Abstract
This study aimed to investigate the adsorption properties of CO2, CH4, and N2 on anthracite. A molecular structural model of anthracite (C208H162O12N4) was established. Simulations were performed for the adsorption properties of single-component and multi-component gases at various temperatures, pressures, and gas ratios. The grand canonical ensemble Monte Carlo approach based on molecular mechanics and dynamics theories was used to perform the simulations. The results showed that the isotherms for the adsorption of single-component CO2, CH4, and N2 followed the Langmuir formula, and the CO2 adsorption isotherm growth gradient was negatively correlated with pressure but positively correlated with temperature. When the CO2 injection in the gas mixture was increased from 1 to 3% for the multi-component gas adsorption, the proportion of CO2 adsorption rose from 1/3 to 2/3, indicating that CO2 has a competing-adsorption advantage. The CO2 adsorption decreased faster with increasing temperature, indicating that the sensitivity of CO2 to temperature is stronger than that of CH4 and N2. The adsorbent potential energies of CO2, CH4, and N2 diminished with rising temperature in the following order: CO2 < CH4 < N2.
Similar content being viewed by others
Introduction
Technologies for infusing CO2 or CO2–N2 mixtures into coal seams to replace CH4 have gradually matured in recent years, improving CH4 extraction rates and enabling the sequestration of large amounts of CO21,2,3,4. These technologies primarily utilize the different adsorption abilities of CO2, CH4, and N2 on coal to improve the CH4 extraction rate.
Gases in coal are mainly adsorbed physically. N2 and CO2 have a wide range of sources, where N2 accounts for approximately 78% of the atmosphere while CO2 is mainly generated through coal oxidation and combustion5. Scholars around the world have conducted multiple gas adsorption experiments6,7,8 and Monte Carlo simulation studies9,10,11 to investigate the adsorption characteristics of different gases on coal. The impact of temperature on gas adsorption has been investigated using the high-pressure volumetric method, which showed that low temperatures hinder gas adsorption on coal12,13. Li et al.14 proposed the theory of the competitive adsorption of multiple gases using the low-temperature nitrogen adsorption method. Zhu et al.15 experimentally verified that the maximum adsorption capacities of CO2, CH4, and N2 on anthracite exceed those on bituminous coal at different temperatures. Xiao et al.16,17 established the relationship between the coal adsorption capacity and temperature for CO2, CH4, and N2. Cheng et al.18,19 applied the grand canonical ensemble Monte Carlo method to investigate the adsorption properties of CO2/N2 on coal. Similarly, Wu et al.20,21 investigated the microscopic mechanism of the competitive adsorption of coal-fired flue gas on coal. The research revealed the competitive adsorption behavior during the gas adsorption. Qu et al.22 developed a macromolecular model of coal and used the grand canonical Monte Carlo (GCMC) and molecular dynamics (MD) molecular simulations to reveal the microscopic mechanisms of CO2, O2, and CH4 adsorption and diffusion on coal molecules at different temperatures, pressures, and molar fractions. Gao et al.23 analyzed the adsorption capacity of CO2/CH4/N2/H2O on different molecular models of brown coal using molecular simulation. Wang et al.24 used molecular simulation to examine the adsorption behavior of CH4/CO2 on oxidized coal deposits. Yu et al.25 conducted experiments and simulations to examine the competitive adsorption mechanism of CO2 and CH4 on low-rank coal mirror groups. Bai et al.26 performed a molecular simulation to investigate the kinetic mechanism of CO2 and N2 replacement of CH4. The results showed that CO2 and N2 mainly replaced CH4 gas by occupying the adsorption site. Liu et al.27 performed CO2-ECBM tests on rectangular coal specimens and monitored the change patterns of pore pressure, gas flow, and outlet concentration with the amount of CO2 injected; they found that infusing CO2 into the coal body can effectively increase the CH4 recovery. Li et al.28 proposed a dynamic evolution model of coal permeability, which responded to the ECBM mining process and confirmed the technical feasibility of N2-BCEM injection.
