Abstract
COVID-19 is a global pandemic that caused a dramatic loss of human life worldwide, leading to accelerated research for antiviral drug discovery. Herbal medicine is one of the most commonly used alternative medicine for the prevention and treatment of many conditions including respiratory system diseases. In this study, a computational pipeline was employed, including network pharmacology, molecular docking simulations, and molecular dynamics simulations, to analyze the common phytochemicals of ginger rhizomes and identify candidate constituents as viral inhibitors. Furthermore, experimental assays were performed to analyze the volatile and non-volatile compounds of ginger and to assess the antiviral activity of ginger oil and hydroalcoholic extract. Network pharmacology analysis showed that ginger compounds target human genes that are involved in related cellular processes to the viral infection. Docking analysis highlighted five pungent compounds and zingiberenol as potential inhibitors for the main protease (Mpro), spike receptor-binding domain (RBD), and human angiotensin-converting enzyme 2 (ACE2). Then, (6)-gingerdiacetate was selected for molecular dynamics (MD) simulations as it exhibited the best binding interactions and free energies over the three target proteins. Trajectories analysis of the three complexes showed that RBD and ACE2 complexes with the ligand preserved similar patterns of root mean square deviation (RMSD) and radius of gyration (Rg) values to their respective native structures. Finally, experimental validation of the ginger hydroalcoholic extract confirmed the existence of (6)-gingerdiacetate and revealed the strong antiviral activity of the hydroalcoholic extract with IC\(_{50}\) of 2.727 \(\upmu \hbox {g}/\hbox {ml}\). Our study provides insights into the potential antiviral activity of (6)-gingerdiacetate that may enhance the host immune response and block RBD binding to ACE2, thereby, inhibiting SARS-CoV-2 infection.
Similar content being viewed by others
Background
Coronavirus disease 2019 (COVID-19) is caused by Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) originated in Wuhan, Hubei, China. The first case diagnosed with COVID-19 was reported on December 1, 2019, while unpublished reports showed that the first confirmed case traced back to November 17 according to the South China Morning Post1. After infecting the first human case with the virus, the disease spread rapidly among people without any animal carrier. So, the World Health Organization (WHO) characterized COVID-19 as a public health emergency of international concern (PHEIC) on January 30, 2020, and a pandemic on March 11, 20202. Although several COVID-19 vaccines have been developed and widely used, WHO reported more than 700 million confirmed cases of COVID-19 including more than 6 million as of August 20233.
SARS-CoV-2 is a member of Betacoronavirus that includes severe acute respiratory syndrome (SARS) and Middle Eastern respiratory syndrome (MERS) coronaviruses which are associated with fatal diseases as well4. They are enveloped viruses with a positive single stranded RNA. They express four structural proteins; Spike (S) protein, envelope (E) protein, membrane (M) protein, and nucleocapsid (N) protein5. The life cycle of SARS-CoV-2 consists of three stages; (1) attachment and entry, (2) replication and synthesis, and (3) viral assembly and release. Spike protein is responsible for the first phase using two domains where S1 called receptor binding domain (RBD) binds to host cell’s receptor and S2 is needed for cell membrane fusion. Structure analysis of RBD of SARS-CoV-2 shows high similarity to SARS-CoV RBD which proposes that SARS-CoV-2 may use human angiotensin-converting enzyme 2 (ACE2) for attachment as SARS-CoV does6. Studies suggest that blocking the interaction between ACE2 and spike protein can suppress the virus entry into the host cell and prevent the development of the disease. Importantly, studies showed that ACE2 catalytic activity is independent of SARS-CoV binding, meaning that it is possible to develop drugs that selectively bind to ACE2 domain used for viral attachment without affecting normal functions in the cell7. The genome of coronaviruses consists of around 30, 000 nucleotides that encode two polyproteins named; pp1a and pp1ab8, representing one of the longest genomes of viruses. They are cleaved by two proteases; papine-like protease (PLpro) and main protease (Mpro). Both proteases are necessary for the virus’ survival in the host cell as they cleave the polyprotein to make RNA of structural and non-structural proteins9,10. It was found that Mpro is conserved among other Betacoronavirus (SARS-CoV and MERS)11. Therefore, the identification of protease inhibitors is a promising approach against coronavirus infection.
Accelerating anti-coronavirus drug development is urgently needed as the virus is rapidly evolved in many variants of concern such as Alpha, Beta, Gamma, Delta, and Omicron that may have increased viral infectivity, increased pathogenicity, different clinical symptoms, or higher resistance to vaccines and treatments12. Since antiquity, natural products and their derivatives have been used in traditional and ancient medicine to treat various diseases and infections. To date, more than 65% of the global population prefers using nature-derived medicines as the first line of treatment for many diseases, owing to their safety and fewer adverse effects13,14. Nature has provided a treasure trove of small molecules for drug discovery and development, especially for infectious diseases. So far, many phytochemicals have proved potential antibacterial and antiviral activities15. Recently, there has been a significant renaissance of interest in the natural product discipline for the rediscovery of novel and bioactive scaffolds. The National Health Commission in China has issued protocols for the use of herbal medicine as monotherapy or in combination with Western medicine16,17 based on previous experience and significant clinical results for the treatment of SARS-CoV and MERS18. One of the most commonly natural products used in Chinese medicine is Ginger (Zingiber officinale) as it has many effects including anticancer19,20, anticoagulant21, anti-inflammatory22, antioxidant23,24,25, antifungal26, and antimicrobial27. Many studies showed that ginger may help prevent or treat several diseases such as cardiovascular diseases28, neurodegenerative diseases29,30, and respiratory diseases31,32. Experimental studies showed the antiviral activity of ginger compounds against human respiratory syncytial virus (HRSV)33 and Chikungunya virus (CHIKV)34. Gingerenone A (Gin A) is a derived compound from ginger roots that can inhibit replication of influenza A virus (IAV) including subtypes (H1N1, H5N1, and H9N2)35. Additionally, a clinical trial showed that feeding patients with acute respiratory distress syndrome (ARDS) with an enteral diet enriched in ginger may improve oxygenation and decrease the duration of mechanical ventilation and length of stay in intensive care unit36. To date, many computational studies have been performed to investigate the potential antiviral activity of different natural products including ginger to help accelerate the drug discovery process against COVID-19. Muhammad et al.37 docked 50 derivatives of shogaols against SARS-CoV-2 Mpro where 39 of them achieved lower binding energies than chloroquine which was docked as a reference ligand. Gingerols were also docked against different SARS-CoV-2 receptors and showed potential inhibitory effects. For instance, (6)-gingerol can bind to Mpro, spike protein, and RNA binding protein38, and Cathepsin K39. Haridas et al.40 proposed (6)-gingerol, (8)-gingerol, (10)-gingerol, (10)-shogaol, (8)-paradol, and (10)-paradol as potential inhibitors of viral entry by binding to the spike RBD and human ACE2 as well. Al-Sanea et al.41 assessed the anti-SARS-CoV-2 activity of methanolic extract of ginger, ginger silver nanoparticles (AgNPs), strawberry methanolic extract, and strawberry AgNPs using MTT assay and showed promising results for both ginger AgNPs and strawberry methanolic extract. Studies in Saudi Arabia42, Bangladesh43, Tunis44, several countries in Africa45,46, and Iran47 indicated that COVID-19 patients who consumed ginger alone or combined with other herbs exhibited lower hospitalization rate or reduced clinical symptoms. According to these previous studies, we performed a combined in silico approach of network pharmacology, molecular docking, and dynamics analyses to identify the most promising antiviral ginger constituents out of 34 ginger compounds and to help understand the mechanism underlying their potential antiviral activity as summarized in Fig. 1.
