Abstract
With the rapid development of imaging technology and comprehensive treatment in modern medicine, the early diagnosis rate of breast cancer is constantly improving, and the prognosis is also improving; As breast cancer patients survive longer, the risk of developing second primary cancers increases. Since both breast and thyroid are Hormone receptor sensitive organs, which are regulated by hypothalamus pituitary target gland endocrine axis, changes in body endocrine status may lead to the occurrence of these two diseases in succession or simultaneously. This study extracted clinical data and survival outcomes of breast cancer patients registered in the Surveillance, Epidemiology and End Results (SEER) database between 2010 and 2019. After matching the case and controls with propensity scores, the selected patients were randomly split into training and test datasets at a ratio of 7:3. Univariate and multivariate COX proportional regression analysis is used to determine independent risk factors for secondary thyroid cancer and construct a column chart prediction model. Age, ethnicity, whether radiotherapy, tumor primary location, N stage, M stage were identified by Cox regression as independent factors affecting secondary thyroid cancer in patients with breast cancer patients, and a risk factor nomogram was established to predict patients’ 3 and 5 year survival probabilities. The AUC values for 3 and 5 years in the training set were 0.713, 0.707, and the c-index was 0.693 (95% CI 0.67144, 0.71456), and the AUC values for 3 and 5 years in the validation set were 0.681, 0.681, and the c-index was 0.673 (95% CI 0.64164, 0.70436), respectively.
Similar content being viewed by others
Introduction
Breast cancer is a malignant tumor that occurs in the Epithelium of the breast gland1. It is the “No. 1 public enemy” that threatens the physical and mental health of women around the world today2. It ranks first in the incidence and death of cancer among women in most countries around the world3. According to the data of the 2023 American Cancer Statistical Report, breast cancer is still one of the three most common cancers among women4 ,occupying the first place in expected new cases among women; With the rapid development of imaging technology and comprehensive treatment in modern medicine5, the early diagnosis rate of breast cancer continues to improve, and the prognosis also improves6.The disease-free survival rate and overall survival rate of breast cancer patients have significantly improved; With the prolongation of the survival period of breast cancer patients, the risk of second primary cancer (SPC) may increase7; Thyroid cancer is a malignant tumor originating from thyroid follicular epithelium8. It is considered to be an inert tumor9 and the most common malignant tumor in the Endocrine system and head and neck tumors10; Since both breast and thyroid are Hormone receptor sensitive organs, which are regulated by hypothalamus pituitary target gland endocrine axis, changes in body endocrine status may lead to the occurrence of these two diseases in succession or simultaneously.
Materials and methods
Data sources
The SEER database containing 13 registry centers prepared by the National Cancer Institute was selected for the data of this study, and a total of 692,555 patients diagnosed with breast cancer from 2010 to 2019 were extracted from the database using the SEER*Stat software program, and a total of 393,722 patients were screened according to the inclusion and exclusion criteria.
Inclusion and exclusion criteria
Inclusion criteria
(1) Patients diagnosed with breast cancer as the first tumor from 2010 to 2019 (2) Patients with no more than 2 tumors and the second type of tumor is thyroid cancer (3) Female patients (4) Patients with complete clinical data(Includes time of diagnosis, age at diagnosis, marital status at diagnosis, site of origin, mode of diagnostic confirmation, mode of case report, chemotherapy or not, radiotherapy or not, molecular typing, Progesterone Receptor, Estrogen Receptor, T,N, and M typing, type of secondary second tumor, interval between secondary second tumors, survival time, survival status)
Exclusion criteria
(1) Those with benign tumors (2) Proven only at autopsy or death (3) Patients with thyroid cancer occurring within 3 months of breast cancer diagnosis
Independent variables
The study data were converted to categorical variables in order to make the study more intuitive and standardized. Race was categorized as black, white, and other; marital status was categorized as married, unmarried; radiation and chemotherapy status was categorized as yes, no; primary tumor location was categorized as mid-breast, quadrant I, quadrant II, quadrant III, quadrant IV, and other; estrogen receptor (ER), and progesterone receptor (PR) status was categorized as estrogen receptor (ER) and progesterone receptor (PR) status positive and negative; and molecular staging included LuminalA, LuminalB, HER2 overexpression and triple-negative; T staging as T0T1, T2, T3 and T4; N staging as N0, N1, N2 and N3; M staging as M0 and M1; and secondary thyroid cancer status as yes or no.