Other international scholars have also studied the adsorption properties of gases consisting of single components and multiple components. Using computer molecular simulations, Laurent et al.29 investigated the absorbent behaviors of CO2 and CH4 in micropores. Perera et al.30 investigated the adsorption capacity of two gases at three temperatures. Abunowara et al.31 analyzed the characteristics of four coal sample adsorption gases in Malaysia using BET, XRD, FESEM, and other techniques, and the coal specimens exhibited a greater adsorption affinity for CO2 than for CH4 and N2. Busch et al.32 studied the gas adsorption of binary mixtures at a specific pressure using Langmuir’s method, which provides an essential idea for this current study on the adsorption of ternary gas mixtures.
The studies highlighted above mainly focused on the adsorption characteristics of binary gas systems, confirming the advantage of CO2 adsorption but ignoring the adsorption characteristics of ternary gas systems. In this article, we quantitatively analyzed the adsorption behavior of CO2, CH4, and N2 on coal and the main influencing factors. The injection of different ratios of CO2, CH4, and N2 into coal seams was simulated for the study.
Methodology
Molecular modeling of anthracite
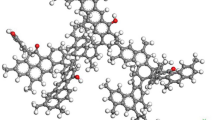
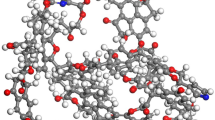
The anthracite molecule (C208H162O12N4)33 (illustrated in Fig. 1) was selected to study the adsorption properties of anthracite for CO2, CH4, and N2. Geometry optimization, energy optimization, and simulated annealing were performed on the structure using the Forcite module in Materials Studio, and the optimized energy parameters are shown in Table 1 to obtain the lowest energy conformation, as shown in Fig. 2. The Amorphous cell module was used to put the two optimized anthracite molecular models into the computational cell (a = 18.607 Å, b = 18.607 Å, c = 18.607 Å), as shown in Fig. 3.
Simulation parameter setting
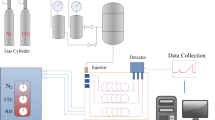
To simulate the adsorption of CO2, CH4, and N2 on anthracite coal, we used a giant regular ensemble Monte Carlo simulation, selecting the Dreiding force field customized for computational accuracy, Charges selects QEq, the Ewald summation method for electrostatic interactions, and the atom-based method for van der Waals interactions. The temperatures used for the Fixed Pressure Task were 263.15 K, 273.15 K, 283.15 K, 293.15 K, 303.15 K, and 313.15 K, and the pressure ranged from 0.01 to 2 MPa.
Results and discussion
Single-component gas adsorption system
Figure 4 illustrates the adsorption isotherms of N2, CO2, and CH4, as single-component gases under varying pressures and temperatures. As observed from the figure, at temperatures of 263.15 K, 273.15 K, 283.15 K, 293.15 K, 303.15 K, and 313.15 K and at pressures ranging from 0.01 to 2 MPa, the adsorption sites on the surface of the anthracite coal become more active with increasing temperature. Consequently, CO2, CH4, and N2 are more readily detached from the coal surface, reducing the adsorption capacity. Similarly, Fig. 5 illustrates the variation of the CO2, CH4, and N2 adsorption with temperature at a pressure of 0.1 MPa. At 263.15 K, a considerably larger number of adsorbed CO2 molecules are in the anthracite molecular model than the number of CH4 and N2 molecules. With the increase of pressure from 0.01 MPa to 2 MPa in the system, the adsorption amount of CO2, CH4 and N2 showed an upward trend, indicating that the elevated pressure can promote the adsorption of CO2, CH4 and N2 by anthracite. The magnitude of adsorption at the same pressure is in the following order: CO2 > CH4 > N2. This result can be explained as follows: first, the molecules of the three gases have different equivalent diameters, where the molecular diameter of CO2 is 0.33 nm, that of N2 is 0.368 nm, and that of CH4 is 0.382 nm. Because the diameter of CO2 molecule is small, the critical temperature and critical pressure of CO2 are larger, which makes the competitive adsorption advantage of CO2 stronger than that of CH4 and N2 in the ternary gas system of CO2, CH4, and N2, and therefore the anthracite molecules preferentially adsorb CO2, so it leads to the adsorption amount of CO2 is larger than that of CH4 and N2. Owing to the small difference between the molecular diameters of CH4 and N2, both have different polarization volume, where that of CH4 is 4.48 × 10–30 m3 and that of N2 is 1.53 × 10–30 m3. The molecules with a higher polarization volume are adsorbed more easily; thus, the amount of CH4 adsorbed is larger than that of N2. Secondly, the critical temperatures are different, the critical temperatures of CO2, CH4 and N2 are 304 K, 191 K and 126 K. The size of the critical temperatures is CO2 > CH4 > N2. As the critical temperature increases, the gas adsorption on the surface of the coal becomes faster and adsorbs more easily. Third, van der Waals forces also play a role: an increase in the pressure of the system is accompanied by an increase in the van der Waals energy, and the stronger the effect of the van der Waals force, the faster the adsorption. Take a temperature of 263.15 K as an example, the van der Waals energy data released by anthracite adsorption of CO2, CH4, and N2 are shown in Table 2.
