Abstract
This study intended to explore the neuropsychological ramifications in childhood acute lymphoblastic leukemia (ALL) survivors in Malaysia and to examine treatment-related sequelae. A case-control study was conducted over a 2-year period. Seventy-one survivors of childhood ALL who had completed treatment for a minimum of 1 year and were in remission, and 71 healthy volunteers were enlisted. To assess alertness (processing speed) and essential executive functioning skills such as working memory capacity, inhibition, cognitive flexibility, and sustained attention, seven measures from the Amsterdam Neuropsychological Tasks (ANT) program were chosen. Main outcome measures were speed, stability and accuracy of responses. Mean age at diagnosis was 4.50 years (SD ± 2.40) while mean age at study entry was 12.18 years (SD ± 3.14). Survivors of childhood ALL underperformed on 6 out of 7 ANT tasks, indicating poorer sustained attention, working memory capacity, executive visuomotor control, and cognitive flexibility. Duration of treatment, age at diagnosis, gender, and cumulative doses of chemotherapy were not found to correlate with any of the neuropsychological outcome measures. Childhood ALL survivors in our center demonstrated significantly poorer neuropsychological status compared to healthy controls.
Similar content being viewed by others
Introduction
Acute lymphoblastic leukemia (ALL) accounts for almost one third of the diagnosis in childhood cancers, making it the most common childhood malignancy1. Advances in treatment of ALL in the past 4 decades have significantly improved the 5-year overall survival (OS) to more than 90% in developed countries2,3. Modern treatment protocols, improvement in risk stratification strategies, risk-directed multiagent chemotherapy regimens and enhanced supportive care contributed to this success. In low-middle income countries (LMIC), the 5-year event-free (EFS) and OS were slightly lower at 74% and 82% respectively as reported in the Intercontinental-BFM2002 research4. In Malaysia, the cure rate of children with standard risk ALL is almost comparable to developed countries while those stratified to high risk (HR) group had a lower OS5.
Central nervous system (CNS)-directed therapy conventionally involved cranial irradiation (CI) which has now been replaced by intrathecal and systemic chemotherapy, due to the former’s many late effects2,3,6,7. Major chemotherapeutic agents used as CNS prophylaxis or in the treatment of CNS involvement in ALL, are methotrexate, cytarabine, and corticosteroids2. These drugs do, however, cause central neurotoxic adverse effects that lead to cognitive and neuropsychological dysfunction8,9,10,11,12,13. Previous studies of neuropsychological outcomes among survivors of childhood ALL reported deficits in processing speed, attention, working memory, executive functions and visuomotor control14,15,16,17,18,19,20,21. Poorer outcomes were linked to more intensive therapy, younger age at diagnosis and female gender15,16. Recent studies reported specific polymorphisms in neurogenesis genes which increase patients’ risk of developing chemotherapy-related neurotoxicity22,23,24,25.The frequencies of these polymorphisms differ between the Caucasian and Asian populations, thus the neurotoxicity risks vary even among those using the same treatment protocols. It is important to investigate the neuropsychological consequences of childhood ALL and its treatment, as they could unfavorably affect quality of life (QOL) and scholastic career26,27,28,29. Survivors of childhood ALL have reported lower QOL compared to healthy population with poorer school performances and emotional functioning26. Consequently, those with attention or memory problems, or task efficiency limitations face the risk of unemployment and were unable to live independently30,31. As reports on neuropsychological dysfunction in LMICs are scarce, the findings from this study may provide insight to clinicians and policymakers to plan an optimal long-term multidisciplinary care for the survivors during follow up. In this study, we also investigated whether disease-related or treatment-related factors were linked to reduced neuropsychological functioning.
Results
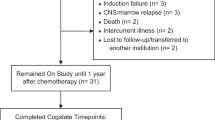
From the database, 137 childhood ALL survivors were identified (Fig. 1). A total of 71 survivors were finally recruited and underwent the ANT assessments with 71 paired healthy controls. Two of the survivors were not able to complete all seven tasks as they became restless and refused to complete the tasks.
Apart from parents’ education level, the demographic data between childhood ALL survivors and healthy controls showed no discernible variations (Table 1). Five of the survivors in the HR group had relapse; four survivors received craniospinal irradiation as part of the treatment protocol (Table 2). High dosage intravenous methotrexate was administered to six patients in the HR group; the mean cumulative dose was 7333.33 mg/m2. Meanwhile, a mean cumulative dose of 8100.00 mg/m2 and 2000.00 mg/m2 of intravenous cytarabine was administered to six patients from the HR group and two patients from the SR group respectively.
A tabulated result of the tasks below can be found in Supplement (Table 1).