Methods
Collection of chemical compounds and target genes
Ginger has many constituents, varying from one country to another based on the place of the origin and whether the rhizomes are fresh or dry48. An extensive literature survey was conducted to retrieve the common phytochemicals of ginger rhizomes through PubMed49 and Google Scholar platforms. Lipinski rule of five (RO5)50 was used for absorption, distribution, metabolism, and excretion (ADME) analysis of ginger compounds using Swiss-ADME online server51. Only compounds meeting the RO5 criteria were included in the study analysis. For target gene retrieval, Similarity ensemble approach (SEA)52, TargetNet53, and SwissTragetPrediction54 databases were used to collect target genes of the corresponding chemicals. Each database has a different naming format for the output, so all queries were mapped to the HUGO Gene Nomenclature Committee (HGNC) gene symbol55. Then, they were pooled to remove any duplicate genes from different resources.
Construction and analysis of the ginger network
Cytoscape v3.7.1 software56 was used to construct a network of herb-chemical-target interactions as an initial network. Protein-protein interactions (PPIs) of the predicted targets were retrieved from STRING v11.0 database57. STRING provides seven resources for physical and functional interactions. Among them, we selected experimental, databases, and coexpression resources with a minimum confidence score of 0.4. STRING network was superimposed on the initial network in Cytoscape to construct the ginger network.
Cytoscape plugin Network Analyzer58 was used to analyze the network and calculate its topological parameters such as degree centrality. To prioritize the hub genes, we removed any gene with a degree score lower than the average. The remaining genes with their corresponding chemicals and ginger compound were referred to as the ginger hub network. Then, “Cluster FI Network” option provided by Cytoscape plugin Reactome FI59 was used to cluster the hub network into sub-networks using spectral partition based network clustering algorithm60 and a size cutoff was used to filter out small modules.
To reveal biological insights into each network module, functional enrichment analysis including gene ontology (GO) terms and pathways was performed using “Analyze Module Functions” option provided by “Reactome FI” plugin. False discovery rate (FDR) value was limited to less than or equal to \(1 \times 10^{-6}\) to prioritize the most significant terms.
Finally, SARS-CoV-2-human PPIs were retrieved from BioGRID 4.4 database61 and superimposed on the ginger hub network to highlight the viral protein interacting with human targets of ginger constituents.
Molecular docking simulations
Molecular docking was performed to explore the potential binding of ginger constituents with SARS-CoV-2 proteins and human ACE2. Crystal structures of target proteins were retrieved from Protein Data Bank in PDB format62 as follows: (i) main protease with unliganded active site (Mpro) [PDB ID: 6Y84]63. Molecular Operating Environment (MOE) v.2019.01 software64 was used for the preparation of proteins and ligands as well as data plotting. MOE SiteFinder module was used to select (Mpro) binding pocket within its only chain. The site containing the catalytic dyade (His41 and Cys145 ) was selected with pocket coordinates (7.268, \(-3.759\), 27.023) and size of 60 alpha spheres, (ii) spike receptor-binding domain (RBD) bound with ACE2 [PDB ID: 6M0J]65, only chain E was kept that represents spike RBD and binding pocket with coordinates (\(-32.288\), 9.259, 28.774) and size of 60 alpha spheres was selected and (iii) human ACE2 receptor [PDB ID: 1R4L] as it is responsible for the virus attachment on the host cell66 Chain A was selected for ACE2 and binding pocket coordinates were centered on (42.699, 6.454, 25.753) with a pocket size of 60 alpha spheres (Table 1). The chemical structures of ligands were extracted from PubChem67 database in SDF format. Water molecules and ions were removed from protein structures and only main-chain amino acids were retained which are essential for binding of the co-crystallized ligand. Polar hydrogen atoms were added by employing the 3D protonation feature in MOE. ASN, GLN, and HIS flips were allowed during 3D protonation. Then, energy minimization was carried out for the three complexes employing AMBER10:EHT force field for energy minimization; where AMBER parameters are suitable for proteins and nucleic acids (ff10); EHT parameters are suitable for small molecules then they were refined to RMS Gradient of 0.1 Kcal/mol/Å. Docking was carried out in the same site of the co-crystallized ligand for both RBD and ACE2 structures while MOE SiteFinder was used to choose the docking site based on a literature search for Mpro structure. For docking scoring, triangle matcher placement was chosen; the first rescoring function was set to be London dG while GBVI/WSA dG was chosen to be the second rescoring function and finally, it was refined using forcefield retaining 30 docked structures for each compound. Root mean square deviation (RMSD) values between the docked conformation and the reference conformation presented in Å were utilized68 to validate the docking performance.
Molecular dynamics (MD) simulations
Among all compounds, (6)-gingerdiacetate displayed the best binding interactions and free energies over the three target proteins. Accordingly, It was chosen to be subjected to 100 nanoseconds (ns) molecular dynamics (MD) simulation against the three target proteins to confirm its stable binding affinity towards them. MD simulations were performed using GROningen MAchine for Chemical Simulations (GROMACS) 2020.6 software package69 where the CHARMM36 forcefield was used for protein topology preparation and the official CHARMM General Force Field server (CGenFF) for ligand topology preparation. The solvation method used here was a dodecahedron box of common simple point charge (SPC) water model where explicit solvent periodic boundary conditions were applied. Charge neutralization using sodium and chloride ions was carried out for the solvated complexes. The systems were subjected to energy minimization to resolve any steric clashes or inappropriate geometry employing the steepest descent method through 5000 steps. System equilibration was also manipulated to ensure a reasonable starting structure using NVT; equilibration under constant number of particles, volume, and temperature (NVT) for 100 picoseconds (ps) using a Berendsen thermostat70. Also, re-equilibration was performed for another 100 ps under constant pressure (Isothermal-isobaric (NPT) ensemble) using the Parrinello-Rahman barostat using a time step of 2 femtoseconds (fs) for each equilibration round71. Finally, MD production phase was carried out for 100 ns using a time step of 2 fs at a constant temperature of 300 \(^{\circ }\)K and constant pressure at 1 atm. For MD trajectories analysis, the complexes were re-centered and re-wrapped within the unit cells using the “trjconv” function of GROMACS. Then, they were analyzed using RMSD of the protein backbone vs the protein-ligand complex referenced to its initial position and the radius of gyration (Rg). GRaphing and Advanced Computation and Exploration of data (Grace) tool was used for data plotting.
Experimental procedure
Plant material
Fresh ginger rhizomes were obtained from the Agricultural Research Center, Ministry of Agriculture, Cairo. A voucher specimen was deposited at the Herbarium of the National Research Centre under the accession number M205. We declare that the collection of plant material is in agreement with relevant institutional, national, and international guidelines and legislation.
Hydro-distillation extraction of ginger volatile oil
Fresh ginger rhizomes (200 g) were hydro-extracted with Clevenger-type apparatus for 3 h. The oily layer was isolated separately by n-hexane, then dried using (0.5 g) anhydrous Na\(_{2}\)SO\(_{4}\), and finally stored in glass vials at \(-20^{\circ }\)C for further analysis.