Statistical methods
R4.2.3 statistical software was used for analysis. Patient standardized incidence rates (SIR) were calculated using the Multiple Principal Standardized Incidence Rates (MP-SIR) module of SEER Stat 8.4.1 software. Due to the large difference in sample sizes between the case and control groups in this study, in order to equalize the distribution of covariates between the groups, equalize confounders, and reduce selection bias, we introduced the propensity score matching (PSM) method11, in which the two groups of patients were matched in a ratio of 1:412. Using the R software package MatchIt, the PSM method was used to match the case and control groups according to the year of diagnosis in a 1:4 ratio, and a total of 392,803 patients with unilateral breast cancer were included as controls, and a 1:4 matching of the case and control groups was accomplished based on the year of diagnosis of breast cancer in the 919 case group of thyroid cancer secondary to breast cancer. Patients served as controls. To ensure that the 919 cases were matched to the 3676 controls, caliper distances were chosen to be as small as possible. Propensity scores were calculated using a logistic regression model, and for better matching, the final matching caliper distance was 0.1. The standardized mean difference (SMD) was used to assess the balance of baseline information between the case and control groups after PSM. We considered SMD less than or equal to 0.1 to indicate a good match11.
Further univariate and multivariate analyses were performed using the COX proportional risk model to determine the risk factors for secondary thyroid cancer in breast cancer patients. R-studio software was used to randomly divide all the data into training and validation sets in the ratio of 7:3, and χ2 test and t-test were performed on different variables in the training and validation sets, and then univariate and multivariate Cox regression analyses were carried out on the data in the training set in order to train the model, and the validation set was used to validate the model13. A nomogram was created using the R packages rms, foreign and survival for the final filtered variables and the ROC curves were used to create the nomograms under the ROC curves. The area under the ROC curve (AUC value) and C-index were used to evaluate the accuracy of the model, the AUC and C-index range from 0 to 1, the closer to 1 indicates that the model is more accurate, and it is usually considered that the model has a better predictive ability when the AUC reaches more than 0.7; the calibration curve was used to evaluate the degree of calibration of the model, and the closer the calibration curve is to the standard curve, the stronger the predictive ability of the model. Variables with a univariate COX regression of P < 0.1 were included in the multifactor analysis, and the multifactor analysis was included in the final model with the criterion that the difference was considered statistically significant at P < 0.0514.
Ethical approval and consent to participate
Not applicable. Data is available in a public database, ethics approval is not applicable.
Results
Standardized incidence rate
The SEER Stat 8.4.1 analysis yielded a SIR result of 14.89 with a 95% CI of 14.02–15.79 for the period 2010–2019, a predicted number of people of 74.16, and a 10 year actual number of people of 1104, with an incidence rate of 159.41/100,000, which leads to the conclusion that patients who already have breast cancer are more likely to develop thyroid cancer than healthy people.
Propensity score matching
919 patients with thyroid cancer secondary to breast cancer after screening as case group and 392,803 patients with solitary breast cancer as control group were included in the propensity score matching, according to the year of diagnosis according to the PSM method to achieve 1:4 matching, and finally a total of 919 cases in the case group and 3676 cases in the control group were obtained, and the results are shown in Table 1. The R language tableone package automatically uses analysis of variance for continuous variables, which is equivalent to t-test since this data is only divided into two groups. The result before matching was P < 0.001 and the result after matching was P = 1, SMD < 0.001, which can be considered as well balanced between the matched case group and the control group.