Baseline speed (BS)
Baseline speed (unit in milliseconds, ms) was derived from a computerized task of alertness. There was no statistically significant difference between the survivors’ and controls’ baseline speed with mean reaction of 339 ± 109 ms and 319 ± 69 ms, respectively (p = 0.11). Although the survivors demonstrated more fluctuation in reaction speed with mean SD of 96 ± 87 ms higher than that of the controls’ 77 ± 42 ms, this discrepancy was not statistically significant (p = 0.071).
Memory search letters (MSL)
This letter detection task measured working memory capacity and distraction. In comparison to the controls, the survivors reacted slower [F(1,69) = 12.235, p < 0.0001, \(\eta_{p}^{2}\) = 0.157] and group (controls vs. survivors) interacted with memory load [F(2,138) = 3.598, p = 0.030, \(\eta_{p}^{2}\) = 0.050], implying that the differences in speed between survivors and controls grew as memory load rose (Fig. 2). When the difficulty level of the task was increased (Part 3: less targets, more distractors), data analysis revealed that the groups varied in speed [F(1,69) = 16.106, p < 0.0001, \(\eta_{p}^{2}\) = 0.189] with survivors being slower. Distraction had no effect on the groups (p = 0.092) indicating that disparities of speed between survivors and controls did not increase with distraction. Additionally, accuracy varied between groups [F(1,69) = 5.747, p = 0.019, \(\eta_{p}^{2}\) = 0.077], with survivors being less accurate. Group did not interact with distraction (p = 0.19), indicating that differences in accuracy did not increase with distraction. The number of errors when the signal contained 0, 1 or 2 distractors) were 1.87 ± 3.85, 3.04 ± 8.22, and 8.66 ± 10.87, respectively for the survivors, and 1.16 ± 2.66, 1.0 ± 2.22, and 5.71 ± 7.20, respectively for the healthy controls. Reaction time (ms) increased as a function of number of distractors (0, 1, 2) from 1123 ± 444, 1314 ± 548, and 1593 ± 664, respectively for the survivors, and 956 ± 324, 1108 ± 434, and 1370 ± 512, respectively for the healthy controls.
Pursuit (PU) and tracking (TR)
Tracking measured accuracy and stability of movement along a planned trajectory while pursuit required concurrent planning and execution of movement while tracking a target with random movement. Accuracy varied between groups [F(1,68) = 5.817, p = 0.019, \(\eta_{p}^{2}\) = 0.079] (Fig. 3). Group interacted with task type, with discrepancies between groups larger on PU than on TR [F(1,68) = 3.586, p = 0.007, \(\eta_{p}^{2}\) = 0.102], indicating that when executive function demands were higher, differences between groups increased. Additionally, the survivors also displayed greater fluctuation in accuracy compared to controls [F(1,68) = 6.030, p = 0.017, \(\eta_{p}^{2}\) = 0.081], and group interacted with task type [F(1,68) = 5.441, p = 0.023, \(\eta_{p}^{2}\) = 0.047], with differences on PU being larger than on TR, demonstrating that when executive function demands were higher, distinctions between groups increased.
Accuracy ± standard error of mean (A) and fluctuation in accuracy ± standard error of mean (B) as a function of task [tracking (TR) vs. pursuit(PU)]. Illustrates the significant group × task interaction. The differences between groups are larger on task PU (higher executive function (EF) demands) than on TR (lower EF demands).
Sustained attention dots (SAD)
This task measured the ability to maintain performance at a certain level during a longer period of time. There was a significant difference in tempo and fluctuation in tempo across groups [F(1,68) = 9.169, p = 0.003, \(\eta_{p}^{2}\) = 0.119], [F(1,68) = 3.691, p = 0.05, \(\eta_{p}^{2}\) = 0.052] respectively, but not in error rate (p = 0.17). The interaction error type by group was not significant (p = 0.39), indicating that error type did not affect outcome. The survivors had a higher fluctuation in tempo (2.35 ± 1.42 s vs. 2.01 ± 1.40 s), and were slower (14.43 ± 6.27 s vs. 12.43 ± 4.97 s) overall.
Visuo-spatial sequencing (VSS)
This task assessed the subjects’ memory of visuospatial temporal patterns. The number of targets that the control group and survivors were able to identify significantly differed [F(1,70) = 8.396, p = 0.005, \(\eta_{p}^{2}\) = 0.107], and group interacted significantly with Recall criterion [F(1,70) = 5.693, p = 0.020, \(\eta_{p}^{2}\) = 0.075], showing that differences in accuracy is greater when working memory demands were higher (reproduction of visuospatial location and temporal order) (Fig. 4).
Shifting attentional set—visual (SSV)
Two components were assessed in SSV, namely cognitive flexibility and inhibition, which are important for executive functioning.
Inhibition
Survivors were generally not slower than controls (p = 0.126), and group did not interact with Inhibition (p = 0.77). In contrast, more errors were made by survivors in comparison to the controls [F(1,69) = 16.043, p < 0.0001, \(\eta_{p}^{2}\) = 0.189]. However, the interaction was not statistically significant (p = 0.124), signifying that differences did not worsen under incompatible conditions.