The isothermal adsorption curves of CO2, CH4, and N2 at different pressure ranges have the form of Langmuir curves. Hence, the Langmuir formula was used to fit the isothermal adsorption curves of CO2, CH4, and N2 for anthracite. The fitting results are presented in Table 3.
The fitting results show that the linear correlation coefficient R2 was greater than 0.94 in all cases, indicating good fitting and confirming the reliability of the simulated data. The adsorption constants k1, k2 decrease with increasing temperature; the adsorption amount also decreases, indicating that low temperatures are favorable for CH4 adsorption.
Figure 4 shows that the adsorption isotherm growth gradient of CO2, CH4, and N2 within the pressure range of 0.01–0.1 MPa is considerably larger than that within the pressure ranges of 0.1–1 MPa and 1–2 MPa. Therefore, CO2 was used as an example to calculate the adsorption isotherm growth gradients of the gases at different temperatures, as shown in Table 4.
At different temperatures, the growth gradients of CO2 adsorption isotherms were 1.985–2.809 for pressures of 0.01–0.1 MPa, 1.468–1.892 for pressures of 0.1–1.0 MPa, and 0.975–1.035 for pressures of 1.1–2.0 MPa. These results show that the CO2 adsorption isotherm growth gradient is the largest and the adsorption rate is the fastest in the pressure range of 0.01–0.1 MPa. This phenomenon occurs because of the large number of adsorption sites on the anthracite surface. Initially, CO2, CH4, and N2 are easily adsorbed on these sites, resulting in a faster adsorption rate, but the gas concentration increases while the adsorption sites are gradually saturated, causing the adsorption rate to decelerate until it reaches equilibrium. Therefore, the larger the growth gradient of the adsorption isotherm, the faster the adsorption rate. This behavior occurs because the growth gradient represents the rate of gas adsorption; the faster the adsorption rate, the greater the number of adsorption sites on the anthracite surface.
Multi-component gas adsorption system
Adsorption amount
The single-component gas adsorption shows that CO2 has certain adsorption advantages over CH4 and N2. Different proportions of CO2, CH4, and N2 were added to the anthracite pore model. As the adsorption rate of the gases with pressure ranging from 0.01 to 0.1 MPa is the fastest in the single-component gas adsorption system, the adsorption characteristics of the three gases are discussed for a pressure of 0.1 MPa. The simulation results are shown in Fig. 6.
A comparison of Fig. 6a–c shows that adding the same ratio of CH4 and different ratios of CO2 and N2 to the anthracite molecule with the temperature of 263.15 K, the relative CO2 adsorption increases from 0.20 to 0.42 mmol/g as the CO2 injection increases from 1 to 3%; The relative adsorption of the same proportion of CH4 decreases with the gradual increase in the CO2 injection. The relative adsorption of CH4 decreases from 0.25 to 0.10 mmol/g while that of N2 in different proportions decreases from 0.15 to 0.08 mmol/g. A comparison of the plots in Fig. 6a,d and e shows that at the temperature of 263.15 K, injecting the same proportion of N2 and different proportions of CO2 and CH4 increases the relative adsorption of CO2 with increasing amount of injected gas from 0.20 to 0.40 mmol/g. The relative adsorption of CH4 decreases from 0.25 to 0.11 mmol/g with decreasing CH4 injection, and the relative adsorption of equal proportions of N2 decreases from 0.15 to 0.09 mmol/g. These results show that the adsorption sensitivity of CO2 is very strong, and the relative CO2 adsorption increases rapidly when the CO2 injection increases from 1 to 3%, which far exceeds the relative adsorption of CH4 and N2. Second, the relative adsorption of CH4 decreases slowly as CO2 injection increases in the system, which is because CO2 has certain adsorption advantages and a stronger adsorption capacity than CH4. Hence, CO2 is preferentially adsorbed, decreasing the relative adsorption of CH4. Third, different proportions of N2 and equal proportions of N2 have less effect on changes in the adsorption amount of the system, indicating that N2 adsorption is more stable. The amount of gas adsorbed is affected not only by temperature and gas injections, but also by the adsorption potential. When a gas is adsorbed on the surface of the coal body, the adsorbent is also attracted to the adsorbate; the closer to the surface, the greater the gravitational force, which is the adsorption potential; thus, the adsorption amount is also related to the adsorption potential.