Baseline information
The 919 patients with thyroid cancer secondary to breast cancer and the 3676 patients only with breast cancer obtained after propensity score matching, for a total of 4595 patients, were included in subsequent model analyses. The baseline data of the 4595 patients are shown in Table 2.
Comparison of baseline characteristics
The 4594 patients obtained after propensity score matching were randomly divided into training set and validation set according to the ratio of 7:3, 3219 patients in the training set and 1376 patients in the validation set. t-test was used for continuous variables, and chi-square test was used for categorical variables to characterize the intergroup differences between the training set and the validation set, and the p-values were all greater than 0.05, which proved that there were no intergroup differences in the results of the randomized splitting, and the results are shown in Table 3.
Results of univariate and multivariate cox regressions
The training set patient data were included in univariate Cox regression analysis for each of the 12 variables. To avoid omission of important variables, 11 variables with P < 0.1 in the univariate Cox regression were included in the multivariate Cox regression. We included the variables with p < 0.1 in the univariate Cox regression analysis to the multivariate Cox model to examine the independent risk factors for second thyroid cancer; when P < 0.05 in multivariate Cox regression analysis, the factor was an independent risk factor affecting patients’ secondary thyroid cancer. The results of this univariate Cox regression showed that age, ethnicity, marital status, primary tumor location, molecular typing, PR status, ER status, whether radiotherapy, whether chemotherapy, T stage, N stage, M stage were the factors affecting the secondary thyroid cancer in breast cancer patients; while the results of multivariate Cox regression showed that age, race, whether radiotherapy, primary tumor location, N-stage, and M-stage were independent risk factors affecting the development of thyroid cancer in patients with breast cancer, and the results are shown in Table 4.
Creation of nomogram
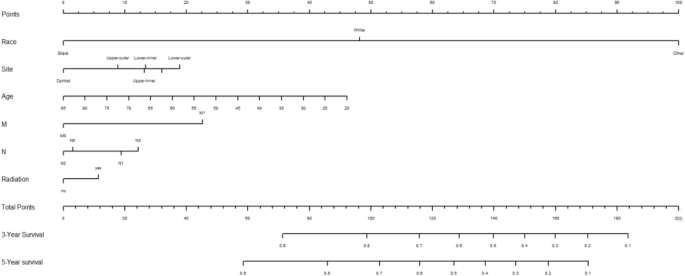
Variables screened in the multifactorial Cox regression analysis (P < 0.05) were included in the cox proportional risk model, and nomograms were created using R-studio software. Each variable was projected upward for breast cancer patients, and then each score on the scale was summed to obtain a total score, based on which the risk of secondary thyroid cancer in breast cancer patients at 3 and 5 years could be predicted, and the higher the total score the higher the risk, the prediction results are shown in Fig. 1. Patients with breast cancer who were younger, received radiotherapy, had N1 staging, M1 staging, were white and other race, and had a primary in the inner lower quadrant were at greater risk of secondary thyroid cancer.