Flexibility
There was no difference in speed between groups (p = 0.130) and group did not interact with flexibility (p = 0.792), suggesting that flexibility demands did not affect outcome differently. Survivors made more errors than controls [F(1,69) = 21.808, p < 0.0001, \(\eta_{p}^{2}\) = 0.24] and the group × flexibility interaction was significant [F(1,69) = 6.386, p = 0.014, \(\eta_{p}^{2}\) = 0.085], which indicates that differences in accuracy between groups increased when flexibility was required (Fig. 5).
Accuracy ± standard error of mean in task shifting attentional set—visual (SSV) as a function of task condition. Illustrates the significant group × task condition interaction. Under the random compatible task condition (cognitive flexibility required) the difference between groups is larger than under the fixed compatible condition (flexibility not required).
The overall task performance of the survivors were presented in z-scores when compared to the norm (Table 3), as differences from well-established norms were expressed in standard deviations. This approach is relevant because deviations from the norm are presented according to clinical criteria and the results are independent of the performance level of the group of healthy controls. In ANT, negative z-scores denote better-than-the-norm performance and positive z-scores denote poorer-than-the-norm performance (slower/faster reaction times and higher/lower number of errors result in positive/negative z-scores, respectively). In this study, it can be concluded that overall task performance was practically within the normal range. Visuospatial memory, resistance against distraction, flexibility, and sustained attention were compromised as only between 52 and 63% of the survivors performed within the normal range (z ≤ 1). It was also concluded that the mean performance was in the normal range for 18 out of 20 performance parameters (mean z-score), and the mean performance stayed below the criterion of a severe deficit (z ≥ 2). However, at the individual level, we observed a variable distribution in the severity of deficits. For instance, the percentage of patients exhibiting a severe deficit ranged from 1.4 to 30%. Notably, parameters such as alertness (BS), executive motor control (PU, TR), and memory search (MSL) were found to be relatively spared, with a lower incidence of severe deficits among the study participants. Conversely, sustained attention (SAD), distraction (MSL), inhibition/flexibility (SSV), and visuospatial memory (VSS) were identified as more compromized, with a higher proportion of individuals exhibiting severe deficits in these cognitive domains.
Relation between task performance and treatment
None of the treatment parameters [age at diagnosis, duration off-treatment, duration on treatment, cumulative IT MTX doses (mg total), cumulative prednisolone/dexamethasone doses and cumulative prednisolone equivalent doses] had significant correlation with any of the ANT performance measures (Table 2 supplement).
Discussion
The neuropsychological results of the survivors were interpreted in comparison to that of the healthy controls. For baseline motor speed (task BS) and distractability (task MSL), the performance of the survivors of childhood ALL at our center was comparable to that of the healthy controls, which indicate that their alertness level (readiness to respond to an external stimulus) was normal. However, they demonstrated significant neuropsychological deficits in working memory, inhibition and flexibility, sustained attention, executive visuo-motor control, and visuospatial memory compared to the controls. Poorer working memory capacity in the survivors was shown by a larger rise in reaction time after an increase in memory load (task MSL) and a larger fall in memory score after applying a stricter recall criterion (task VSS).
Response inhibition and flexibility (task SSV), both playing an important role in executive functioning, were also significantly affected in our survivors. Without inhibitory control, an individual will be impulsive and unable to finish one task at a time; whereas without flexibility, one faces difficulty juggling multiple tasks and dealing with changing situations or decisions. Deficits in both working memory and executive functioning have been reported among adult ALL survivors’ years after they had been off treatment18,32. We did not find any correlation between treatment intensity and the working memory or executive functioning, however a larger cohort study may be required to determine the effect of intensified treatment on the survivors32,33.
Impairment in sustained attention among our survivors was demonstrated in the SAD task, which was consistent with studies by Buizer et al.16 and Langer et al.34. Buizer et al.16 reported significant impairment in attention predominantly in those who received intensified treatment whereas Langer et al.34 reported significantly poorer performance in survivors who received CI. Buizer et al.15 and Knight et al.23 both reported significantly worse visuo-motor control (tasks pursuit and tracking) among the ALL survivors compared to healthy controls, signifying deficit in higher cognitive function. Schuitema et al.35 also reported similar observation, 25 years after completion of treatment, with patients who received CI during treatment performing poorer compared to those on chemotherapy alone. Female sex and longer duration from treatment completion were associated with worse performance, however age at diagnosis was not found to influence the outcome15. Our survivors also experienced deficit in visuo-motor control, however we did not identify any factors associated with the deficit.
The frontal and prefrontal cortices of the brain are involved in mediating working memory and executive functioning, thus play an important part in learning processes and carrying out activities of daily living36,37,38. Sustained attention was controlled by the prefrontal and parietal cortices39 whereas visuo-motor function was linked with the frontal, parietal, and temporal white matter tracts35. As the myelination of frontal, prefrontal cortex and cerebellar-prefrontal networks takes a protracted course during childhood, the less mature brain in younger children is more susceptible to damage by CNS-directed therapy33,40,41.
