, M., Arioka, M. & Kitamoto, K. Functional analysis of the ATG8 homologue Aoatg8 and role of autophagy in differentiation and germination in Aspergillus oryzae. Eukaryot. Cell 5, 1328–1336 (2006).
Kikuma, T. & Kitamoto, K. Analysis of autophagy in Aspergillus oryzae by disruption of Aoatg13, Aoatg4, and Aoatg15 genes. FEMS Microbiol. Lett. 316, 61–69 (2011).
Zhou, D. et al. The autophagy-related gene Aolatg4 regulates hyphal growth, sporulation, autophagosome formation, and pathogenicity in Arthrobotrys oligospora. Front. Microbiol. 11, 592524 (2020).
Sumita, T., Izumitsu, K. & Tanaka, C. Characterization of the autophagy-related gene BmATG8 in Bipolaris maydis. Fungal Biol. 121, 785–797 (2017).
Ren, W. et al. The autophagy gene BcATG8 regulates the vegetative differentiation and pathogenicity of Botrytis cinerea. Appl. Environ. Microbiol. 84, e02455 (2018).
Sumita, T., Izumitsu, K. & Tanaka, C. A serine/threonine kinase gene BcATG1 is involved in conidiation and sclerotial development in Botrytis cinerea. Mycoscience 57, 107–117 (2016).
Ren, W. et al. Ubiquitin-like activating enzymes BcAtg3 and BcAtg7 participate in development and pathogenesis of Botrytis cinerea. Curr. Genet. 64, 919–930 (2018).
Yu, H. et al. The autophagy genes ChATG4 and ChATG8 are required for reproductive development, virulence, and septin assembly in Cochliobolus heterostrophus. Phytopathology 112, 830–841 (2022).
Nguyen, L. N. et al. Autophagy-related lipase FgATG15 of Fusarium graminearum is important for lipid turnover and plant infection. Fungal Genet. Biol. 48, 217–224 (2011).
Lv, W. et al. Genome-wide functional analysis reveals that autophagy is necessary for growth, sporulation, deoxynivalenol production and virulence in Fusarium graminearum. Sci. Rep. 7, 11062 (2017).
Josefsen, L. et al. Autophagy provides nutrients for nonassimilating fungal structures and is necessary for plant colonization but not for infection in the necrotrophic plant pathogen Fusarium graminearum. Autophagy 8, 326–337 (2012).
Wang, Y. et al. The autophagy-related proteins FvAtg4 and FvAtg8 are involved in virulence and fumonisin biosynthesis in Fusarium verticillioides. Virulence 13, 764–780 (2022).
Deng, Y. Z., Ramos-Pamplona, M. & Naqvi, N. I. Autophagy-assisted glycogen catabolism regulates asexual differentiation in Magnaporthe oryzae. Autophagy 5, 33–43 (2009).
Pinan-Lucarré, B., Paoletti, M., Dementhon, K., Coulary-Salin, B. & Clavé, C. Autophagy is induced during cell death by incompatibility and is essential for differentiation in the filamentous fungus Podospora anserina. Mol. Microbiol. 47, 321–333 (2003).
Orii, M., Tsuji, T., Ogasawara, Y. & Fujimoto, T. Transmembrane phospholipid translocation mediated by Atg9 is involved in autophagosome formation. J. Cell Biol. 220, e202009194 (2021).
Polyansky, A. et al. Phospholipid imbalance impairs autophagosome completion. EMBO J. 41, e110771 (2022).
Kubodera, T. et al. Thiamine-regulated gene expression of Aspergillus oryzae thiA requires splicing of the intron containing a riboswitch-like domain in the 5′-UTR. FEBS Lett. 555, 516–520 (2003).
Kunz, P. J., Barthel, L., Meyer, V. & King, R. Vesicle transport and growth dynamics in Aspergillus niger : Microscale modeling of secretory vesicle flow and centerline extraction from confocal fluorescent data. Biotechnol. Bioeng. 117, 2875–2886 (2020).