GC–MS analysis of ginger volatile oil
GC–MS analysis was performed using an Agilent Technologies system (7890B), equipped with a mass spectrometer detector (5977A), headspace sampler (7697A), and HP-5MS capillary column (30 m \(\times \) 0.25 mm internal diameter and \(0.25\,\upmu \hbox {m}\) film thickness). Hydrogen gas was used as the carrier gas for the analysis at a flow rate of 1.0 ml/min with a splitless injection mode. The following temperature program was adopted: 50 \(^{\circ }\)C for 2 min; rising at 10 \(^{\circ }\)C /min to 250 \(^{\circ }\)C; rising at 15 \(^{\circ }\)C/min to 300 \(^{\circ }\)C and held for 10 min. The injector and detector were held at 280 \(^{\circ }\)C and 300 \(^{\circ }\)C, respectively. Mass spectra were obtained by electron ionization (EI) at 70 eV; using a spectral range of m/z 30–550 and solvent delay 5 min. Identification of different constituents was determined by comparing the fragmentation pattern with those stored in Wiley and NIST Mass Spectral Library data.
Headspace GC–MS for volatile analysis
Three grams of fresh ginger rhizomes were placed into a 20 ml headspace vial and immediately sealed with silicone rubber septa and aluminum caps for the absorption of the volatile compounds. They were transferred to the headspace and heated up to 80 \(^{\circ }\)C for 20 min while being agitated, and then introduced directly into the GC injector with a loop temperature of 120\(^{\circ }\)C, and transfer line temperature of 140 \(^{\circ }\)C. GC–MS analysis was executed on an Agilent Technologies system (7890B), equipped with a mass spectrometer detector (5977A), headspace sampler (7697A), and HP-5MS capillary column (30 m \(\times \) 0.25 mm internal diameter and \(0.25\,\upmu \hbox {m}\) film thickness). Analyses were carried out as previously described in72.
Ginger extraction for LC–MS analysis
Fresh ginger rhizomes were cut into small parts (250 g) and extracted with methanol: water (7:3 v/v). The solvent was then evaporated under reduced pressure till dryness and kept refrigerated for further analysis.
LC–MS/MS analysis of ginger hydroalcoholic extract
Instrument: The analysis of the sample was performed using liquid chromatography–electrospray ionization–tandem mass spectrometry (LC–ESI–MS/MS) with an ExionLC AC system for separation and SCIEX Triple Quad 5500+ MS/MS system equipped with electrospray ionization (ESI) for detection.
Conditions: The separation was performed with a Ascentis\(\circledR \) Express 90 Å C18 Column (2.1 \(\times \) 150 mm, \(2.7\,\upmu \hbox {m}\)). For MS/MS analysis, negative ionization mode was applied with a scan (EMS–IDA–EPI) from 100 to 1000 Da for MS\(_{1}\) with the following parameters: curtain gas: 25 psi; IonSpray voltage: \(-4500\); source temperature: 500 \(^{\circ }\)C; ion source gas 1 & 2 were 45 psi and from 50 to 1000 Da for MS\(_{2}\) with a declustering potential: \(-80\); collision energy: \(-35\); collision energy spread: 15. Compounds’ identification was performed using MS-DIAL. The mobile phases consisted of two eluents A: 5 mM ammonium formate pH 8; B: acetonitrile (LC grade). The mobile phase gradient was programmed as follows: 5% B at 0–1 min, 5–100% B from 1–20 min, 100% B from 20 to 25 min, 5% at 25.01, 5% from 25.01–30 min. The flow rate was 0.3 ml/min and the injection volume was 5 \(\upmu \hbox {l}\).
For MS/MS analysis, positive ionization mode was applied with a scan (EMS-IDA-EPI) from 100 to 1000 Da for MS\(_{1}\) with the following parameters: curtain gas: 25 psi; IonSpray voltage: 5500; source temperature: 500 \(^{\circ }\)C; ion source gas 1 & 2 were 45 psi and from 50 to 1000 Da for MS\(_{2}\) with a declustering potential: 80; collision energy: 35; collision energy spread: 15. The mobile phases consisted of two eluents A: 5 mM ammonium formate pH 3; and B: acetonitrile (LC grade).
Quantitative determination of pungent substances in the ginger crude extract
The separation of gingerol and shagaol was carried out by Waters alliance HPLC equipped with a photodiode array detector and oven. The mobile phases consisted of two eluents A: water 0.5% Acetic acid; B: acetonitrile 0.5% acetic acid (HPLC grade). The mobile phase gradient was programmed as follows: (65% B–35% A) at 0-9.5 min, (75 % B–25% A) from 10 to 16 min, (65% B–35%A) from 16.10 to 20 min. The flow rate was 2.0 ml/min and the injection volume was 25 \(\upmu \hbox {l}\). Column C18 (250 \(\times \) 4.6 mm 5 \(\upmu \)m), column temperature 25 \(^{\circ }\)C
MTT cytotoxicity assay
To assess the half-maximal cytotoxic concentration (CC50), stock solutions of the HD oil were prepared in 10% DMSO in ddH\(_{2}\)O and diluted further to the working solutions with DMEM. The cytotoxic activity of the extracts was tested in VERO-E6 cells by using the 3-(4, 5-dimethylthiazol -2-yl)-2, 5-diphenyltetrazolium bromide (MTT) method with minor modifications73.
Determination of the antiviral activity of ginger, determination of the Inhibitory concentration 50 (IC50)
Determination of the antiviral activity of ginger hydroalcoholic extract and the hydrodistilled oil against SARS-CoV-2 was performed as described in73.
Results
Screening of ginger compounds and predicted targets
In general, ginger contains over 400 chemical compounds that vary from one place to another74. We retrieved a total of 34 constituents that were found as the most common phytochemicals of ginger rhizomes. All compounds meet the RO5 criteria and were used for network and docking analyses as listed in Table S1. In total, 5225 predicted targets were retrieved from SEA, TargetNet, and SwissTargetPrediction databases for the 34 ginger compounds (Table S2). Pooling and filtration resulted in 784 distinct genes where 29 genes are common among the three databases. Across all databases, the pungent compounds including gingerols and shogaols75 have the highest number of predicted targets.
Biological insights into the mechanism of ginger constituents
Human protein-protein interactions (5849 queries) were downloaded from STRING connecting 696 targets (Table S3). PPIs network was merged with the herb-chemical-target network, resulting in the ginger network that consists of 731 nodes (1 herb, 34 chemicals, and 696 target proteins) connected by 9976 edges. The initial network was filtered based on degree centrality by removing any node with a degree below the average (23), resulting in 284 nodes (1 herb, 34 chemicals, and 249 hub targets) connected by 5740 edges. The hub network was clustered into 7 highly connected groups of genes defined as network modules (Table S4). Two modules of size 2 were removed as they were too small while the other five modules ranged in size from 21 to 107 nodes as shown in Fig. 2.
Functional enrichment analysis was performed to identify the most enriched cellular components (CC), biological processes (BP), molecular functions (MF), and pathways of each network module. The analysis resulted in 28, 102, and 65 for GO terms as CC, BP, and MF, respectively, and 409 pathways with FDR range from \(1 \times 11^{-16}\) to \(1 \times 10^{-6}\). The top GO terms and pathways of each module are given in Table 2 and Table 3, respectively. GO analysis identified that module 0 is enriched in plasma membrane (CC), signal transduction and inflammatory response (BP), and protein kinase and ATP binding (MF). Module 1 is enriched in mitochondrion (CC) as it is associated with aerobic respiration-related processes. Module 2 is mainly involved in cell cycle and cell division. Module 3 is associated with regulation of RNA metabolic pathways while module 4 is involved in lipid metabolism and xenobiotic metabolic process. Reactome pathway analysis provided more detailed terms such as PI3K-Akt, Rap1, Ras, MAPK, PDGFR-beta, and cAMP signaling pathways that are significantly enriched in module 0. Enriched pathways with each module are matching with the previously mentioned GO terms. Additionally, Reactome identified infectious disease pathways that are significantly enriched with gene sets of module 0 such as Human cytomegalovirus infection, Hepatitis B, and Human immunodeficiency virus 1 infection, and module 2 such as Human T-cell leukemia virus 1 infection.