The effect of duration off-treatment on the neuropsychological outcome remains controversial. While patients receiving CI showed a definite decline in performance with time, studies on neuropsychological outcomes for those receiving chemotherapy alone were less robust. We postulate that after an acute insult to the brain following chemotherapy, there may be a period of gradual catch-up due to neural plasticity of an immature brain leading to these improvements. However, it remains to be seen whether this improvement can be sustained over time and with the increasing environmental demands. Due to the short off-treatment duration in our cohort, a follow-up study would be informative to examine the long-term outcome in our survivors.
Bisen-Hersh et al.42 highlighted academic difficulties among the survivors of childhood ALL, emphasizing on impairment in attention, working memory and processing speed. They concluded that these impairments were significant with neurophysiological and pre-clinical evidence indicating white matter abnormalities and acquired brain damage from intravenous and IT methotrexate and CI42. Krull et al.43 reported that 2 decades after diagnosis, neurocognitive impairment was prevalent among the survivors receiving lower dose CI, as well as those who received chemotherapy only. This neurocognitive impairment affects their functional outcomes when they return to school after cancer treatment, and later in adult life when they graduate and seek full-time employment43,44. Survivors of childhood leukemia were reported to have lower grades in school, especially those with younger age at diagnosis44. Unfortunately, our study may not be powered to detect the impact of younger age at diagnosis on task performance. With the current education approach in Asian countries which revolves around memorization and exam-orientated learning, it is important for us to recognize that these survivors will experience hardship to catch-up with the peers due to their neuropsychological deficits.
This study had several limitations. An attempt to match the healthy controls to the survivors based on socio-economic status was not feasible as the controls were recruited from schools around the suburban/city area. Our study included a mixture of participants who underwent chemotherapy-only and combined therapy (CI). While this approach allowed for a broader representation of survivors, it complicates the interpretation of treatment effects as they introduce heterogeneity, making it challenging to attribute observed cognitive outcomes solely to one form of treatment. Correlation analysis between task performance and certain treatment parameters for example CI and cumulative chemotherapy (high dose methotrexate/cytarabine) doses was not performed due to the small number of survivors in these group. Therefore, we were unable to determine any significant association between these variables and neuropsychological deficits. Additionally, in interpreting the results of our study, the norms utilized for the ANT were derived from a Dutch population. While the ANT is a widely employed cognitive assessment tool, the cultural and demographic variations between the reference population and our study participants may cause a degree of variability in the interpretation of individual performance. The wide age range of 7–18 may have caused potential variability in cognitive outcomes due to developmental differences. Furthermore, the small sample size may restrict the statistical power needed to detect subtle effects. While we aimed to capture a more diverse range of survivors, the limitations between inclusivity and statistical power should be acknowledged.
We conclude that survivors of childhood ALL in our center showed significant neuropsychological deficit compared to healthy controls. The major domains of deficit were in working memory, sustained attention, and executive functioning (in particular attentional flexibility). It is essential to develop treatment protocols that are effective but less harmful, such as targeted delivery drugs, to preserve neuropsychological function in survivors of childhood ALL.
Methods
This single-center, case-control study was conducted in Pediatric Hematology and Oncology Unit at Department of Pediatrics, Faculty of Medicine, The National University of Malaysia (UKM) over a 2-year period. Ethical approval was acquired from the Research Ethics Committee Universiti Kebangsaan Malaysia and the study was conducted in accordance with the Declaration of Helsinki. Written informed consent was taken from the parents prior to participation.
Treatment protocols
The chemotherapy protocols used for the treatment of childhood ALL in UKM were modified UKALL XI in 1999–2000, modified UKALL97 or 97(99) during 2001–2008, and modified UKALL2003 from year 2009 onwards. Patients on modified UKALL 97, 97(99) or modified 2003 protocol were assigned to either standard risk (SR) (Regimen A) or high risk (HR) (Regimen B and C), based on their risk stratification. Prednisolone and dexamethasone were used in the modified UKALL97(99) and UKALL2003 respectively, whereas 6-mercaptopurine was used in both modified protocols. All other chemotherapy drugs and treatment duration followed the original Regimen A, B, and C protocols. Chemotherapy drugs consisted of: intravenous vincristine, daunorubicin, cyclophosphamide, doxorubicin; intramuscular E. coli asparaginase; subcutaneous cytarabine; oral prednisolone or dexamethasone, oral methotrexate, oral 6-mercaptopurine and intrathecal methotrexate ± intrathecal cytarabine. All patients with relapse in this study received modified ALL-REZ-BFM 90 protocol. Craniospinal irradiation (24 Gy in 15 fractions of 1.6 Gy each) was given to patients with CNS relapse. Meanwhile, patients diagnosed with infantile leukemia received modified Interfant-99 protocol.
