Abstract
The organic enrichment effects on the meiofauna and nematofauna were assessed for field sediment and other experimental ones enriched with organic matters conducted in the laboratory for 4 weeks. Also, dissolved oxygen (DO) and pH were monitored for each one. The abundance and diversity of meiofaunal groups and nematofauna varied. Strong significant correlations were found between DO and the studied items. Nematoda was the most abundant group in the field sediment and other experimental ones; their counts increased with the increase in organic enrichments and were dominated by deposit feeders. Amphipoda, Ostracoda and predator/omnivore nematodes disappeared in highly organic-enriched sediments. Changes in DO and organic enrichments might be the more attributable reasons for the alteration of the meiobenthic assemblages. The generic compositions of Nematoda provide a good indicator for environmental alterations.
Similar content being viewed by others
Introduction
Meiofauna inhabit intertidal and subtidal soft bottom habitats in water bodies worldwide. Conditions that influence the meiofauna are different from those that affect other components of benthic organisms within the same region. The main significant aspect influencing these animals is possibly the sediment texture, which plays a crucial role in determining the size of interstitial space suitable for dwelling1. Coarse sediment grain provides more interstitial space, while fine ones have less interstitial space. Additionally, the granulometry of sediment controls the ability to retain and interchange the water. The exchange of gasses is unrestricted in coarse sediments and reduced in fine-ones2,3.
In term of abundance; nematodes, copepods and polychaetes are supposed to be the main meiobenthic groups. Copepoda are usually the second most abundant meiobenthic animals in marine samples2. Free living marine nematodes have been used successfully as indicators of biological health and ocean pollution4,5,6,7 for at least the past 40 years8. Before the sample collections, it was generally thought that nematodes were the predominant large group of meiofauna. Additionally, it was believed that the abundance and distribution of meiofauna were strongly influenced by nematodes. For this study, the eulittoral zone of a sandy beach was chosen as the sampling area. This decision was based on the understanding that the eulittoral zone provides the most favorable habitat for meiofauna, as it exhibits greater species diversity compared to other supra/sub-littoral zones, as proposed by Huston's dynamic equilibrium hypothesis9.
Armonies and Reise10 revealed that there may be an optimal equilibrium among organic supply, oxygen levels, and water retention in the eulittoral zone, which resulted in a high number of taxa per meter interval. This finding was further supported by various studies11,12. Due to its unique reproductive strategy, meiofauna is regarded as a significant component in the detrital food chain. It plays a crucial role in both the energy flow of the ecosystem and the ecological assessment of environmental quality. Increasingly, ecologists are utilizing meiobenthic organisms as sensitive indicators to evaluate changes in the environment and community structures7,13.
The aim of this study is to evaluate the impact of organic enrichment on meiofaunal community structure and generic corpro of Nematodes.
Materials and methods
Field sampling and laboratory procedures
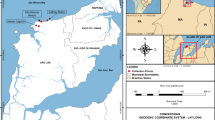
In March 2023, the sandy sediment site (30° 24′ 0.59″ N, 32° 18′ 38.8″ E) was investigated. This site is located on the west side of the Great Bitter Lake, east of Abou-Soultan Power Station and north of Fayed city (Ismailia, Egypt). Agriculture and recreation activities are the main land uses of this region. Meiofauna were sampled from the eulittoral zone (Mid tidal mark) using a hand metal cylindrical corer with an inner diameter of 3.5 cm. The corer device was pushed into the sediment by hand to a depth 10 cm and then pulled out to collect a total volume of 100 cm3. Three replicate cores were sampled for the field study. Each sample was transferred into polyethylene bags directly at the site. The sediment samples were preserved in 5% buffered formalin in the field. Water temperature, dissolved oxygen DO, pH and salinity were measured using an YSI multi-parameter device (YSI, Yellow Springs, OH, USA). Enteromorpha intestinalis (green algae) was also collected from the adjacent hard substrate.