Zhou, L. et al. Superresolution and pulse-chase imaging reveal the role of vesicle transport in polar growth of fungal cells. Sci. Adv. 4, e1701798 (2018).
Katayama, T. & Maruyama, J. Trace copper-mediated asexual development via a superoxide dismutase and induction of AobrlA in Aspergillus oryzae. Front. Microbiol. 14, 1135012 (2023).
Ogawa, M., Tokuoka, M., Jin, F. J., Takahashi, T. & Koyama, Y. Genetic analysis of conidiation regulatory pathways in koji-mold Aspergillus oryzae. Fungal. Genet. Biol. 47, 10–18 (2010).
Maruyama, J.-I. & Kitamoto, K. Multiple gene disruptions by marker recycling with highly efficient gene-targeting background (ΔligD) in Aspergillus oryzae. Biotechnol. Lett. 30, 1811–1817 (2008).
Green, M. R. & Sambrook, J. The inoue method for preparation and transformation of competent escherichia coli: “Ultracompetent” cells. Cold Spring Harb. Protoc. 2020, pdb.prot101196 (2020).
Oakley, B. R. et al. Cloning, mapping and molecular analysis of the pyrG (orotidine-5’-phosphate decarboxylase) gene of Aspergillus nidulans. Gene 61, 385–399 (1987).
Takahashi, T., Hatamoto, O., Koyama, Y. & Abe, K. Efficient gene disruption in the koji -mold Aspergillus sojae using a novel variation of the positive-negative method. Mol. Genet. Genom. 272, 344–352 (2004).
Motohashi, K. Evaluation of the efficiency and utility of recombinant enzyme-free seamless DNA cloning methods. Biochem. Biophys. Rep. 9, 310–315 (2017).
Okegawa, Y. & Motohashi, K. Evaluation of seamless ligation cloning extract preparation methods from an Escherichia coli laboratory strain. Anal. Biochem. 486, 51–53 (2015).
Okegawa, Y. & Motohashi, K. A simple and ultra-low cost homemade seamless ligation cloning extract (SLiCE) as an alternative to a commercially available seamless DNA cloning kit. Biochem. Biophys. Rep. 4, 148–151 (2015).
Löfgren, L. et al. The BUME method: A novel automated chloroform-free 96-well total lipid extraction method for blood plasma. J. Lipid Res. 53, 1690–1700 (2012).
Nakao, N. et al. Natural ligand-nonmimetic inhibitors of the lipid-transfer protein CERT. Commun. Chem. 2, 20 (2019).
Wickham, H. et al. Welcome to the Tidyverse. J. Open Source Softw. 4, 1686 (2019).
Acknowledgements
We thank Masahiro Ogawa for the helpful discussions. This work was done using the facilities of the Agro-Biotechnology Research Center at the University of Tokyo. The R40Δku5-2 and the R40Δku5-2ΔpyrG strain were kindly gifted from Jun-ichi Maruyama and Takuya Katayama.
Funding
This work was supported by JSPS KAKENHI Grant Number JP18H02119 and JST ACT-X Grant Number JPMJAX21B3.
Author information
Authors and Affiliations
Contributions
T. S.: Conceptualization, Validation, Formal analysis, Investigation, Writing—Original Draft, Writing—Review & Editing, Visualization. R. I.: Conceptualization, Formal analysis, Writing—Original Draft, Writing—Review & Editing, Visualization, Project administration, Funding acquisition. R. F.: Writing—Review & Editing. H. H.: Writing—Review & Editing, Project administration, Funding acquisition, Supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Suzawa, T., Iwama, R., Fukuda, R. et al. Phosphatidylcholine levels regulate hyphal elongation and differentiation in the filamentous fungus Aspergillus oryzae. Sci Rep 14, 11729 (2024). https://doi.org/10.1038/s41598-024-62580-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-62580-4
- Springer Nature Limited