Subjects
Childhood ALL survivors aged 7–18 years, who had completed their treatment for a minimum of 1 year and remained in remission were eligible for the study. This cohort of survivors could inevitably include some children whose school performances were below average before their illness and possibly remained below average even without the impact of ALL and its treatment. Evidence have shown weak to no correlation between ANT task performance and intelligence (i.e., IQ measure) in children33,34,35,36.
A control group of healthy school children was randomly selected from schools in Kuala Lumpur based on the following criteria: no known medical illness, average to good school performance as per school reports, good school attendance, same schooling year of patients and same gender. As the performance on the selected neuropsychological tasks, i.e., reaction time tasks was known to be affected by age and gender32,33, the healthy controls were matched to the survivors based on these two parameters. A ‘match' was defined as individuals within the same calendar year of birth and of the same biological gender.
In ANT tasks, the requested answer was a keypress on the basis of a simple instruction. The tasks were language-culture-free and the possibility that education or social circumstances affecting the results was minimal.
In selecting the age range for our study, we chose to include subjects aged 7 and above due to the educational context in Malaysia. At the age of 7, students typically commence their primary education, specifically in Primary 1. At this stage, students are expected to possess foundational skills such as basic reading abilities and numerical knowledge. By focusing on this age range, we aimed to capture a cohort that has undergone initial educational exposure, ensuring a baseline level of cognitive development and readiness for the tasks involved in our study. Consequently, subjects below the age of 7 were excluded to maintain consistency with the educational milestones and developmental expectations associated with the early years of formal education in Malaysia. We also restricted the upper age limit to 18 years to ensure that only individuals within the pediatric age group were covered, considering the distinctive challenges and considerations associated with pediatric cancer survivorship. This age range is also consistent with the typical age group used in most pediatric oncology studies to facilitate comparability across research findings.
Tool
The Amsterdam Neuropsychological Tasks (ANT) program is a computerized neuropsychological test used to assess fundamental processes that underlie the execution of advanced cognitive processes, i.e., alertness, sustained attention, working memory, inhibition, cognitive flexibility, and visuomotor control32. The ANT program uses task paradigms in which task demands are manipulated. For example, increasing memory load in a memory search task causes an increase in reaction time and/or number of errors, i.e., the task effect. By comparing the task effects between a control and the study group, the manipulated process (working memory) can be used to interpret the disparities between these groups. It enables precise assessment of the speed and accuracy of certain components of executive function and attention32. This program has been used in different clinical diagnoses, known to cause a diffuse impact on brain function, with satisfactory sensitivity and validity, such as ADHD, multiple sclerosis, phenylketonuria, and neurofibromatosis37,38,39,40,41,42. Buizer et al.13 and Schuitema et al.14 used ANT to examine neuropsychological outcomes of childhood leukemia following treatment with chemotherapy, or craniospinal irradiation and chemotherapy respectively22,43. We have also used the ANT program to evaluate neuropsychological outcome among survivors of childhood brain tumor in our center44.
In the current study, we selected seven tasks, administered in a fixed order: baseline speed (BS), memory search letters (MSL), sustained attention dots (SAD), tracking (TR), pursuit (PU), shifting attentional set–visual (SSV), and visuo-spatial sequencing (VSS). These tasks allowed us determine a more specified profile of neuropsychological (dys)functioning, including alertness (BS), working memory (MSL, VSS), sustained attention (SAD), inhibition and cognitive flexibility (SSV), and executive visuomotor control (PU, TR). The ANT tasks’ validity and test–retest reliability were both good and well-documented42. Detailed tasks descriptions can be found in the supplement.
The investigators completed in-depth training on how to conduct the ANT tasks prior to the study commencement. All tests were conducted consistently in the morning in a quiet room at the clinic. A laptop screen was used to display all test stimuli. Participants had to respond by clicking a mouse button or using the mouse as a tracking device. For right-handed participants, the right and left mouse buttons were designated as ‘Yes’ and ‘No’ responses respectively, and vice versa for left-handed participants. Each test was preceded by a briefing, during which the participants received verbal instructions as well as a visual presentation of the different probe and stimulus types. This was followed by two practice chances for each activity to ensure that they understood the instructions well. In general, participants were told to respond as quickly and precisely as they could while performing the tests. The outcome variables for all reaction time (RT) tasks were speed and accuracy of responses for each signal type; unless otherwise stated, the employed post-response interval (PRI: duration between responses and next stimulus start) was 1200 ms and signals were given in pseudo-random sequence38.