In the lab, the sediment samples were filtered through a 50-μm screen, and the organisms were collected. The individuals of meiofauna were counted and subsequently sorted into major taxa using a stereo microscope (Prior S2000, magnification 100 ×). Nematodes were examined under a compound microscope (Carl Zeiss 1000 × magnification) and identified to the genus level by pictorial keys14,15,16. According to Wieser17, all nematode individuals were categorized to one of the three trophic groups: deposit, epistrate and predators/omnivores feeders.
Laboratory experimental design
Extra sediment samples for experiments were taken from the surface layer (0–10 cm in depth) and immediately transferred to the laboratory. The sediment sample was put into each of the nine jars. These jars were divided into three groups based on their levels of organic enrichment, namely, high-organic enrichment, low-organic enrichment, and control (without enrichment) (Fig. 1). There were three replicates for each enrichment group. In the high-organic enrichment group, 10 g dry weight of green algae (frozen at − 20 °C before treatment, then thawed and dried at 120 °C for 6 h and then powdered with a grinding machine) was added into each of the 3 Jars. On the other hand, 2 g of green algae was added for the low-organic enrichment group. The control group did not receive any amount of algae. So, ratios of added algae among these enrichments were 5: 1:0. The sediment samples were mixed and 100 ml of homogenized sediment was put into each jar. Subsequently, 150 ml of pre-filtered seawater was added to each jar. Finally, each jar was supplied with continuous aeration for 4 weeks. The experiments were carried out under dim light at room temperature to prevent the growth of microalgae. At the end of the experiment, all microcosms were collected, and the samples were preserved in 5% formalin. Also, the pH and dissolved oxygen (DO) levels were measured in each jar.
Data analysis
The differences in meiofaunal abundance and other major groups among the different groups were examined using a one-way analysis of variance (ANOVA) with a confidence level of 95%. Additionally, Pearson's correlation analysis was conducted to assess the relationships between the total meiofaunal abundance, other major taxa, and the pH and dissolved oxygen (DO). Post-Hoc Tests’ between pH and DO among organic enrichment level were calculated. The above analyses were performed using the statistical software SPSS 18.0 (2002) while PRIMER v6.0 software18 used for univariate measures of the diversity indices for meiofauna taxa and nematofauna for field meiofauna samples and other sediments with different organic enrichment levels.
Results
Field study
Water temperature, Salinity, pH and dissolved oxygen values at the study site were 24.3 °C, 38.6‰, 8.2 and 8.7 mg/l, respectively. Meiofaunal community was composed of 8 major taxa (Amphipoda, Polychaeta, Bivalvia, Copepoda, Gastropoda, Nematoda, Oligochaeta and Ostracoda). As observed in Fig. 2, Nematoda (75%) was the most abundant group followed by Copepoda (9%) and Polychaeta (8%). Nematoda recorded the highest average density (170.1 ± 56.3 individual/100 cm3) while Gastropoda recorded the lowest one (0.9 ± 1.1 individual/100 cm3) (Table 1). For further comparison with the results of experimental study, densities of all taxa at the sampling sites were expresses in number of individual per 100 cm3.
Laboratory experiments
Dissolved oxygen concentration (DO) and pH
The highest DO and pH values were recorded in without organic enrichment jars (8.8 ± 0.2 mg/l and 7.9 ± 0.1, respectively), while the lowest readings were recorded in high organic enrichment jars (2.9 mg/l ± 0.1 and 7.2 ± 0.1, respectively) (Fig. 3).The differences in DO and pH among the enrichment levels were significant (P = 0.000 and P = 0.001, respectively). Post-Hoc Tests' Multiple Comparisons revealed significant variations between all enrichment levels in both DO and pH, except for those between sediments without organic enrichment and low organic enrichment ones (P = 0.55).
Meiofaunal abundance
The highest density of total meiofauna was recorded in the high organic enrichment jars (261 ± 25.7 individual/100 cm3) while the lowest density was recorded in low organic enrichment jars (220.7 ± 19.3 individual/100 cm3) (Table 1). The meiofaunal community was represented by 6 Major taxa in the experimental jars (Amphipoda, Polychaeta, Copepoda, Nematoda, Oligochaeta and Ostracoda). Nematoda was the common taxa in all experiments; their density was increased with the increasing of organic enrichment levels and ranged between 72 and 79% (Fig. 4). Amphipoda, Bivalvia and Gastropoda were absent in organic enrichment jars. (Table 1). One way ANOVA showed a significant variations in taxa density among different enrichments (Table 1).