Adsorption potential
Polanyi34 and Dubinin35 proposed the adsorption potential theory. The theory posits that an adsorption potential field encircles a solid, and gas molecules are adsorbed within this field through the influence of attractive forces. Consequently, the connection between the adsorption potential and adsorption amount was used to analyze the adsorption features of three gases: CO2, CH4, and N2.
According to Polanyi, the relationship between adsorption potential and pressure is as follows:
In this context, ε represents the adsorption potential [J/mol]; R represents the ideal gas constant, taken as 8.3144 J/(mmol·K); T represents the absolute temperature [K]; P0 represents the vapor pressure at saturation of the gas corresponding to temperature T [MPa]; and P represents the pressure [MPa].
According to Dubinin, the formula for calculating P0 is,
In this context, Pc represents the critical pressure; the critical pressures of CO2, CH4, and N2 are taken as 7.38 MPa, 4.60 MPa, and 3.40 MPa, respectively. Tc represents the critical temperature, and the critical temperatures of CO2, CH4, and N2 are taken as 304.13 K, 190.56 K, and 126.20 K, respectively.
When we combine the simulation data and substitute Eq. (2) into Eq. (1), the relationship between the adsorption amount and adsorption potential energy of multi-component gases at different temperatures can be calculated, which is illustrated in Fig. 7.
Figure 7a and b demonstrate that by injecting the same proportion of CH4 and N2 into the system, the CO2 adsorption increases rapidly at the temperature of 263.15 K. This phenomenon occurred because of the increased CO2 concentration in the system resulting from the increase in CO2 injection in the system from 1 to 3%. Because of the adsorption advantage of CO2 itself over CH4 and N2, the relative adsorption of CO2 rises rapidly. When the system temperature increases from 263.15 to 283.15 K, the CO2 adsorption rapidly decreases because CO2 is more temperature-sensitive than CH4 and N2. The adsorption levels off when the temperature increases from 283.15 to 313.15 K. This is because the adsorption decreases with increasing temperature, which is consistent with the conclusion of the single-component gas adsorption. The adsorption potential energy of CO2 increases from 8.78 to 11.35 kJ/mol, that of CH4 from 9.79 to 12.56 kJ/mol, and that of N2 from 10.93 to 13.91 kJ/mol, indicating that the adsorption potential energy of each gas component increases with increasing temperature, and the adsorption potential energy of the gas components follows the order: CO2 < CH4 < N2. As shown in Fig. 7a, CO2 adsorption decreases from 1.01 to 0.24 mmol/g, CH4 adsorption decreases from 0.48 to 0.16 mmol/g, and N2 adsorption drops from 0.31 to 0.13 mmol/g. As shown in Fig. 7b, CO2 adsorption decreases from 0.85 to 0.22 mmol/g, CH4 adsorption decreases from 0.47 to 0.13 mmol/g, N2 adsorption decreases from 0.31 to 0.13 mmol/g, and the adsorption amounts of the gas components are in the order of CO2 > CH4 > N2. Therefore, the adsorption amount has a negative correlation with the adsorption potential energy, i.e. the quantity of gas adsorbed reduces as the adsorption potential energy increases. From a thermodynamic perspective, anthracite adsorbs the most CO2, followed by CH4 and N2. This phenomenon occurs because a rise in temperature increases the thermal movement of the solid surface molecules, weakening the intermolecular interaction forces and, consequently, reducing the surface energy. Thus, the CO2, CH4, and N2 molecules are not easily adsorbed on the surface of the coal molecules, which reduces the adsorption amount.