Data analysis
Tests of homogeneity of variance and normality were conducted and assumptions were identified. GLM repeated measures ANOVA(RM-ANOVAs) were used to analyze the results. As each survivor was individually paired with a control subject, Group (controls vs. survivors) was entered as within-subject (WS) factor, using the SPSS program version 27. RM-ANOVAs were run by inputting the mean speed and fluctuation in speed in task BS, mean tempo, fluctuation in tempo, mean error rate, and post-error slowing in task SAD, respectively. As values of a second WS factor, different task manipulations (see supplement) were recorded for each task. MSL: memory load (parts 1–3), distraction within part 3 (0–2 distracters); SAD: bias (misses vs. false alarms); SSV: attentional flexibility [not-required (part 1)], —required (part 3, compatible trials), and inhibition [compatible (part 1)], incompatible (part 2); VSS: recall criterion (correct order relevant vs. irrelevant). Task type (TR vs. PU) was entered as a WS factor, while mean deviation and fluctuation in deviation were inputted in RM-ANOVAs. The group × task manipulation interactions was the main focus in the RM-ANOVAs.
Correlational evaluations were conducted to investigate the potential relationships between task performance (using z-scores generated by the program) and age at diagnosis, gender, on and off treatment duration, and treatment intensity. The regression functions are based on norm samples of participants with normal development, including 6.770 (BS), 3.240 (MSL), 2.340 (PU), 3.260 (TR), 3.180 (SAD), 830 (VSS), and 3.440 (SSV). As a result, they are regarded as reliable predictions of performance level33. The survivors were divided into of the following three categories: z ≤ 1 (within the normal range or better than the norm), 1 < z < 2 (between 1 and 2 SD deviating from the norm: mild impairment), z ≥ 2 (more than 2 SD deviating from the norm: severe impairment)43.
Chi-square test was used to analyze categorical data (Table 1), which were reported in frequency and percentage. Data that were not normally distributed were analyzed using Mann–Whitney U test. A significant p-value was determined at < 0.05. Effect sizes were computed using partial eta squared with \(\eta_{p}^{2}\) ∼ 0.03 denoting a weak effect, \(\eta_{p}^{2}\) ∼ 0.06 denoting a moderate effect and \(\eta_{p}^{2}\) ≥ 0.14 considerably a large effect50.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Pui, C. H. & Evans, W. E. Acute lymphoblastic leukemia. N. Engl. J. Med. 339, 605–615. https://doi.org/10.1056/nejm199808273390907 (1998).
Pui, C. H. et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N. Engl. J. Med. 360, 2730–2741. https://doi.org/10.1056/NEJMoa0900386 (2009).
Pui, C. H. et al. Extended follow-up of long-term survivors of childhood acute lymphoblastic leukemia. N. Engl. J. Med. 349, 640–649. https://doi.org/10.1056/NEJMoa035091 (2003).
Stary, J. et al. Intensive chemotherapy for childhood acute lymphoblastic leukemia: results of the randomized intercontinental trial ALL IC-BFM 2002. J. Clin. Oncol. 32, 174–184. https://doi.org/10.1200/jco.2013.48.6522 (2014).
Alias, H., Doris Lau, S. C., Loh, C.-K., Harrison, C. J. & Eswaran, J. Improved treatment of childhood ALL in Malaysia. Blood 134, 5828–5828. https://doi.org/10.1182/blood-2019-129402 (2019).
Daams, M. et al. Long-term effects of cranial irradiation and intrathecal chemotherapy in treatment of childhood leukemia: a MEG study of power spectrum and correlated cognitive dysfunction. BMC Neurol. 12, 84. https://doi.org/10.1186/1471-2377-12-84 (2012).
Pui, C. H. & Evans, W. E. Treatment of acute lymphoblastic leukemia. N. Engl. J. Med. 354, 166–178. https://doi.org/10.1056/NEJMra052603 (2006).
Asato, R. et al. Nuclear magnetic resonance abnormalities of the cerebral white matter in children with acute lymphoblastic leukemia and malignant lymphoma during and after central nervous system prophylactic treatment with intrathecal methotrexate. Cancer 70, 1997–2004. https://doi.org/10.1002/1097-0142(19921001)70:7%3c1997::aid-cncr2820700732%3e3.0.co;2-g (1992).
Hwang, T. L., Yung, W. K., Estey, E. H. & Fields, W. S. Central nervous system toxicity with high-dose Ara-C. Neurology 35, 1475–1479. https://doi.org/10.1212/wnl.35.10.1475 (1985).
Openshaw, H., Slatkin, N. E., Stein, A. S., Hinton, D. R. & Forman, S. J. Acute polyneuropathy after high dose cytosine arabinoside in patients with leukemia. Cancer 78, 1899–1905 (1996).
Pääkkö, E. et al. White matter changes on MRI during treatment in children with acute lymphoblastic leukemia: Correlation with neuropsychological findings. Med. Pediatr. Oncol. 35, 456–461. https://doi.org/10.1002/1096-911x(20001101)35:5%3c456::aid-mpo3%3e3.0.co;2-1 (2000).