Diversity indices of meiofauna
Generally, all diversity indices for meiofaunal assemblages were low. The lowest values of total recorded taxa (S), Shannon–Wiener (H′) and Species Richness (SR) were recorded in the high organic enrichment sediments while the highest diversity indices were recorded in the Field meiofaunal assemblages (Table 2).
Nematofauna
The highest number of nematode genera (17 genera) was recorded in the field samples, while the least number (7 genera) was recorded in the high organic enrichment sediments. Daptonema was the most abundant genus (62.1 ± 11.6 individual/100 cm3) in the high organic enrichement sediment. In contrast, 10 genera (Acantholaimus, Actinonema, Dichromadora, Halicoanolaimus, Metasphaerolaimus Paralongicyatholaimus, Sabatieria, Sphaerolaimus, Syringolaimus, and Tricoma) were disappeared in the high level of organic enrichment jars (Table 3). Deposit feeder nematodes were dominated all feeding types and their density ranged from 49% in field samples and 82.6% in high organic enrichement jars. In the other hand, Predator/omnivores was the least feeding type and disappeared in the organic enriched sediments (Fig. 5).
Pearson's correlation coefficients between dissolved oxygen, pH, total meiofauna, group abundance, number of recorded taxa and nematode feeding types were calculated for field samples and the experimental enrichment jars. The total recorded taxa showed positively significant strong correlations with DO and pH (r = 0.97, P = 0.004 and r = 0.95, P = 0.05; respectively), and also total abundance of meiofauna exhibited the same pattern (r = 0.98, P = 0.02). Moreover, predator/omnivore nematodes showed a significant strong correlation with DO (r = 0.96, P = 0.04). The deposit feeding nematodes showed negative strong correlations with the other feeding types. Furthermore the other correlations were not significant. Dendrogram of these correlations revealed two main clusters; the first one constituted of DO, pH, no. of recorded groups, epistrate feeders% and predator/omnivores%. In contrast, deposit feeders, total abundance of meiofauna and nematodes densities constitute the second cluster (Fig. 6).
Diversity indices of nematofauna
The highest diversity indices of nematofaunal assemblages were recorded for the field sediment while the least ones were recorded for the high organic enrichment ones (Table 4).
Discussion
Three different levels of organic enrichment were conducted to assess the impact of organic enrichment on the meiofaunal community in the laboratory. The design in this study was based on Webb19; Armenteros et al.20. All nine experimental jars were kept in dark to avoid the growth of photo-autotrophs such as microalgae. The experimental design might to possibly is magnifying the effects of organic enrichment due to the stagnant situations Nevertheless, the experimental setup was considered valuable as it ensured that the essential characteristics of the samples remained consistent between the laboratory experiments and the field study, as noted by Sundbäck et al.21.
The organic enrichment of the sediments seemed to control the meiofaunal community structure. Among three different organic content levels, densities of different groups and nematofauna were significantly different. The abundance of total meiofauna and nematode were the highest in high level of organic enrichment sediments, compared with other levels of enrichment. This outcome was deemed satisfactory since it was anticipated that the addition of organic matter would lead to an increase in meiofaunal densities, including both nematodes and other meiofaunal groups.
Wang et al.2 and Austen and Widdicombe22; established that organic enrichment leads to the rising in meiobenthic densities, which supports the general model of Huston8. Also, Pinckney et al.23 stated that the eutrophication and organic pollution will lead to increased food supply and a rise in the benthic densities. The abundance and biomass of nematodes are strongly influenced by both food availability and bacterial density. Pascal et al.24 discovered, through trophic studies conducted on a tidal mudflat, that nematodes exhibit a preference for microalgae as a food source, in addition to bacterial food. Consequently, a significant increase in meiofaunal densities occurs with high organic enrichment. According to Montagna et al.13, Rieper-Kirchner25, Gyedu-Ababioa and Baird26; Krueger and McSorley27 and Nugteren et al.28, nematodes are particularly attracted to accumulations of bacteria on plant debris. Furthermore, macrobenthic annelids prefer detritus as their primary food source. Therefore, the introduction of organic enrichment and the subsequent indirect enhancement of bacterial food resources are expected to lead to a general increase in meiofaunal densities, with a specific emphasis on nematodes and annelids.