Finally, superimposing SARS-CoV-2-human PPIs on the ginger hub network highlighted 71 target genes interacting with 27 viral proteins (Table S5). Top 10 viral proteins include structural proteins such as membrane (M), envelope (E), and spike (S) which are connected to 30, 18, and 12 hub targets, respectively. Accessory proteins (ORF7b, ORF14, ORF7a, ORF9b, and ORF3a), and non-structural proteins (nsp6 and nsp4) are also ranked within top 10 with degree of 11 to 26. The top connected human targets to viral proteins belong to module 0 (SRC, PTPN1, and RAC1), module 1 (NDUFS1, NDUFS2, and NDUFV2), and CES1 from module 4 as they are connected to at least seven viral proteins. For an integrative view, a full network was constructed to combine all types of nodes (1 herb, 34 chemicals, 245 corresponding hub targets, and 27 SARS-CoV-2 viral proteins) with different interaction types as shown in Fig. 3.
Identification of candidate chemicals against COVID-19 infection based on docking analysis
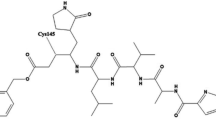
Molecular docking simulations showed that gingerols and shogaols which are the major phenolic compounds in ginger performed better than other compounds against the three target proteins (Table S6). The six top-ranked constituents based on docking score \(\Delta \)G (Kcal / Mol) with two SARS-CoV-2 receptors and human receptor protein ACE2 are listed in Table 4. (6)-Gingerdiacetate has the best docking scores of \(-8.19\) Kcal/mol and \(-7.23\) Kcal/mol against Mpro (6Y84) and ACE2 (1R4L), respectively. For the RBD of spike protein (6M0JE), (10)-gingerol and (8)-gingerol scored \(-6.91\) Kcal/mol and \(-6.55\) Kcal/mol, respectively, which are slightly lower than (6)-gingerdiacetate score of \(-6.22\) Kcal/mol, meaning that they have better affinity towards RBD than the other 2 targets unlike (6)-gingerdiacetate that shows better affinity towards Mpro and ACE2. The interactions formed between the three top-ranked ligands and the target proteins were visualized using MOE to select the most favorable compound for further analysis. We found that (6)-gingerdiacetate formed four hydrogen bonds with Mpro pocket (Ser40, Asn142, Cys145) as shown in Fig. 4a while each of (10)-gingerol and (8)-gingerol formed only two hydrogen bonds. Although (10)-gingerol has a better score with RBD compared to (6)-gingerdiacetate, it only formed hydrophobic interactions with the protein structure, without forming any hydrogen bonds. Similarly, (6)-gingerdiacetate and (10)-gingerol have almost the same docking score with ACE; however, only (6)-gingerdiacetate formed hydrogen interactions with ACE2 (Fig. 4c). Accordingly, (6)-gingerdiacetate showed the best interactions with the best binding scores against the three target proteins. In turn, to assess the postulate that (6)-gingerdiacetate is the best candidate as a potential inhibitor targeting SARS-CoV-2 Mpro, spike RBD and Human ACE2, 100 ns MD simulations were carried out to confirm the stability of the three complexes.
Two-dimensional representation of protein-ligand interactions between (6)-gingerdiacetate and the target proteins; (a) Mpro (PDB ID: 6Y84); (b) RBD of spike protein (PDB ID: 6M0JE); (c) human ACE2 (PDB ID: 1R4L). Images were created using MOE v.2019.01.
MD analysis of the best docked ligand with target proteins
According to docking analysis, (6)-gingerdiacetate was selected for MD simulations as it has the minimum binding energy scores and best hydrogen interactions as well with the viral and human receptors. MD trajectories over 100 ns for the three complexes were analyzed to investigate the stability of the proteins with bound ligand using RMSD parameter as shown in Fig. 5. Interestingly, Mpro-ligand complex showed similar deviation as the native structure in the simulation period from 25 ns to 55 ns with very low fluctuations, but afterward it shows a very high fluctuation till the end of simulation with RMSD between 0.5 nm to 1 nm, indicating that the ligand-complex lost its stability after about 55 ns and the ligand left the binding pocket of the receptor as shown in Fig. 5a. On the other hand, RBD and ACE2-ligand complexes were stable and showed similar RMSD patterns to their native structures (Fig. 5b, c), respectively) with very low fluctuation \(\sim 0.05\) nm for the RBD throughout the whole 100 ns simulation. ACE2 complex reached stability throughout the last 50 ns in the simulation with \(\sim 0.05\) nm fluctuation as well. To confirm the complexes’ stability and evaluate the effect of ligand binding on the protein structure, radius of gyration (Rg) was calculated for each protein in apo and ligand-bound form as shown in Fig. 6. Similar to RMSD plots, Mpro-ligand complex shows less stability than the native system at \(\sim 60\) ns confirming its unstable binding in long simulation (Fig. 6a). RBD and its respective ligand complex have the same pattern of Rg values which ranges from 1.82 nm to 1.86 nm (Fig. 6b). The binding of the ligand to ACE2 did not change its structure as both systems exhibited almost the same pattern as well with a bit higher values of Rg (2.5 nm to 2.6 nm), confirming the stability of these two complexes (Fig. 6c). Overall, MD analysis of 100 ns for RBD and ACE2 systems show consistency with their corresponding docking results while only the first 50 ns of Mpro are consistent with docking analysis.
Radius of gyration (Rg) plots of target proteins in apo structure vs (6)-gingerdiacetate-protein complexes over 100 ns simulation run: (a) apo and ligand-bound Mpro, (b) apo and ligand-bound RBD; (c) apo and ligand-bound ACE2. Apo structures and protein-ligand complexes are colored in black and red, respectively.
GC–MS analysis of the hydro-distilled oil (HD) of ginger rhizomes
Results of the GC–MS analysis of the hydro-distilled (HD) oil of ginger rhizomes are listed in Table 5. Overall, 33 volatile components were identified belonging to different metabolite classes such as monoterpenes and sesquiterpenes hydrocarbons, and their oxygenated congeners. Compounds identification was based on their mass spectral data (MS) and relative retention time compared to those of Wiley spectral library collection and NIST library databases.
Headspace GC–MS analysis of fresh ginger rhizomes
Further, headspace GC–MS analysis was performed to compare the volatiles of HD oil to those of the fresh ginger rhizomes, Table 6.
Phytochemical analysis of hydroalcoholic extract of ginger by LC/MS–MS
For the characterization of the nonvolatile constituents present in the hydroalcoholic extract of ginger rhizomes, liquid chromatography coupled to tandem mass spectrometry (LC–MS/MS) was recruited. The LC–MS/MS analysis of the hydroalcoholic extract of ginger rhizomes reflected the prevalence of 6-, 8-, and 10-gingerol, (6)-gingerdiacetate, and 6-, 8- and 10-shogaol, with fragmentation patterns agreeing with that reported in the literature (Table 7).