Validation of nomogram
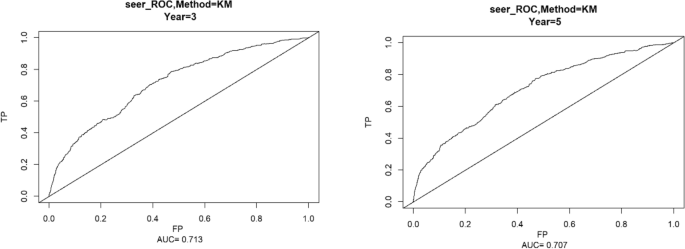
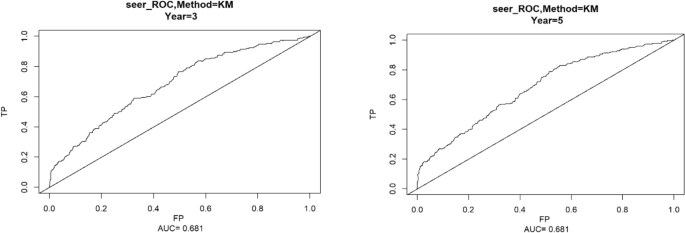
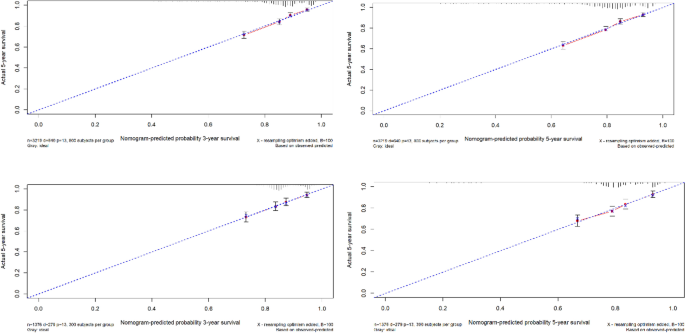
Using the ROC curve AUC value and c-index to evaluate the differentiation of the model, we found that, in the Cox proportional risk regression model, the AUC values of three and five years for the patients in the training set (Fig. 2) were 0.713, 0.707, and the c -index was 0.693 (95% CI 0.67144, 0.71456), respectively, and the AUC of three and five years for the patients in the validation set (Fig. 3) values were 0.681, 0.681,c-index was 0.673 (95% CI 0.64164, 0.70436 respectively), we can consider the model as having moderate predictive power. The calibration plots were plotted using the data from the validation set to evaluate the fit of the established model, and the results showed that in the Cox proportional risk regression model, the calibration curves of 3 and 5 years were close to the dotted line with a 45 ° inclination in the middle, which indicated that the model fit was better.The calibration curves of 3 and 5 years are shown in Fig. 4.
Utility and discussion
Breast cancer is known as the world’s number one “red face killer”, according to the latest cancer data released by the World Health Organization, the number of new cases of breast cancer (2.26 million) has exceeded that of lung cancer (2.2 million), and has become the most prevalent cancer in the world4. In recent years, more and more general hospitals have set up thyroid breast surgery departments, mainly because it is generally recognized that both the thyroid and the breast are target organs for hormone regulation by the hypothalamic-pituitary endocrine axis, and there are some common pathogenic factors in the two. Therefore, the use of a combination of clinical diagnostics and intelligent means to determine the risk factors for secondary thyroid cancer in breast cancer patients is important for both doctors and patients, and it can guide breast cancer patients for follow-up screening and help clinicians develop treatment plans for the general population and breast cancer survivors.
Although the number of breast cancer patients has been increasing year by year, the low incidence of dual primary cancers, the fact that patients with second primary cancer may be seen in different hospitals, the difficulty of data collection and the long time required for follow-up make the collection of data for the study of dual primary cancers of the breast still difficult. Therefore, this study chooses the U.S. National Cancer Database (SEER), which covers about 30% of the U.S. population, updates patient data in March to April every year, and has a large number of patients with a long follow-up period, making it a more ideal source of data for the study of dual primary cancers.
A total of 692,555 patients diagnosed with breast cancer in the SEER database from 2010 to 2019 were included in this study, and the SEERSTATA software was used to calculate the SIR > 1 for thyroid cancer secondary to breast cancer, and it can be assumed that patients with breast cancer are more likely to develop thyroid cancer compared to cancer-free populations, which is consistent with the findings of previous literature. Due to the large difference in sample size between the case and control groups, in order to minimize bias, we matched 919 patients with thyroid cancer secondary to breast cancer as the case group and 393,722 patients with solitary breast cancer as the control group using propensity score matching. Because the diagnostic criteria, examination instruments, and staging criteria may differ between years, we performed 1:4 propensity score matching according to the year of diagnosis to minimize confounding bias and to include as many control cases as possible to ensure representativeness12. The matched 4595 patients were randomly divided into training set and validation set in the ratio of 7:3, and the obtained training set was used to train the cox regression model, and the validation set was used to verify the stability of the model15,16. The results of univariate cox regression showed that the 11 factors included except ER were independent factors for secondary thyroid cancer in breast cancer patients, and the results of multivariate cox regression showed that age, ethnicity, location of primary tumor, whether or not to have radiotherapy, N-stage, and M-stage were independent factors for secondary thyroid cancer in breast cancer patients.