McLachlan et al.29 revealed that, along the coasts of South Africa, meiofaunal abundance was correlated positively with the amount of detritus in the soft bottom habitats. Also, Moreno et al.30 found a same correlation for a Mediterranean coast, with low content of organic matter that provides relatively poor meiofauna. In comparisons with the eulittoral zones of the North Sea, with a high content of organic matter, is populated densely31.
The concentration of free sulfide should be monitored and analyzed as they may elucidate the meiobenthic fauna in terms of densities and diversities in the present study. Sutherland et al.32 found that a high variation of meiofauna was occurred at the presence of high amount of free sulfide. Also, they stated that nematodes exhibited a minor drop in abundance with increasing organic enrichment. In microcosm experiment, Webb19 and Armenteros et al.20 found different findings from the results in this study. They found high meiofaunal and nematode abundances in low organic level treatments, and the lowest ones were found in high organic level treatments. Moreover, they reported that nematodes and total meiofauna decreased significantly in the high organic enrichment treatments. Furthermore, they suggested that the chemical stressors such as ammonia and hydrogen sulfide derived from reduced conditions in sediments might be important factors affecting the meiobenthic assemblages.
Giere2 and Wang et al.3 stated that the increase in organic matter will enhance meiofaunal abundance but will also change the community composition and their small scale distribution patterns. Nematoda and Copepoda are responding differently to the environmental alteration. Nematoda are the most resistant group in habitats with low level of oxygen33,34,35. Wang et al.3 and Pascal et al.36 stated that Harpacticoid Copepoda, Amphipoda and Ostracoda are sensitive to the organic pollution and dissolved oxygen. This status was supported in the current study by investigating the meiofaunal densities relationship to oxygen level and organic content availability. Hypoxia occurred in both organic enrichment levels experiments, which propose the nematode assemblage, might adapt to naturally enriched sediments. The number of recorded taxa and nematode genera were decreased with the increase of organic enrichment. Also Amphipoda and ostracoda were disappeared in the enriched sediments.
In high organic enriched sediment of the present study, 4 groups of meiofauna were found namely; Nematoda (80%), Copepodaes (12%), Polychaeta (6%) and Oligochaeta (2%). Also, Shannon–Wiener and Species Richness value were the least in these high organic enriched sediments. Heip37 and Mahmoudi et al.38 reported that Shannon–Wiener indices generally have lower values in polluted situations. Herman et al.39 and Fraschetti et al.40 have reported that taxon diversity tends to be lower under polluted conditions, primarily due to the disappearance of rare taxa such as Ostracoda, Gastrotricha, Halacarida, Hydrozoa, and Tardigrada. In their study along the Belgian coast, Herman et al.39 examined meiobenthic communities at 18 stations and found up to seven different higher taxa in sandy stations, while more than 50% of the other stations had only one or two taxa. Amjad and Gray41 also observed a decrease in the number of meiofaunal taxa along an organic enrichment gradient. Hodda and Nicholas42 discovered a significant correlation between the relative abundance of Oligochaeta and pollution levels, although this group never dominates marine meiobenthic communities.
Also, Keller43,44,45 found that the effect of sewage on the meiobenthic community structures along Marseille coast, France (the heavily polluted coastal regions) were macrofauna are absent, and a relatively poor densities of nematodes, copepods and acari were recorded. All of these findings were supporting the present results.