Quantitative determination of the pungent principles of ginger hydroalcoholic extract by HPLC
Quantitative determination of the pungent principles revealed the prevalence of 10-gingerol followed by the 6-gingerol as listed in Table 8 and shown in Fig. 7
In vitro antiviral activity against SARS-CoV-2 virus
Initially, the cytotoxicity assay was performed to determine the safety of the ginger and the HD oil. The CC\(_{50}\) of the ginger hydroalcoholic extract on Vero E6 cells was 3.480 \(\upmu \hbox {g}/\hbox {ml}\) while the CC\(_{50}\) of the HD ginger oil on Vero E6 cells was 56.1 \(\upmu \hbox {g}/\hbox {ml}\) (Fig. 8). Following, the extract and the HD oil were tested for the inhibition of the SARS-CoV-2 virus replication. Inhibitory concentration (IC\(_{50}\)) was calculated to determine the dose that causes inhibition of 50% of the virus replication. The crude ginger extract showed strong antiviral activity as reflected by its IC\(_{50}\) value calculated as 2.727 \(\upmu \hbox {g}/\hbox {ml}\) whereas the HD ginger oil showed low antiviral activity as reflected by its IC\(_{50}\) value calculated as 42.8 \(\upmu \hbox {g}/\hbox {ml}\) (Fig. 8).
Dose response and inhibition curves for ginger hydro-distilled oil (a) and ginger hydroalcoholic extract (b) showing the half maximal cytotoxic concentration; CC\(_{50}\) in Vero E6 cells and inhibitory concentration 50% (IC\(_{50}\)) against NRC-03-nhCoV which was calculated by using the nonlinear regression analysis of the GraphPad Prism.
Discussion
Natural products are considered as major sources for drug discovery for infectious diseases and other therapeutic areas15,79. Many nature-based existing drugs have been clinically investigated to be used in the treatment of COVID-19 such as the anti-malaria drugs; chloroquine and hydroxychloroquine. However, it was found that treatment with chloroquine caused acute toxicity cases as reported by the United States80 and Nigeria81 media. Hydroxychloroquine showed an inhibitory effect on SARS-CoV-2 infection in vitro and less toxicity effect as well but receiving an overdose may result in poisoning82. Therefore, investigating other natural products that have higher efficiency and minimal side effects is needed. In this study, we conducted a computational analysis of network pharmacology, molecular docking, and molecular dynamics simulations to identify potent ginger compounds that could be capable of preventing viral replication by targeting the viral or the host proteins, followed by experimental validation to predict the antiviral activity of ginger bioactive compounds.
Analysis of the integrative network (herb-chemicals-targets-viral proteins) provides insights into the therapeutic effects of ginger compounds. Functional enrichment analysis showed that hub targets are significantly involved in (i) cellular processes such as cell cycle and cell communication, (ii) innate immune response and inflammatory response, (iii) regulation of biological processes such as RNA transcription by polymerase II, cell migration, and apoptosis, and (iv) cellular metabolic process such as respiratory electron transport chain, xenobiotic metabolic process, and lipid metabolism. Reactome pathway analysis showed significantly enriched infectious disease pathways and signaling pathways that are closely linked to modulation of the immune system such as chemokine signaling pathways, Rap1 signaling pathway, Ras signaling pathway, and MAPK signaling pathway83. These results suggest that ginger compounds may treat or reduce the severity of COVID-19 by protecting the host cell and enhancing the defense mechanism against viral infection.
Molecular docking was performed to assess the binding between both viral proteins and human ACE2 against the candidate chemicals. Docking results suggest (6)-gingerdiacetate, (10)-gingerol, and (8)-gingerol as promising compounds because of their high negative binding scores with all three receptors. A closer look at their interaction with the proteins’ pockets postulates that among 34 compounds, (6)-gingerdiacetate was the top hit against the three target receptors with the highest binding energy score and the best interactions. The binding of (6)-gingerdiacetate with target receptors is stabilized by different interaction types as shown in Fig. 4. To confirm the stability of the identified compound with the three target proteins a 100 ns MD simulation was performed. MD analysis confirms the very high stability of (6)-gingerdiacetate with both spike RBD and human ACE2 while it loses its stability after \(\sim 50\) ns from binding with SARS-CoV-2 Mpro.
Moreover, we conducted experimental assays for ginger analysis. GC–MS analysis of HD oil of ginger showed that sesquiterpene hydrocarbons as the predominant class (\(\approx \) 43 %), followed by oxygenated monoterpenes (\(\approx \) 38%). Whereas the monoterpene hydrocarbons and the oxygenated sesquiterpenes were found at much lower percentages (\(\approx \) 10%, and \(\approx \) 8% respectively). Concerning the predominant sesquiterpenes, \(\beta \)-sesquiphellandrene was the major detected compound (29.38%) agreeing with the former reports84. \(\beta \)-sesquiphellandrene is believed to be a major contributor to the anticancer properties of ginger and showed in vitro antiviral activity against rhinovirus 1B84. While \(\alpha \)-curcumene contributed to 8.12% of the volatile constituents of the HD ginger oil and is reported as an antioxidant and anti-inflammatory agent85. Other detected sesquiterpene hydrocarbons were \(\beta \)-elemene, \(\gamma \)-elemene, valencene, \(\gamma \)-muurolene, \(\beta \)-bisabolene, and \(\alpha \)-farnesene. The second abundant volatile class was the oxygenated monoterpenes accounting for almost 38% of the oil constituents. Citral isomers constituted 13.0 & 7.3% of the volatile content which is thought to be partly responsible for the antiviral activity of Lippia citriodora against the yellow fever virus86. Following was eucalyptol (1, 8 cineole) comprising 7% of the volatiles. Clinically, eucalyptol inhalation was effective in asthmatic patients through its anti-inflammatory and analgesic properties87 and showed antiviral activity against influenza A virus88. Whereas oxygenated sesquiterpenes comprised only 7.8% of the total volatiles, and were exemplified by sesquisabinene hydrate isomers, elemol, zingiberenol, and others as tabulated in Table 5. Comparably, the headspace GC-MS analysis revealed the predominance of monoterpene hydrocarbons (70.31%) followed by sesquiterpene hydrocarbon (14.31%), with the occurrence of other miscellaneous compounds such as aldehydes, ketones, and alcohols. Sabinene was the major detected compound in the fresh sample rhizomes of ginger (30.64%) followed by camphene (25.13%) then zingiberene (7.94%) which was previously reported to interact with key residues in the catalytic domain of the Mpro in the docking analyses89. While \(\beta \)-myrcene, D-limonene, sabinene, eucalyptol (1,8-Cineole), \(\beta \)-bisabolene, \(\alpha \)-farnesene are common in both hydro-distilled (HD) oil and fresh ginger rhizomes albeit with different percentages. Concerning the nonvolatile constituents, 6-gingerol along with 8-, and 10-gingerols were predominant metabolites, in addition to (6)-gingerdiacetate and shogaols compounds (Table 7). Considering the in vitro antiviral potential of ginger rhizomes, the hydroalcoholic extract showed better antiviral activity than the HD oil. Such higher activity of the hydroalcoholic extract could be attributed to the occurrence of (6)-gingerdiacetate.
While most of the previous studies focused on gingerols and shogaols, suggesting them as potent candidates against COVID-1937,38,39,40,89,90, there is limited research on other gingerols derivatives such as (6)-gingerdiacetate that exhibited a higher antioxidant effect than (6)-ginerdiol, (6)-shogaol, (6)-gingerol, and (6)-dehydrogingerdione91. As COVID-19 infection induces cytokine storm as an inflammatory response that causes damage to lung tissues and cell death92, antioxidant therapy can be a potential approach for management or treatment of COVID-1993,94,95. Our computational and experimental findings shed light on the potential effect of (6)-gingerdiacetate as a drug candidate for inhibiting SARS-CoV-2 invasion to host cells.