Contrary to the general impression that age, as a continuous variable with an OR value of less than 1, is considered a protective factor against secondary thyroid cancer from breast cancer, the younger the age, the more likely it is to be secondary thyroid cancer, and previous studies by other scholars have also demonstrated that patients with secondary thyroid cancer after breast cancer were younger compared with those who had breast cancer only17, which we believe may be related to the recent years of rejuvenation of the incidence of breast cancer, the early diagnosis and early treatment that makes the breast cancer patient. We believe that this may be related to the recent rejuvenation of breast cancer incidence, prolonged survival due to early diagnosis and early treatment, and the modern lifestyle, where a good prognosis increases the likelihood of a secondary second tumor. However, in some scholars’ studies, age is a risk factor for survival in patients with thyroid cancer secondary to breast cancer relative to patients with solitary breast cancer, and older patients are more likely to have lower survival rates18. In conclusion, age is an extremely important factor affecting breast cancer patients with secondary thyroid cancer and warrants further study.
In terms of race, the SEER database has more white profiles due to region, and multivariate cox results show that whiteness and other ethnicities are independent risk factors for secondary thyroid cancers in patients with breast cancer. This may be related to the level of medical care, conditions, and other factors that were co-incorporated into the study, with genetic susceptibility playing a different role in different factors. Mariotto A.B. et al. suggest that the higher incidence of second primary cancers among white women is due to the higher overall survival and screening rates among white women compared to black female populations, and that more comprehensive medical coverage and higher levels of medical care make it easier to diagnose the disease, which would inevitably lead to an increase in the number of diagnoses if these cancers were diagnosed at an earlier stage, which would be consistent with the results of the present study19. If these cancers were diagnosed at an early stage, this would inevitably lead to an increase in the number of diagnoses. However the study by Shuting Li et al. suggests that black women with breast cancer should be given attention13. However, in a study by Karan Seegobin et al., it was found that there was no significant difference in the incidence of secondary breast and gynecologic cancers between Caucasians and Blacks20 ,which may be due to the different target second primary cancer disease types in the study, and further research is needed in the future.
Breast cancer patients who receive radiation therapy are more likely to develop secondary thyroid cancers compared to those who do not, a finding that is generally consistent with current clinical opinion that radiation therapy can affect thyroid hormone secretion and thyroid function21. The relationship between radiation therapy and the risk of second primary cancers has long been recognized, and it has been demonstrated that radiation therapy is associated with an increased risk of second primary malignancies after exposure21. In several observational studies of breast cancer follow-up, the incidence of subsequent secondary acute myeloid leukemia was increased in patients with breast cancer, which may be related to the dose intensity of chemotherapy, the use of adjuvant radiotherapy, and the use of granulocyte colony-stimulating factor (GCSF)22. Data from the DBCG (The Danish Breast Cancer Cooperative Group) registry estimate that the proportion of second primary cancers after breast cancer associated with radiation therapy is about 9%. This is consistent with the results from the US SEER database registry23. However Grantzau’s analysis found that breast cancer radiotherapy was associated with a small but significant increase in the risk of second cancers for lung, esophageal, and soft tissue cancers, but was not significantly associated with second cancers for thyroid cancer24. Another study on the health of the population in Taiwan compared the risk of TC in BC patients who received radiotherapy and those who did not, and the risk of TC in women who received radiotherapy was not significantly higher than that in women who did not receive radiotherapy. This may be related to the selection of data from different ethnic groups in different regions.