The nematofauna diversity varied among different levels of organic enrichment in this study; Shannon–Wiener and Species Richness values were exhibited the least values for the sediments of high organic enrichment. Also, dominance of deposit feeders was found due to the increase of organic content. Sabeel and Vanreusel46 stated that the alteration in nematode community composition can be attributed to changes in sediment characteristics. Williams47, Fleeger et al.48 and Mohammad49 found that good relation between the distribution of nematode species directly to pore-space, while more recent studies suggest that the shift in size class is more likely the response of organisms to other sediment-related physic–chemical parameters such as oxygen concentration32,50,51, abundance of microalgal biomass52 and sediment disturbance53. Small nematodes such as Daptonema and Microlaimus are considered as colonizers i.e. small individuals that show rapid growth, and early reproduction46,53. This is in accordance with results of the current study where Daptonema and Microlaimus were showed increase of their abundances with increasing organic enrichment levels. These results are appearing shift in dominance of large bodied species with low turnover rates toward dominance of small-bodied species with high turnover rates (as opportunist or r-strategist species).
In the current study, predator nematode genera were absent in the organic enriched sediments and displaying a positively strong significant correlation with oxygen level. This finding is consistent with Vitiello and Aissa54 who studied the nematode communities in polluted lagoon of Tunisia and found only three nematode genera were characteristic of the organically polluted communities. Also, they found that the absence of the predator nematode in this area while they accounted for about 15% of the communities in the unpolluted region. Also, Giere2 reported that the oxygen requirements of nematode was the highest for predator ones. This status might be elucidating the absence of these genera in the high organic enriched sediment in the present study.
Conclusion
Changes in some water quality parameters can affect the meiofauna/nematofauna assemblages due to the organic enrichment of sediment. Its community structures considered good indicators for environmental impacts and healthiness. Further detail studies (the biomass of nematodes, concentrations of free sulfide and unionized ammonia) on the composition of nematode communities in the organic-enrichment microcosm experiments are needed. Also, longer experiment period (more than four weeks) perhaps could be significantly useful for future works.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Mohammad, D. A. Meiobenthic assemblages in some coral reef sites in marsa alam (Red Sea, Egypt) with emphasis on free living nematodes. Egypt. J. Aquat. Bio. Fish. 26(6), 495–510 (2022).
Giere, O. Meiobenthology: The Microscopic Motile Fauna of Aquatic Sediments 2nd edn, 527 (Springer, 2009).
Wang, J., Zhou, H., Zhang, Z., Cong, B. & Xu, S. Effects of organic enrichment on sandy beach meiofauna: a laboratory microcosm experiment. J. Ocean Univ. China 10, 246–254 (2011).
Gray, J. et al. The role of ecology in marine pollution monitoring. Ecology Panel report. Rapp. P.-V. Reun. Cons. Int. Explor. Mer. 179, 237–252 (1980).
Heip, C., Vincx, M. & Vranken, G. The ecology of marine nematodes. Oceanogr. Mar. BioI. Ann. Rev. 23, 399–489 (1985).
Bongers, T. & Ferris, H. Nematode community structures as a bioindicator in environmental monitoring. Trends Ecol. Evol. 14, 224–228 (1999).
Kennedy, A. & Jacoby, C. Biological indicators of marine environmental health: Meiofauna—A neglected benthic component?. Environ. Monit. Assess. 54, 47–68 (1999).
Ridall, A. & Ingels, J. Suitability of free-living marine nematodes as bioindicators: Status and future considerations. Front. Mar. Sci. 8, 1–16 (2021).
Huston, M. A. general hypothesis of species diversity. Am. Nat. 113, 81–101 (1979).
Armonies, W. & Reise, K. Faunal diversity across a sandy shore. Mar. Ecol. Prog. Ser. 196, 49–57 (2000).
Ott, J. A. Determination of fauna boundaries of nematodes in an intertidal sand flat. Int. Ernationale Rev. ges. Hydrobiol. Hydrogr. 57(4), 645–663 (1972).
Short, A. Handbook of Beach and Shoreface Morphodynamics 379 (John Wiley, 1999).
Montagna, P., Bauer, J., Harind, D. & Spies, R. Meiofaunal and microbial trophic interactions in a natural submarine hydrocarbon seep. Vie et milieu. 45, 17–25 (1995).
Tarjan, A. Illustrated Guide to the Marine Nematodes 135 (IFAS Univ of Florida, 1980).