Conclusions
In summary, we performed network analysis, molecular docking, and molecular dynamics simulations to predict the anti-SARS-CoV-2 activity of the common bioactive constituents of ginger. Network pharmacology revealed that ginger compounds may indirectly affect the virus survival in the host cell through multi-biological processes including cell cycle, cell communication, apoptosis, and inflammatory responses. Docking and MD simulations identified (6)-gingerdiacetate as a promising compound that binds to SARS-CoV-2 spike RBD and human ACE2 and may prevent the viral attachment to the host cell. Lastly, (6)-gingerdiacetate was detected experimentally in the ginger hydroalcoholic extract that showed higher antiviral activity than the HD ginger oil. Such higher activity of the hydroalcoholic extract could be attributed to the occurrence of (6)-gingerdiacetate. Yet further studies are warranted on the individual compounds post-isolation to validate the therapeutic effects of the candidate chemical against the pandemic.
Data availibility
All data and results supporting the conclusions of this article are included within the article and its supplementary information file.
Abbreviations
- COVID-19:
-
Coronvirus disease 2019
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- WHO:
-
World Health Organization
- PHEIC:
-
Public health emergency of international concern
- SRAS:
-
Severe acute respiratory syndrome
- MERS:
-
Middle Eastern respiratory syndrome
- RBD:
-
Receptor binding domain
- ACE2:
-
Angiotensin-converting enzyme 2
- \({\hbox {PL}}^{\hbox {pro}}\) :
-
Papine like protease
- \({\hbox {M}}^{\hbox {pro}}\) :
-
Main protease
- HRSV:
-
Human respiratory syncytial virus
- CHIKV:
-
Chikungunya virus
- IAV:
-
Influenza A virus
- Gin A:
-
Gingerenone A
- ARDS:
-
Acute respiratory distress syndrome
- AgNPs:
-
Silver nanoparticles
- Ro5:
-
Rule of five
- ADME:
-
Absorption, distribution, metabolism, and excretion
- SEA:
-
Similarity ensemble approach
- HGNC:
-
HUGO Gene Nomenclature Committee
- PPIs:
-
Protein–protein interactions
- GO:
-
Gene ontology
- FDR:
-
False discovery rate
- PDB:
-
Protein Data Bank
- MOE:
-
Molecular Operating Environment
- RMSD::
-
Root mean square deviation
- MD::
-
Molecular dynamics
- GROMACS:
-
GROningen MAchine for Chemical Simulations
- CGenFF:
-
CHARMM General Force Field server
- Rg:
-
Radius of gyration
- Grace:
-
GRaphing and Advanced Computation and Exploration of data
- CC:
-
Cellular components
- BP:
-
Biological processes
- MF:
-
Molecular functions
- NSP:
-
Non-structural protein
References
Ma, J. Coronavirus: China’s first confirmed Covid-19 case traced back to November 17. In South China Morning Post. https://www.scmp.com/news/china/society/article/3074991/coronavirus-chinas-first-confirmed-covid-19-case-traced-back (2020).
Cucinotta, D. & Vanelli, M. Who declares covid-19 a pandemic. Acta Bio Med. Atenei Parmensis 91(1), 157 (2020).
W.H. Organization. WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int/ (2022)
Alamri, M.A., Tahir Ul Qamar, M., Mirza, M.U., Bhadane, R., Alqahtani, S.M., Muneer, I., Froeyen, M., & Salo-Ahen, O.M. Pharmacoinformatics and molecular dynamics simulation studies reveal potential covalent and FDA-approved inhibitors of SARS-COV-2 main protease 3clpro. J. Biomol. Struct. Dyn. 1–13 (2020)
Cui, J., Li, F. & Shi, Z.-L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 17(3), 181–192 (2019).
Harapan, H., Itoh, N., Yufika, A., Winardi, W., Keam, S., Te, H., Megawati, D., Hayati, Z., Wagner, A.L., & Mudatsir, M. Coronavirus disease 2019 (covid-19): A literature review. J. Infect. Public Health (2020).
Kuba, K. et al. A crucial role of angiotensin converting enzyme 2 (ace2) in SARS coronavirus-induced lung injury. Nat. Med. 11(8), 875–879 (2005).
Naqvi, A.A.T., Fatima, K., Mohammad, T., Fatima, U., Singh, I.K., Singh, A., Atif, S.M., Hariprasad, G., Hasan, G.M., & Hassan, M.I. Insights into SARS-COV-2 genome, structure, evolution, pathogenesis and therapies: Structural genomics approach. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 165878 (2020).
Kiemer, L., Lund, O., Brunak, S. & Blom, N. Coronavirus 3cl pro proteinase cleavage sites: Possible relevance to SARS virus pathology. BMC Bioinform. 5(1), 1–9 (2004).
Ratia, K. et al. Severe acute respiratory syndrome coronavirus papain-like protease: Structure of a viral deubiquitinating enzyme. Proc. Natl. Acad. Sci. 103(15), 5717–5722 (2006).
Wu, C. et al. Analysis of therapeutic targets for SARS-COV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B 10(5), 766–788 (2020).
Chai, R. et al. Traditional Chinese medicine: An important broad-spectrum anti-coronavirus treatment strategy on covid-19 background. Tradit. Med. Res. 7(3), 19 (2022).
Chopra, B. & Dhingra, A. K. Natural products: A lead for drug discovery and development. Phytother. Res. 35(9), 4660–4702 (2021).
Tasleem, M. et al. Investigation of antidepressant properties of yohimbine by employing structure-based computational assessments. Curr. Issues Mol. Biol. 43(3), 1805–1827 (2021).
Atanasov, A. G. et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 33(8), 1582–1614 (2015).
Ang, L., Lee, H. W., Choi, J. Y., Zhang, J. & Lee, M. S. Herbal medicine and pattern identification for treating covid-19: A rapid review of guidelines. Integr. Med. Res. 9(2), 100407 (2020).
Ho, L. T., Chan, K. K., Chung, V. C. & Leung, T. H. Highlights of traditional Chinese medicine frontline expert advice in the china national guideline for covid-19. Eur. J. Integr. Med. 36, 101116 (2020).
Yang, Y., Islam, M. S., Wang, J., Li, Y. & Chen, X. Traditional Chinese medicine in the treatment of patients infected with 2019-new coronavirus (SARS-COV-2): A review and perspective. Int. J. Biol. Sci. 16(10), 1708 (2020).
Miyoshi, N. et al. Dietary ginger constituents, Galanals a and b, are potent apoptosis inducers in human t lymphoma Jurkat cells. Cancer Lett. 199(2), 113–119 (2003).
Das, A., Kasoju, N., Bora, U. & Rangan, L. Chemico-biological investigation of rhizome essential oil of Zingiber moran-native to northeast India. Med. Chem. Res. 22(9), 4308–4315 (2013).
Wang, C. C., Chen, L. G., Lee, L. T. & Yang, L.-L. Effects of 6-gingerol, an antioxidant from ginger, on inducing apoptosis in human leukemic hl-60 cells. In vivo (Athens, Greece) 17(6), 641–645 (2003).
Johji, Y. et al. Cholagogic effect of ginger and its active constituents. J. Ethnopharmacol. 13(2), 217–225 (1985).
Kumboonma, P., Senawong, T., Saenglee, S., Yenjai, C. & Phaosiri, C. Identification of phenolic compounds from Zingiber offinale and their derivatives as histone deacetylase inhibitors and antioxidants. Med. Chem. Res. 26(3), 650–661 (2017).
Sida, S., Samakradhamrongthai, R. S. & Utama-Ang, N. Influence of maturity and drying temperature on antioxidant activity and chemical compositions in ginger. Curr. Appl. Sci. Technol. 19(1), 28–42 (2019).