In addition, some researchers have suggested that the ER/PR signaling pathway may be a common etiology of breast and thyroid carcinogenesis, and studies of its mechanisms, including the ER pathway and CHEK2 gene mutations in thyroid tissues. In our study, only PR receptor status was an independent factor for secondary thyroid cancer in breast cancer patients. Some researchers have suggested that patients with secondary thyroid cancer have a higher rate of both ER and PR positivity than patients with breast cancer only25,26.ER has two isoforms, Erα and Erβ, and overexpression of ERα in thyroid cancer tissues and lack of expression of ERβ in peripheral tissues was reported in 201127 , and under-expression or deletion of ER can be considered a hallmark of thyroid cancer28. Undifferentiated thyroid stem and progenitor cells express lower levels of ERβ compared to differentiated human thyroid cells29. Therefore, low levels of ER expression may suggest dedifferentiation of thyroid cancer27,30. The fact that this paper did not conclude that ER has an effect on secondary thyroid cancer in breast cancer patients may be related to the fact that gene-related variables were not included in this study, and that the SEER database contains only baseline and treatment information and no genetic data, which is a limitation that exists in this study31,32,33.
In AJCC staging, T stage represents the size and extent of the tumor, N stage represents lymph node metastasis, and M stage represents distant spread, with more stages representing more seriousness and worse prognosis. In this study, it was concluded that in N staging, breast cancer patients with N1 stage were more likely to develop secondary thyroid cancer compared to other stages, which may be related to the survival period, and we believe that only if the survival period is long enough, there is a possibility of developing a second primary tumor, while patients with N1 stage had lymph node metastasis, but it was not as serious as N2, N3, and N4 stage, so they were more likely to have a longer survival period; while patients with N0 stage had not No lymph node metastasis has occurred, and from the perspective of disease development, the possibility of secondary cancer is less than that of stage N1.In M stage, M0 stage represents no distant metastasis, and M1 stage represents distant metastasis, and we generally know that tumors with metastases are more likely to cause other cancers.
To summarize, in this study, univariate and multivariate cox analyses were used to screen the influencing factors of secondary thyroid cancer in breast cancer patients, respectively, and a column-line diagram was successfully established, and the calibration curves of the training and validation sets were well fitted, and the AUC and c-index reached significance. It is suggested that the column-line diagram has good predictive ability for the risk of secondary thyroid cancer in breast cancer patients 3 and 5 years after the onset of the disease. However, since the data were obtained from the United States, more studies are needed to verify whether the results obtained by applying this data can be applied to the Chinese population, and the results of this study can provide some references for clinicians14.
This study also has some limitations; first, this is a retrospective study, and selection bias due to incomplete data is inevitable. Although we used PSM to avoid selection bias, potential confounders cannot be excluded. Secondly, the Cox model fits well, but the AUC of the validation set is less than 0.7, which represents that the predictive ability of the model is still lacking, which may be related to the fact that breast cancer patients have a good prognosis and a long survival period, while only ten years of data have been observed in the present study, so in the future, we need more follow-up data to improve the model. Because the SEER database itself provides a limited amount of information and the database does not provide any information about genes, we were unable to study the genetic correlation of breast cancer secondary to thyroid cancer at the genetic level. The database has not been updated with data on tumor grading since 2017, so it is not possible to analyze the grading situation, which deserves further study in the future.
Conclusions
In summary, our study suggests that breast cancer patients are more likely to develop thyroid cancer compared to the general population. Among them, age, ethnicity, marital status, primary tumor location, molecular typing, PR status, ER status, whether or not radiotherapy, whether or not chemotherapy, T stage, N stage, and M stage are the factors affecting the secondary thyroid cancer in breast cancer patients; age, ethnicity, whether or not radiotherapy was performed, primary location of the tumor, N stage, and M stage are the independent factors affecting the secondary thyroid cancer in patients with breast cancer patients: the younger the age, the whiter the ethnicity, the whites and the other ethnicities, undergoing radiotherapy, internal lower quadrant and other locations, N1 stage, and M1 stage are more likely to develop thyroid cancer in breast cancer patients.