Platt, H. & Warwick, R. Free-living marine nematodes. Part II. British chromadorids, Brill/Backhuys, Leiden Sanders HL (1968) Marine benthic diversity: A comparative study. Am. Nat. 102, 243–282 (1988).
Warwick, R., Platt, H. & Somerfield, P. Free-living marine nematodes part III- Monhysterids. In Pictorial Key to World Genera and Notes for the Identification of British Species (eds Barnes, D. & Crothers, J.) (Linn. Soc. Lon, NY, 1998).
Wieser, W. Die Beziehung zwischen Mundhohlengestalt, Ernahrungsweise und Vorkommen bei freilebenden marinen Nematoden. Ark. Zool. 4(2), 439–484 (1953).
Clarke, K. & Gorley, R. Primer v6: User manual/tutorial (Primer E Ltd., 2006).
Webb, D. Response of macro- and meiobenthos from a carbon-poor sand to phytodetrital sedimentation. J. Exp. Mar. Bio. Ecol. 203, 259–271 (1996).
Armenteros, M. et al. Effects of organic enrichment on nematode assemblages in a microcosm experiment. Mar. Environ. Res. 70, 374–382 (2010).
Sundbäck, K., Jonsson, B., Nilsson, P. & Lindström, I. Impact of accumulating drifting macroalgae on a shallow-water sediment system: an experimental study. Mar. Ecol. Prog. Ser. 58, 261–274 (1990).
Austen, M. & Widdicombe, S. Comparison of the response of meio- and macrobenthos to disturbance and organic enrichment. J. Exp. Mar. Bio. Ecol. 330, 96–104 (2006).
Pinckney, J., Paerl, H., Tester, P. & Richardson, T. The role of nutrient loading and eutrophication in estuarine ecology. Environ. Health Perspect. 109, 699–706 (2001).
Pascal, P., Dupuy, C., Richard, P., Rzeznik-Orignac, J. & Niquil, N. Bacterivory of a mudflat nematode community under different environmental conditions. Mar. Bio. 154, 671–682 (2008).
Rieper-Kirchner, M. Microbial degradation of North Sea macroalgae: field and laboratory studies. Bot. Mar. 32, 241–252 (1989).
Gyedu-Ababio, T. K. & Baird, D. Response of meiofauna and nematode communities to increased levels of contaminants in a laboratory microcosm experiment. Ecotoxico. Environ. Saf. 63, 443–450 (2006).
Krueger, R. & McSorley, R. Nematode management in organic agriculture 1–9 (University of Florida: IFAS Extension, 2008).
Nugteren, P. et al. Spatial distribution of detrital resources determines the outcome of competition between bacteria and a facultative detritivorous worm. Limnol. Oceanogr. 54(5), 1413–1419 (2009).
McLachlan, A., Woolridge, T. & Dye, A. The ecology of sandy beaches in southern Africa. S. Afr. J. of Zool. 16, 219–231 (1981).
Moreno, M. et al. Across-shore variability and trophodynamic features of meiofauna in a microtidal beach of the NW Mediterranean. Estu. Coast. Shelf Sci. 66, 357–367 (2006).
Mclntyre, A. D. Ecology of the marine meiobenthos. Biol. Rev. 44, 245–290 (1969).
Sutherland, T., Levings, C., Petersen, S., Poon, P. & Piercey, B. The use of meiofauna as an indicator of benthic organic enrichment associated with salmonid aquaculture. Mar. Pollut. Bull. 54, 1249–1261 (2007).
Moore, C. & Bett, B. The use of meiofauna in marine pollution impact assessement. Zool. J. Linn. Soc. 96, 263–280 (1989).
Neira, C. et al. Nematode community structure along a central Chile margin transect influenced by the oxygen minimum zone. Deep-Sea Res. I Oceanogr. Res. Pap. 78, 1–15 (2013).
Broman, E. et al. Uncovering diversity and metabolic spectrum of animals in dead zone sediments. Commun. Biol. 3(1), 106 (2020).
Pascal, P., Fleeger, J., Galvez, F. & Carman, K. The toxicological interaction between ocean acidity and metals in coastal meiobenthic copepods. Mar. Pollut. Bull. 60(12), 2201–2208 (2010).