Ezez, D., & Tefera, M. Effects of solvents on total phenolic content and antioxidant activity of ginger extracts. J. Chem. 2021 (2021)
Nasri, H. et al. Preventive and curative effects of ginger extract against histopathologic changes of gentamicin-induced tubular toxicity in rats. Int. J. Prevent. Med. 4(3), 316 (2013).
Bordia, A., Verma, S., & Srivastava, K. Effect of ginger (Zingiber officinale Rosc.) and fenugreek (Trigonella foenumgraecum L.) on blood lipids, blood sugar and platelet aggregation in patients with coronary artery disease. Prostaglandins Leukotrienes Essent. Fatty Acids 56(5), 379–384 (1997)
Akinyemi, A. J. et al. Effect of dietary supplementation of ginger and turmeric rhizomes on angiotensin-1 converting enzyme (ACE) and arginase activities in l-name induced hypertensive rats. J. Funct. Foods 17, 792–801 (2015).
Park, G. et al. 6-Shogaol, an active compound of ginger, protects dopaminergic neurons in Parkinson’s disease models via anti-neuroinflammation. Acta Pharmacol. Sin. 34(9), 1131–1139 (2013).
Huh, E. et al. Ginger fermented with schizosaccharomyces pombe alleviates memory impairment via protecting hippocampal neuronal cells in amyloid beta 1–42 plaque injected mice. Food Funct. 9(1), 171–178 (2018).
Townsend, E. A. et al. Effects of ginger and its constituents on airway smooth muscle relaxation and calcium regulation. Am. J. Respir. Cell Mol. Biol. 48(2), 157–163 (2013).
Townsend, E. A., Zhang, Y., Xu, C., Wakita, R. & Emala, C. W. Active components of ginger potentiate \(\beta \)-agonist-induced relaxation of airway smooth muscle by modulating cytoskeletal regulatory proteins. Am. J. Respir. Cell Mol. Biol. 50(1), 115–124 (2014).
San Chang, J., Wang, K. C., Yeh, C. F., Shieh, D. E. & Chiang, L. C. Fresh ginger (Zingiber officinale) has anti-viral activity against human respiratory syncytial virus in human respiratory tract cell lines. J. Ethnopharmacol. 145(1), 146–151 (2013).
Kaushik, S., Jangra, G., Kundu, V., Yadav, J. P. & Kaushik, S. Anti-viral activity of Zingiber officinale (ginger) ingredients against the chikungunya virus. Virus Dis. 31(3), 270–276 (2020).
Wang, J., Prinz, R. A., Liu, X. & Xu, X. In vitro and in vivo antiviral activity of gingerenone a on influenza a virus is mediated by targeting Janus kinase 2. Viruses 12(10), 1141 (2020).
Shariatpanahi, Z. V. et al. Effect of enteral feeding with ginger extract in acute respiratory distress syndrome. J. Crit. Care 28(2), 217–221 (2013).
Muhammad, S., Amin, S., Iqbal, J., Al-Sehemi, A.G., Alarfaji, S.S., Ilyas, M., Atif, M., & Ullah, S. Insighting the therapeutic potential of fifty (50) shogaol derivatives against mpro of SARS-COV-2. J. Comput. Biophys. Chem. 2250020 (2022)
Rathinavel, T. et al. Phytochemical 6-gingerol—A promising drug of choice for covid-19. Int. J. Adv. Sci. Eng. 6(4), 1482–1489 (2020).
Oso, B. J., Adeoye, A. O. & Olaoye, I. F. Pharmacoinformatics and hypothetical studies on allicin, curcumin, and gingerol as potential candidates against covid-19-associated proteases. J. Biomol. Struct. Dyn. 40(1), 389–400 (2022).
Haridas, M. et al. Compounds of Citrus medica and Zingiber officinale for covid-19 inhibition: In silico evidence for cues from ayurveda. Future J. Pharmaceut. Sci. 7(1), 1–9 (2021).
Al-Sanea, M. M. et al. Strawberry and ginger silver nanoparticles as potential inhibitors for SARS-COV-2 assisted by in silico modeling and metabolic profiling. Antibiotics 10(7), 824 (2021).
Aldwihi, L. A. et al. Patients’ behavior regarding dietary or herbal supplements before and during Covid-19 in Saudi Arabia. Int. J. Environ. Res. Public Health 18(10), 5086 (2021).
Azam, M. N. K., Al Mahamud, R., Hasan, A., Jahan, R. & Rahmatullah, M. Some home remedies used for treatment of covid-19 in Bangladesh. J. Med. Plants Stud. 8(4), 27–32 (2020).
Wannes, W. A. & Tounsi, M. S. Can medicinal plants contribute to the cure of Tunisian Covid-19 patients. J. Med. Plants 8(5), 218–226 (2020).
Orisakwe, O. E., Orish, C. N. & Nwanaforo, E. O. Coronavirus disease (covid-19) and Africa: Acclaimed home remedies. Sci. Afr. 10, 00620 (2020).
Iwuoha, V. C., Ezeibe, E. N. & Ezeibe, C. C. Glocalization of covid-19 responses and management of the pandemic in Africa. Local Environ. 25(8), 641–647 (2020).
Mesri, M. et al. The effects of combination of Zingiber officinale and echinacea on alleviation of clinical symptoms and hospitalization rate of suspected covid-19 outpatients: A randomized controlled trial. J. Complem. Integr. Med. 18(4), 775–781 (2021).
Mishra, R. K., Kumar, A. & Kumar, A. Pharmacological activity of Zingiber officinale. Int. J. Pharmaceut. Chem. Sci. 1(3), 1073–1078 (2012).
Canese, K. & Weis, S. Pubmed: The bibliographic database. NCBI Handb. 2, 1 (2013).
Lipinski, C. A., Lombardo, F., Dominy, B. W. & Feeney, P. J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 23(1–3), 3–25 (1997).
Daina, A., Michielin, O. & Zoete, V. Swissadme: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 7(1), 1–13 (2017).
Gfeller, D., Michielin, O. & Zoete, V. Shaping the interaction landscape of bioactive molecules. Bioinformatics 29(23), 3073–3079 (2013).
Yao, Z.-J. et al. Targetnet: A web service for predicting potential drug-target interaction profiling via multi-target SAR models. J. Comput.-Aided Mol. Des. 30(5), 413–424 (2016).
Daina, A., Michielin, O. & Zoete, V. Swisstargetprediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 47(W1), 357–364 (2019).
Seal, R.L., Gordon, S.M., Lush, M.J., Wright, M.W., & Bruford, E.A. genenames.org: The HGNC resources in 2011. Nucleic Acids Res. 39(suppl_1), 514–519 (2010)
Shannon, P. et al. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 13(11), 2498–2504 (2003).
Szklarczyk, D. et al. String v11: Protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 47(D1), 607–613 (2019).
Assenov, Y., Ramírez, F., Schelhorn, S.-E., Lengauer, T. & Albrecht, M. Computing topological parameters of biological networks. Bioinformatics 24(2), 282–284 (2008).
Wu, G., Feng, X. & Stein, L. A human functional protein interaction network and its application to cancer data analysis. Genome Biol. 11(5), 53 (2010).
Newman, M. E. Modularity and community structure in networks. Proc. Natl. Acad. Sci. 103(23), 8577–8582 (2006).
Stark, C., Breitkreutz, B.-J., Reguly, T., Boucher, L., Breitkreutz, A., & Tyers, M. Biogrid: A general repository for interaction datasets. Nucleic Acids Res. 34(suppl_1), 535–539 (2006)
Berman, H. M. et al. The protein data bank. Nucleic Acids Res. 28(1), 235–242 (2000).