Data availability
The datasets analyzed during the current study are available in the SEER*Stat software (version 8.4.2, download from https://seer.cancer.gov/data/options.html). A registration form needs to be completed before using and filtercriteria need to be added. The datasets are also available from the corresponding author on reasonable request.
References
Huang, J. et al. Effect of breast cancer as the first or second primary cancer on the prognosis of women with thyroid cancer: A SEER database analysis. Transl. Cancer Res. 911, 6955–6962 (2020).
Bolf, E. L., Sprague, B. L. & Carr, F. E. A linkage between thyroid and breast cancer: A common etiology?. Cancer Epidemiol. Biomark. Prev. 284, 643–649 (2019).
Bakos, B. et al. Co-occurrence of thyroid and breast cancer is associated with an increased oncogenic SNP burden. BMC Cancer 21, 1–11 (2021).
Siegel, R. L., Miller, K. D., Wagle, N. S. & Jemal, A. A Cancer statistics, 2023.. CA Cancer J. Clin. 73(1), 17–48 (2023).
Zhang, W. et al. Metastasis patterns and prognosis in young breast cancer patients: A SEER database analysis[j]. Front. Oncol. 12, 872862 (2022).
基于SEER数据库对乳腺癌...原发癌危险因素的回顾性分析_齐欣.pdf>.
Fu, J. et al. The clinical and genetic features in patients coexisting primary breast and thyroid cancers. Front. Endocrinol. 14, 1136120 (2023).
Dong, L., Lu, J., Zhao, B., Wang, W. & Zhao, Y. Review of the possible association between thyroid and breast carcinoma. World J. Surg. Oncol. 16, 1–7 (2018).
Ahn, J.-H. et al. The association between vitamin D supplementation and the long-term prognosis of differentiated thyroid cancer patients: A retrospective observational cohort study with propensity score matching. Front. Endocrinol. 14, 1163671 (2023).
Lim, H., Devesa, S. S., Sosa, J. A., Check, D. & Kitahara, C. M. Trends in thyroid cancer incidence and mortality in the United States, 1974–2013. JAMA https://doi.org/10.1177/10732748221135447 (2017).
Cui, J. et al. Association of dietary pattern and Tibetan featured foods with high-altitude polycythemia in Naqu, Tibet: A 1:2 individual-matched case-control study. Front. Nutr. https://doi.org/10.3389/fnut.2022.946259 (2022).
Wang, J., Ning, Y., Du, Y. & Kang, Y. Lymphadenectomy benefits small cell carcinoma of ovary: A population-based analysis. Curr. Oncol. 2910, 7802–7815 (2022).
Li, S. et al. Clinicopathological features, survival and risk in breast cancer survivors with thyroid cancer: An analysis of the SEER database. BMC Public Health 19, 1 (2019).
Wang, R. et al. Risk factor analysis and nomogram establishment and verification of brain astrocytoma patients based on SEER database. Sci. Rep. 13, 1 (2023).
Qiu, X. et al. Chemotherapy combined with radiotherapy can benefit more unresectable HCC patients with portal and/or hepatic vein invasion: A retrospective analysis of the SEER database. Front. Oncol. https://doi.org/10.3389/fonc.2023.1098686 (2023).
Zhou, Y. et al. The prognostic significance of further axillary dissection for sentinel lymph node micrometastases in female breast cancer: A competing risk analysis using the SEER database. Front. Oncol. https://doi.org/10.3389/fonc.2022.1012646 (2022).
Pu, C.-C., Yin, L. & Yan, J.-M. Risk factors and survival prediction of young breast cancer patients with liver metastases: A population-based study. Front. Endocrinol. https://doi.org/10.3389/fendo.2023.1158759 (2023).
Donin, N. et al. Risk of second primary malignancies among cancer survivors in the United States, 1992 through 2008. Cancer 12219, 3075–3086 (2016).
Mariotto, A. B., Rowland, J. H., Ries, L. A. G., Scoppa, S. & Feuer, E. J. Multiple cancer prevalence: A growing challenge in long-term survivorship. Cancer Epidemiol. Biomark. Prev. 163, 566–571 (2007).