Potential energy distribution
The potential energy distribution of the anthracite adsorption of CO2, CH4, and N2 was obtained through a simulation, and the relationship between the preferential adsorption potential and the amount of gas injected was analyzed. The results are illustrated in Fig. 8 for the pressure of 0.1 MPa and temperature of 263.15 K. The data on the initial potential energy distribution of the gases are shown in Table 5.
Adsorption energy is the energy produced during adsorption. Molecules decelerate and eventually stop at the surface of the adsorption medium during adsorption, which releases some energy. Thus, the greater the absolute value of the adsorption energy, the greater the intermolecular interactions and preferential adsorption.
A comparison of (a) and (b) in Fig. 8 shows that for different injection ratios of CO2, CH4, and N2 into the system, the absolute magnitude of the potential energy peak of CO2 increases with the increasing quantity of gas injected the system. In the two gas systems with certain CH4 and N2 ratios, the potential energy peak of the optimal adsorption site of CO2 decreases from − 8.85 kcal/mol to − 9.65 and − 9.15 kcal/mol, respectively. The potential energy peak of the optimal adsorption site of CH4 decreased from − 3.95 kcal/mol to − 5.05 and − 6.95 kcal/mol, respectively. The potential energy peak of the optimal adsorption site of N2 decreased from − 3.65 kcal/mol to − 4.65 and − 3.95 kcal/mol, respectively. A comparison of (a) and (b) shows that the optimal adsorption site for injecting the same proportion of CH4 into the system is higher than that of N2; thus, the preferential adsorption potential of CO2 is greater than that of CH4, and that of CH4 is greater than that of N2. This result is consistent with the order of magnitude of the amount of the three gases adsorbed. The absolute values of the potential energy peaks for both CH4 and N2 showed a rising pattern with the increase in CO2 injection; hence, CO2 can promote the adsorption behavior of CH4 and N2 on anthracite.
Conclusions
-
(1)
The adsorption behavior of the single-component gas system is in accordance with Langmuir’s law of adsorption, and the values of adsorption constants k1, k2 are generally negatively correlated with temperature. From the perspective of the adsorption rate, CO2, CH4, and N2 attained the fastest adsorption rate in the single-component gas adsorption system at temperatures of 263.15 K, 273.15 K, 283.15 K, 293.15 K, 303.15 K, and 313.15 K, as well as pressures in the range of 0.01 to 2 MPa. In different pressure ranges, the adsorption rate of CO2 increased with increasing temperature because adsorption is typically exothermic, and when the adsorption process has not reached equilibrium, elevated temperatures accelerate the rate of adsorption. The adsorption amount was positively correlated with pressure and negatively correlated with temperature, and the adsorption amounts were in this order of magnitude: CO2 > CH4 > N2.
-
(2)
In the multi-component gas system, at the same pressure, the proportion of the relative adsorption amount of CO2 in the system increased from 1/3 to 2/3 with the increase in CO2 injection from 1 to 3%, whereas the relative adsorption of CH4 and N2 decreased. The relative CO2 adsorption was positively correlated with the amount of gas injected but negatively correlated with temperature. The relative adsorption amounts were in this order of magnitude: CO2 > CH4 > N2.
-
(3)
In the multi-component gas system, under identical pressures, the adsorption potential energy of CO2, CH4, and N2 at 263.15 K was 8.78 kJ/mol, 9.79 kJ/mol, and 10.93 kJ/mol, respectively, with the values increasing with increasing temperature. The adsorption potential energy of CO2, CH4, and N2 was positively correlated with temperature and negatively correlated with the adsorption amount. The adsorption potential energy of the three gases was in this order of magnitude: CO2 < CH4 < N2.
-
(4)
In the multi-component gas system, at the same temperature and pressure, the potential energy peaks of CO2, CH4, and N2 were − 8.85 kcal/mol, − 3.95 kcal/mol, and − 3.65 kcal/mol, respectively, and the absolute values at the optimal adsorption sites were in this order of magnitude: CO2 > CH4 > N2. The absolute values of the potential energy peaks of the optimal adsorption sites for CH4 and N2 increased as the injection of CO2 into the system increased. Furthermore, the preferential adsorption potentials followed the order of CO2 > CH4 > N2.
Data availability
All data supporting the findings of this study are available from the corresponding author Jiaxing Lin upon request.