Owen, C., Lukacik, P., Strain-Damerell, C., Douangamath, A., Powell, A., Fearon, D., Brandao-Neto, J., Crawshaw, A., Aragao, D., & Williams, M. et al. Covid-19 main protease with unliganded active site (2019-NCOV, coronavirus disease 2019, SARS-COV-2). CSB Protein Data Bank (PDB) ID 6, 3–7 (2020)
C.C.G.C. Inc. Molecular Operating Environment (MOE) (Chemical Computing Group, 2019)
Lan, J. et al. Structure of the SARS-COV-2 spike receptor-binding domain bound to the ace2 receptor. Nature 581(7807), 215–220 (2020).
Towler, P. et al. Ace2 X-ray structures reveal a large hinge-bending motion important for inhibitor binding and catalysis. J. Biol. Chem. 279(17), 17996–18007 (2004).
Kim, S. et al. Pubchem substance and compound databases. Nucleic Acids Res. 44(D1), 1202–1213 (2016).
Kufareva, I. & Abagyan, R. Methods of protein structure comparison. In Homology Modeling. 231–257 (Springer, 2011)
Abraham, M. J. et al. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1, 19–25 (2015).
Golo, V. & Shaĭtan, K. Dynamic attractor for the Berendsen thermostat an the slow dynamics of biomacromolecules. Biofizika 47(4), 611–617 (2002).
Tuble, S. C., Anwar, J. & Gale, J. D. An approach to developing a force field for molecular simulation of martensitic phase transitions between phases with subtle differences in energy and structure. J. Am. Chem. Soc. 126(1), 396–405 (2004).
Hegazi, N. M., Tantawy, M. A., Emam, M., Bakry, S. M. & Hussein, S. A. A. Headspace gas chromatography/mass spectrometry analysis endorses melaleuca species as abundant source of medicinal eucalyptol and its proposed anti-obesity activity. Egypt. J. Chem. 65(1), 607–618 (2022).
Marín-Palma, D. et al. Curcumin inhibits in vitro SARS-COV-2 infection in Vero e6 cells through multiple antiviral mechanisms. Molecules 26(22), 6900 (2021).
Grzanna, R., Lindmark, L. & Frondoza, C. G. Ginger-an herbal medicinal product with broad anti-inflammatory actions. J. Med. Food 8(2), 125–132 (2005).
Awang, D.V. Tyler’s Herbs of Choice: The Therapeutic Use of Phytomedicinals. (CRC Press, 2009)
Tao, Y., Li, W., Liang, W. & Van Breemen, R. B. Identification and quantification of gingerols and related compounds in ginger dietary supplements using high-performance liquid chromatography- tandem mass spectrometry. J. Agric. Food Chem. 57(21), 10014–10021 (2009).
Jiang, H., Sólyom, A.M., Timmermann, B.N., & Gang, D.R. Characterization of gingerol-related compounds in ginger rhizome (Zingiber officinale Rosc.) by high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 19(20), 2957–2964 (2005).
Nishidono, Y. et al. Identification of the chemical constituents in ginger (Zingiber officinale) responsible for thermogenesis. Nat. Prod. Commun. 13(7), 1934578–1801300722 (2018).
Harvey, A. L., Edrada-Ebel, R. & Quinn, R. J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 14(2), 111–129 (2015).
Solomon, S. FDA Letter to Stakeholders: Do Not Use Chloroquine Phosphate Intended for Fish as Treatment for COVID-19 in Humans. https://www.fda.gov/animal-veterinary/product-safety-information/fda-letter-stakeholders-do-not-use-chloroquine-phosphate-intended-fish-treatment-covid-19-humans (FDA, 2020).
Busari, S., & Adebayo, B. Nigeria Records Chloroquine Poisoning After Trump Endorses it for Coronavirus Treatment. https://edition.cnn.com/2020/03/23/africa/chloroquine-trump-nigeria-intl/index.html (CNN, 2020).
Liu, J. et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-COV-2 infection in vitro. Cell Discov. 6(1), 1–4 (2020).
Khanal, P. et al. Combination of system biology to probe the anti-viral activity of andrographolide and its derivative against covid-19. RSC Adv. 11(9), 5065–5079 (2021).
Siripoltangman, N. & Chickos, J. Vapor pressure and vaporization enthalpy studies of the major components of ginger, \(\alpha \)-zingiberene, \(\beta \)-sesquiphellandrene and (-) ar curcumene by correlation gas chromatography. J. Chem. Thermodyn. 138, 107–115 (2019).
Motterlini, R., Foresti, R., Bassi, R. & Green, C. J. Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Radic. Biol. Med. 28(8), 1303–1312 (2000).
Astani, A., Reichling, J. & Schnitzler, P. Comparative study on the antiviral activity of selected monoterpenes derived from essential oils. Phytother. Res. 24(5), 673–679 (2010).
Juergens, L. J., Worth, H. & Juergens, U. R. New perspectives for mucolytic, anti-inflammatory and adjunctive therapy with 1, 8-cineole in COPD and asthma: Review on the new therapeutic approach. Adv. Ther. 37(5), 1737–1753 (2020).
Brochot, A., Guilbot, A., Haddioui, L. & Roques, C. Antibacterial, antifungal, and antiviral effects of three essential oil blends. Microbiologyopen 6(4), 00459 (2017).
Ahkam, A. H., Hermanto, F. E., Alamsyah, A., Aliyyah, I. H. & Fatchiyah, F. Virtual prediction of antiviral potential of ginger (Zingiber officinale) bioactive compounds against spike and mpro of SARS-COV2 protein. Berkala Penelitian Hayati J. Biol. Res. 25(2), 52–57 (2020).
Goswami, D., Kumar, M., Ghosh, S.K., & Das, A. Natural product compounds in Alpinia officinarum and ginger are potent SARS-COV-2 papain-like protease inhibitors. ChemRxiv (2020).
Kikuzaki, H. Ginger for drug and spice purposes. Herbs Bot. Teas 75 (2000)
Delgado-Roche, L. & Mesta, F. Oxidative stress as key player in severe acute respiratory syndrome coronavirus (SARS-COV) infection. Arch. Med. Res. 51(5), 384–387 (2020).
Soto, M. E., Guarner-Lans, V., Soria-Castro, E., Manzano Pech, L. & Pérez-Torres, I. Is antioxidant therapy a useful complementary measure for covid-19 treatment? An algorithm for its application. Medicina 56(8), 386 (2020).
DE FLORA, S., Balansky, R., & La Maestra, S. Antioxidants and covid-19. J. Prevent. Med. Hyg. 62(1 Suppl 3), 34 (2021).
Fazio, S., Affuso, F. & Bellavite, P. A review of the potential roles of antioxidant and anti-inflammatory pharmacological approaches for the management of mild-to-moderate symptomatic covid-19. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 28, 936292–1 (2022).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
A.S. and A.H. performed the computational procedure, conducted the literature review, and drafted the various versions of the manuscripts. N.M.H. and M.F. proposed the Ginger herb, provided the initial references related to the Ginger herb, supplied the list of the chemical constituents of the target Ginger herb, performed the experimental procedure, and helped draft the manuscript. M.E. proposed the topic and its research framework and supervised the research work. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Samy, A., Hassan, A., Hegazi, N.M. et al. Network pharmacology, molecular docking, and dynamics analyses to predict the antiviral activity of ginger constituents against coronavirus infection. Sci Rep 14, 12059 (2024). https://doi.org/10.1038/s41598-024-60721-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-60721-3
- Springer Nature Limited