Heip, C. Meiobenthos as a tool in the assessment of marine environmental quality. Rapp. P.-V. Reun. Cons. Explor. Mer. 179, 182–187 (1980).
Mahmoudi, M. et al. How effective is wastewater treatment? A case study under the light of taxonomic and feeding features of meiobenthic nematodes. Environ. Sci. Pollut. Res. 29, 2566–2578 (2021).
Herman, R., Vincx, M. & Heip, C. Meiofauna of tbe Belgian coastal waters: Spatial and temporal variability and productivity. In concerted actions oceanography. M. Sci. Policy Bruss. Belg. 3, 65–80 (1985).
Fraschetti, S. et al. Structural and functional response of meiofauna rocky assemblages to sewage pollution. Mar. Pollut. Bull. 52(5), 540–548 (2006).
Amjad, B. & Gray, J. Use of the nematode-copepod ratio as an index of organic pollution. Mar. Pollut. Bull. 14, 178–181 (1983).
Hodda, M. & Nicholas, W. Meiofauna associated with mangroves in the Hunter River Estuary and Fullerton Cove, Soutb-eastern Australia. Aust. J. Mar. Freshw. Res. 36, 41–50 (1985).
Keller, M. Effets du deversement en mer du grand collecteur de biologique l’agglomeration Marseillaise sur les populations meiobenthiques. C. R. Acad. Sc. Paris, T. 299, Serie III 19, 765–768 (1984).
Keller, M. Distribution quantitative de la meiofaune dans l’aire d’epandage de I’Egout de Marseille. Mar. Bio. 89, 293–302 (1985).
Keller, M. Structure des peuplements meiobenthiques dans Ie secteur pollue par Ie rejet en mer de I’Egout de Marseille. Ann. Lnst. Oceanogr Paris 62, 13–36 (1986).
Sabeel, R. & Vanreusel, A. Potential impact of mangrove clearance on biomass and biomass size spectra of nematode along the Sudanese Red Sea coast. Mar. Environ. Res. 103, 46–55 (2015).
Williams, R. The abundance and biomass of the interstitial fauna of a graded series of shell-gravels in relation to the available space. J. Anim. Ecol. 41, 623–646 (1972).
Fleeger, J., Grippo, M. & Pastorick, S. What is the relative importance of sediment granulometry and vertical gradients to nematode morphometrics?. Mar. Bio. Res. 7, 122–134 (2011).
Mohammad, D. A. Free-living nematodes in some mangrove sites on the southern Egyptian Red Sea coast with emphasis on their horizontal distribution. Egypt. J. Aquat. Bio. Fish. 27(1), 509–530 (2023).
Snelgrove, P. & Butman, C. Animal-sediment relationships revisited: Cause versus effect. Oceanogr. Mar. Biol. An Annu. Rev. 32, 111–177 (1994).
Steyaert, M. et al. Responses of intertidal nematodes to short-term anoxic events. J. Exp. Mar. Biol. Ecol. 345, 175–184 (2007).
Schwinghamer, P. Generating ecological hypotheses from biomass spectra using causal analysis: A benthic example. Mar. ecol. prog. ser. Oldendorf 13(2), 151–166 (1983).
Vanaverbeke, J., Steyaert, M., Vanreusel, A. & Vincx, M. Nematode biomass spectra as descriptors of functional changes due to human and natural impact. Mar. Ecol. Prog. Ser. 249, 157–170 (2003).
Vitiello, P. & Aissa, P. Structure des peuplements de nematodes en milieu lagunaire pollue. Actes due 110 Congres Nat. Soc. sav. Montpellier Sci. 2, 115–126 (1985).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
D.M. generated the initial idea; M.S. designed the study, gathered and analysed the data; D.M. and A.A. wrote the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mohammad, D.A., AL-Farga, A. & Sami, M. Experimental study of organic enrichment on meiofaunal diversity. Sci Rep 14, 10681 (2024). https://doi.org/10.1038/s41598-024-60690-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-60690-7
- Springer Nature Limited