Vezmar, S., Becker, A., Bode, U. & Jaehde, U. Biochemical and clinical aspects of methotrexate neurotoxicity. Chemotherapy 49, 92–104. https://doi.org/10.1159/000069773 (2003).
Waber, D. P. et al. Cognitive sequelae in children treated for acute lymphoblastic leukemia with dexamethasone or prednisone. J. Pediatr. Hematol. Oncol. 22, 206–213. https://doi.org/10.1097/00043426-200005000-00004 (2000).
Annett, R. D. et al. Neuropsychological functioning of children treated for acute lymphoblastic leukemia: Impact of whole brain radiation therapy. Psychooncology 24, 181–189. https://doi.org/10.1002/pon.3586 (2015).
Buizer, A. I., De Sonneville, L. M., van den Heuvel-Eibrink, M. M., Njiokiktjien, C. & Veerman, A. J. Visuomotor control in survivors of childhood acute lymphoblastic leukemia treated with chemotherapy only. J. Int. Neuropsychol. Soc. 11, 554–565. https://doi.org/10.1017/s1355617705050666 (2005).
Buizer, A. I., de Sonneville, L. M., van den Heuvel-Eibrink, M. M. & Veerman, A. J. Chemotherapy and attentional dysfunction in survivors of childhood acute lymphoblastic leukemia: effect of treatment intensity. Pediatr. Blood Cancer 45, 281–290. https://doi.org/10.1002/pbc.20397 (2005).
Campbell, L. K. et al. A meta-analysis of the neurocognitive sequelae of treatment for childhood acute lymphocytic leukemia. Pediatr. Blood Cancer 49, 65–73. https://doi.org/10.1002/pbc.20860 (2007).
Jansen, N. C. et al. Neuropsychological outcome in chemotherapy-only-treated children with acute lymphoblastic leukemia. J. Clin. Oncol. 26, 3025–3030. https://doi.org/10.1200/jco.2007.12.4149 (2008).
Kanellopoulos, A. et al. Neurocognitive outcome in very long-term survivors of childhood acute lymphoblastic leukemia after treatment with chemotherapy only. Pediatr. Blood Cancer 63, 133–138. https://doi.org/10.1002/pbc.25690 (2016).
Peterson, C. C. et al. A meta-analysis of the neuropsychological sequelae of chemotherapy-only treatment for pediatric acute lymphoblastic leukemia. Pediatr. Blood Cancer 51, 99–104. https://doi.org/10.1002/pbc.21544 (2008).
Waber, D. P. et al. Neuropsychological outcomes from a randomized trial of triple intrathecal chemotherapy compared with 18 Gy cranial radiation as CNS treatment in acute lymphoblastic leukemia: Findings from Dana-Farber Cancer Institute ALL Consortium Protocol 95–01. J. Clin. Oncol. 25, 4914–4921. https://doi.org/10.1200/jco.2007.10.8464 (2007).
Wang, S. M., Sun, L. L., Zeng, W. X., Wu, W. S. & Zhang, G. L. Effects of a microRNA binding site polymorphism in SLC19A1 on methotrexate concentrations in Chinese children with acute lymphoblastic leukemia. Med. Oncol. 31, 62. https://doi.org/10.1007/s12032-014-0062-0 (2014).
Yazıcıoğlu, B. et al. Influence of folate-related gene polymorphisms on high-dose methotrexate-related toxicity and prognosis in Turkish children with acute lymphoblastic leukemia. Turk. J. Haematol. 34, 143–150. https://doi.org/10.4274/tjh.2016.0007 (2017).
Śliwa-Tytko, P., Kaczmarska, A., Lejman, M. & Zawitkowska, J. Neurotoxicity associated with treatment of acute lymphoblastic leukemia chemotherapy and immunotherapy. Int. J. Mol. Sci. 23, 5515 (2022).
Gandy, K. et al. Genetic variants, neurocognitive outcomes, and functional neuroimaging in survivors of childhood acute lymphoblastic leukemia. JNCI Cancer Spectr. https://doi.org/10.1093/jncics/pkad039 (2023).
Kunin-Batson, A., Kadan-Lottick, N. & Neglia, J. P. The contribution of neurocognitive functioning to quality of life after childhood acute lymphoblastic leukemia. Psychooncology 23, 692–699. https://doi.org/10.1002/pon.3470 (2014).
Harshman, L. A. et al. Population-based exploration of academic achievement outcomes in pediatric acute lymphoblastic leukemia survivors. J. Pediatr. Psychol. 37, 458–466. https://doi.org/10.1093/jpepsy/jsr119 (2012).
Cheung, Y. T. et al. Leukoencephalopathy and long-term neurobehavioural, neurocognitive, and brain imaging outcomes in survivors of childhood acute lymphoblastic leukaemia treated with chemotherapy: A longitudinal analysis. Lancet Haematol. 3, e456–e466. https://doi.org/10.1016/s2352-3026(16)30110-7 (2016).