Seegobin, K. et al. Pilot study on the occurrence of multiple cancers following cancer-related therapy at the University of Florida, Jacksonville (2011–2016). J Investig. Med. 667, 1050–1054 (2018).
Praga, C. et al. Risk of acute myeloid leukemia and myelodysplastic syndrome in trials of adjuvant epirubicin for early breast cancer: Correlation with doses of epirubicin and cyclophosphamide. J. Clin. Oncol. 2318, 4179–91 (2005).
Smith, R. E., Bryant, J., DeCillis, A. & Anderson, S. Acute myeloid leukemia and myelodysplastic syndrome after doxorubicin-cyclophosphamide adjuvant therapy for operable breast cancer: The national surgical adjuvant breast and bowel project experience. J. Clin. Oncol. 217, 1195–204 (2003).
Sokołowski, M., Mazur, G. & Butrym, A. Breast cancer and synchronous multiple myeloma as a diagnostic challenge: Case report and review of literature. Curr. Probl. Cancer 422, 231–234 (2018).
Grantzau, T. & Overgaard, J. Risk of second non-breast cancer among patients treated with and without postoperative radiotherapy for primary breast cancer: A systematic review and meta-analysis of population-based studies including 522,739 patients. Radiother. Oncol. 1213, 402–413 (2016).
Li, S. et al. Clinicopathological features, survival and risk in breast cancer survivors with thyroid cancer: An analysis of the SEER database. BMC Public Health 191, 1592 (2019).
Ji, J., Zhang, X., Yuan, S., Liu, H. & Yang, L. Survival impact of gastrectomy and chemotherapy for gastric signet ring cell carcinoma with different metastatic lesions: A population-based study. Asian J. Surg. 47(4), 1769–1775 (2024).
Di Vito, M. et al. Overexpression of estrogen receptor-α in human papillary thyroid carcinomas studied by laser-capture microdissection and molecular biology. Cancer Sci. 10210, 1921–7 (2011).
Božović, A., Mandušić, V., Todorović, L. & Krajnović, M. Estrogen receptor beta: The promising biomarker and potential target in metastases. Int. J. Mol. Sci. 22(4), 1656 (2021).
Xu, S., Chen, G., Peng, W., Renko, K. & Derwahl, M. Oestrogen action on thyroid progenitor cells: Relevant for the pathogenesis of thyroid nodules?. J. Endocrinol. 2181, 125–33 (2013).
Thomas, C. & Gustafsson, J. The different roles of ER subtypes in cancer biology and therapy. Nat. Rev. Cancer 118, 597–608 (2011).
Lal, G. et al. Risk of subsequent primary thyroid cancer after another malignancy: Latency trends in a population-based study. Ann. Surg. Oncol. 196, 1887–96 (2012).
Sun, L. M., Lin, C. L., Liang, J. A., Huang, W. S. & Kao, C. H. Radiotherapy did not increase thyroid cancer risk among women with breast cancer: A nationwide population-based cohort study. Int. J. Cancer 13712, 2896–903 (2015).
An, J. H. et al. A possible association between thyroid cancer and breast cancer. Thyroid 2512, 1330–8 (2015).
Acknowledgements
We would like to thank Editage for English language editing.
Author information
Authors and Affiliations
Contributions
Y.D. (first author): design, original draft preparation, software analysis, data analysis and interpretation, manuscript writing, final approval of manuscript. R.W.: data interpretation, final approval of manuscript. J.C: collection data, final approval of manuscript. C.J.: methodology, final approval of manuscript. Y.C.: methodology, final approval of manuscript. X.L.: corresponding author, guide the revision of the article, final approval of manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Diao, Y., Wang, R., Cui, J. et al. Risk factors for secondary thyroid cancer in patients with breast cancer: a propensity‑matched SEER analysis. Sci Rep 14, 12679 (2024). https://doi.org/10.1038/s41598-024-59209-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-59209-x
- Springer Nature Limited