References
Fan, N. et al. Numerical study on enhancing coalbed methane recovery by injecting N2/CO2 mixtures and its geological significance. Energy Sci. Eng. 8, 1104–1119 (2020).
Jeong, S. R. et al. Review of the adsorption equilibria of CO2, CH4, and their mixture on coals and shales at high pressures for enhanced CH4 recovery and CO2 sequestration. Fluid Phase Equil. 564, 113591 (2023).
Lin, J., Ren, T., Cheng, Y. P., Nemcik, J. & Wang, G. D. Cyclic N2 injection for enhanced coal seam gas recovery: A laboratory study. Energy 188, 116115 (2019).
Talapatra, A., Halder, S. & Chowdhury, A. I. Enhancing coal bed methane recovery: Using injection of nitrogen and carbon dioxide mixture. Petrol. Sci. Technol. 39, 49–62 (2021).
Deng, J., Xiao, Y., Li, Q. W., Lu, J. H. & Wen, H. Experimental studies of spontaneous combustion and anaerobic cooling of coal. Fuel 157, 261–269 (2015).
Davarpanah, A. & Mirshekari, B. Experimental investigation and mathematical modeling of gas diffusivity by carbon dioxide and methane kinetic adsorption. J. Ind. Eng. Chem. Res. 58, 12392–12400 (2019).
Xue, Y. et al. Experimental investigation of mechanical properties, impact tendency, and brittleness characteristics of coal mass under different gas adsorption pressures. Geomech. Geophys. Geo-Energy Geo-Resour. https://doi.org/10.1007/s40948-022-00439-6 (2022).
Zhu, H. Q., Guo, S., Xie, Y. Y. & Zhao, H. R. Molecular simulation and experimental studies on CO2 and N2 adsorption to bituminous coal. Environ. Sci. Pollut. Res. 28, 15673–15686 (2021).
Hao, M., Qiao, Z., Zhang, H., Wang, Y. L. & Li, Y. L. Thermodynamic analysis of CH4/CO2/N2 adsorption on anthracite coal: Investigated by molecular simulation. Energy Fuels 35, 4246–4257 (2021).
Hao, M., Zhao, Y. C., Wei, C. M., Zhang, H. & Wang, Y. L. Multi-component gases competitive adsorption on residual coal under goaf conditions based on Monte Carlo simulation. Chem. Phys. Lett. 771, 138557 (2021).
Wu, S. Y., Deng, C. B. & Wang, X. F. Molecular simulation of flue gas and CH4 competitive adsorption in dry and wet coal. J. Nat. Gas Sci. Eng. 71, 102980 (2019).
Qu, G. et al. Experiment and molecular simulation study on the adsorption of CH4 and CO2 by coal with high-metamorphic. J. Saf. Environ. 22, 142–147 (2022).
Zhang, Z., Zhao, D., Zhang, C., Chen, Y. & Tang, C. Isothermal adsorption/desorption characteristics of soft coal at different temperatures. J. Liaoning Tech. Univ. (Nat. Sci.) 40, 510–517 (2021).
Li, S., Li, Z., Liu, P., Bai, Y. & Yan, M. Experimental study of competitive adsorption characteristics and mechanism of N2/CH4/CO2 mixture on coal. J. China Univ. Min. Technol. 52, 446–456 (2023).
Zhu, C. J. et al. Experimental comparison of CO2, CH4, and N2 adsorption capacity on typical Chinese coals at different temperatures. Energy Sour. A Recov. Util. Environ. Effects https://doi.org/10.1080/15567036.2020.1806954 (2020).
Xiao, T. et al. Experimental research on adsorption characteristics of N2, CH4, and CO2 in coal under different temperatures and gas pressures. Energy Sci. Eng. 11, 637–653 (2023).
Zhou, W. et al. Experimental study of the effects of gas adsorption on the mechanical properties of coal. Fuel 281, 118745 (2020).
Cheng, G., Tang, X. & Si, J. Adsorption analysis of CO2/N2 in bituminous coal and anthracite. J. North China Inst. Sci. Technol. 20, 34–43 (2023).
Zhu, H. Q. et al. Molecular simulation study on adsorption and diffusion behaviors of CO2/N2 in lignite. Acs Omega 5, 29416–29426 (2020).