Yilmaz, M. C. et al. Determination of school-related problems in children treated for cancer. J. Sch. Nurs. 30, 376–384. https://doi.org/10.1177/1059840513506942 (2014).
Kirchhoff, A. C. et al. Physical, mental, and neurocognitive status and employment outcomes in the childhood cancer survivor study cohort. Cancer Epidemiol. Biomark. Prev. 20, 1838–1849. https://doi.org/10.1158/1055-9965.Epi-11-0239 (2011).
Kunin-Batson, A. et al. Predictors of independent living status in adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Pediatr. Blood Cancer 57, 1197–1203. https://doi.org/10.1002/pbc.22982 (2011).
van der Plas, E. et al. Brain structure, working memory and response inhibition in childhood leukemia survivors. Brain Behav. 7, e00621. https://doi.org/10.1002/brb3.621 (2017).
Ashford, J. et al. Attention and working memory abilities in children treated for acute lymphoblastic leukemia. Cancer 116, 4638–4645. https://doi.org/10.1002/cncr.25343 (2010).
Langer, T. et al. CNS late-effects after ALL therapy in childhood. Part III: neuropsychological performance in long-term survivors of childhood ALL: Impairments of concentration, attention, and memory. Med. Pediatr. Oncol. 38, 320–328. https://doi.org/10.1002/mpo.10055 (2002).
Schuitema, I. et al. Accelerated aging, decreased white matter integrity, and associated neuropsychological dysfunction 25 years after pediatric lymphoid malignancies. J. Clin. Oncol. 31, 3378–3388. https://doi.org/10.1200/jco.2012.46.7050 (2013).
Anderson, V. Assessing executive functions in children: Biological, psychological, and developmental considerationst. Pediatr. Rehabil. 4, 119–136. https://doi.org/10.1080/13638490110091347 (2001).
D’Esposito, M. et al. Functional MRI studies of spatial and nonspatial working memory. Brain Res. Cogn. Brain Res. 7, 1–13. https://doi.org/10.1016/s0926-6410(98)00004-4 (1998).
Smith, E. E. & Jonides, J. Working memory: A view from neuroimaging. Cogn. Psychol. 33, 5–42. https://doi.org/10.1006/cogp.1997.0658 (1997).
Pardo, J. V., Fox, P. T. & Raichle, M. E. Localization of a human system for sustained attention by positron emission tomography. Nature 349, 61–64. https://doi.org/10.1038/349061a0 (1991).
Ciesielski, K. T., Lesnik, P. G., Benzel, E. C., Hart, B. L. & Sanders, J. A. MRI morphometry of mamillary bodies, caudate nuclei, and prefrontal cortices after chemotherapy for childhood leukemia: Multivariate models of early and late developing memory subsystems. Behav. Neurosci. 113, 439–450. https://doi.org/10.1037//0735-7044.113.3.439 (1999).
Fuster, J. M. Frontal lobe and cognitive development. J. Neurocytol. 31, 373–385. https://doi.org/10.1023/a:1024190429920 (2002).
Bisen-Hersh, E. B., Hineline, P. N. & Walker, E. A. Disruption of learning processes by chemotherapeutic agents in childhood survivors of acute lymphoblastic leukemia and preclinical models. J. Cancer 2, 292–301. https://doi.org/10.7150/jca.2.292 (2011).
Krull, K. R. et al. Neurocognitive outcomes decades after treatment for childhood acute lymphoblastic leukemia: A report from the St Jude lifetime cohort study. J. Clin. Oncol. 31, 4407–4415. https://doi.org/10.1200/jco.2012.48.2315 (2013).
Andersen, K. K., Duun-Henriksen, A. K., Frederiksen, M. H. & Winther, J. F. Ninth grade school performance in Danish childhood cancer survivors. Br. J. Cancer 116, 398–404. https://doi.org/10.1038/bjc.2016.438 (2017).
Acknowledgements
We would like to acknowledge Dr. Syed Alhafiz Syed Hashim for his contribution to the recruitment of subjects and data collection for this study and Dr. Mohammad Shukri Khoo for technical contribution. We would like to thank the Faculty of Medicine, Universiti Kebangsaan Malaysia for the research Grant (FF-2013-391 and GGPM-2013-106).
Author information
Authors and Affiliations
Contributions
H.A. designed the study. H.A., D.L.S.C. and N.M.R. performed the study, analyzed the data, and wrote the initial manuscript. H.A. and d.S.L.M.J. revised the manuscript and made substantial modification during revision. All authors read and approved final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alias, H., Mohd Ranai, N., Lau, S.C.D. et al. Neuropsychological task outcomes among survivors of childhood acute lymphoblastic leukemia in Malaysia. Sci Rep 14, 7915 (2024). https://doi.org/10.1038/s41598-024-58128-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-58128-1
- Springer Nature Limited