Wu, S. Y., Jin, Z. X. & Deng, C. B. Molecular simulation of coal-fired plant flue gas competitive adsorption and diffusion on coal. Fuel 239, 87–96 (2019).
Zhou, W. N. et al. Molecular simulation of CO2/CH4/H2O competitive adsorption and diffusion in brown coal. RSC Adv. 9, 3004–3011 (2019).
Qu, L. A., Wang, Z. Z. & Liu, L. Molecular simulation study based on adsorption of gas (CO2, O2, CH4) on Coal. Fire-Switzerland 6, 355 (2023).
Gao, D. M., Hong, L., Wang, J. R. & Zheng, D. Molecular simulation of gas adsorption characteristics and diffusion in micropores of lignite. Fuel 269, 117443 (2020).
Wang, S., Hu, Y., Yang, X. S., Liu, G. D. & He, Y. R. Examination of adsorption behaviors of carbon dioxide and methane in oxidized coal seams. Fuel 273, 117599 (2020).
Yu, S., Bo, J. & Li, J. H. Retraction: Simulations and experimental investigations of the competitive adsorption of CH4 and CO2 on low-rank coal vitrinite (Retraction of Vol 23, art no 280, 2017). J. Mol. Model. https://doi.org/10.1007/s00894-019-4074-8 (2019).
Bai, Y., Lin, H. F., Li, S. G., Yan, M. & Long, H. Molecular simulation of N2 and CO2 injection into a coal model containing adsorbed methane at different temperatures. Energy 219, 119686 (2021).
Liu, Z. D., Cheng, Y. P., Wang, Y. K., Wang, L. & Li, W. Experimental investigation of CO2 injection into coal seam reservoir at in-situ stress conditions for enhanced coalbed methane recovery. Fuel 236, 709–716 (2019).
Li, B., Zhang, J. X., Ding, Z. B., Wang, B. & Li, P. A dynamic evolution model of coal permeability during enhanced coalbed methane recovery by N2 injection: Experimental observations and numerical simulation. RSC Adv. 11, 17249–17258 (2021).
Laurent, B. et al. Adsorption-induced deformation of microporous materials: Coal swelling induced by CO2-CH4 competitive adsorption. Langmuir ACS J. Surf. Colloids 28, 2659–2670 (2012).
Perera, M. S. A., Ranjith, P. G., Choi, S. K., Airey, D. & Weniger, P. Estimation of gas adsorption capacity in coal: A review and an analytical study. Int. J. Coal Prep. Util. 32, 25–55 (2012).
Abunowara, M. et al. Experimental measurements of carbon dioxide, methane and nitrogen high-pressure adsorption properties onto Malaysian coals under various conditions. Energy https://doi.org/10.1016/j.energy.2020.118575 (2020).
Busch, A. & Gensterblum, Y. CBM and CO2-ECBM related sorption processes in coal: A review. Int. J. Coal Geol. 87, 49–71 (2011).
Li, B. Research of adsorption-deformation-seepage-diffusion characteristics of CO2/CH4/N2 in coals with different coal ranks (in Chinese). Dissertation for Doctor Degree, Liaoning Technical University (2022). doi:https://doi.org/10.27210/d.cnki.glnju.2022.000869.
Polanyi, M. The potential theory of adsorption: Authority in science has its uses and its dangers. Science 141, 1010–1013 (1963).
Dubinin, M. The potential theory of adsorption of gases and vapors for adsorbents with energetically nonuniform surfaces. Chem. Rev. 60, 235–241 (1960).
Acknowledgements
The authors would like to thank all the reviewers who participated in the review, as well as MJEditor (www.mjeditor.com) for providing English editing services during the preparation of this manuscript.
Funding
This work is supported by the National Natural Science Foundation of China (Grant No.52074147), Liaoning Natural Science Foundation of China (Grant No. LJKQZ20222334).
Author information
Authors and Affiliations
Contributions
The data were analyzed by H.L. and G. D., Z. D. analyzed the images, and L.J. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hong, L., Lin, J., Gao, D. et al. Molecular modeling of CO2 affecting competitive adsorption within anthracite coal. Sci Rep 14, 7586 (2024). https://doi.org/10.1038/s41598-024-58483-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-58483-z
- Springer Nature Limited