Abstract
Magnetite nanoparticles are small, strongly magnetic iron oxide particles which are produced during high-temperature combustion and friction processes and form part of the outdoor air pollution mixture. These particles can translocate to the brain and have been found in human brain tissue. In this study, we estimated associations between within-city spatial variations in concentrations of magnetite nanoparticles in outdoor fine particulate matter (PM2.5) and brain cancer incidence. We performed a cohort study of 1.29 million participants in four cycles of the Canadian Census Health and Environment Cohort in Montreal and Toronto, Canada who were followed for malignant brain tumour (glioma) incidence. As a proxy for magnetite nanoparticle content, we measured the susceptibility of anhysteretic remanent magnetization (χARM) in PM2.5 samples (N = 124 in Montreal, N = 110 in Toronto), and values were assigned to residential locations. Stratified Cox proportional hazards models were used to estimate hazard ratios (per IQR change in volume-normalized χARM). ARM was not associated with brain tumour incidence (HR = 0.998, 95% CI 0.988, 1.009) after adjusting for relevant potential confounders. Although we found no evidence of an important relationship between within-city spatial variations in airborne magnetite nanoparticles and brain tumour incidence, further research is needed to evaluate this understudied exposure, and other measures of exposure to magnetite nanoparticles should be considered.
Similar content being viewed by others
Introduction
Outdoor air pollution, especially fine particulate matter (PM2.5), is among the leading causes of death and disease worldwide and is implicated in the development of numerous cancers1. Recently, research interest has turned to examining the effects of pollutants on the brain. A major hypothesized mechanism for the health effects associated with particulate matter exposures is the ability of inhaled particles to induce oxidative stress and an inflammatory response in the body2; inflammation has also been implicated in the development of brain cancer3,4. However, although there is a biologically plausible explanation for a relationship between exposures to particulate matter and brain cancer incidence, evidence in the literature is mixed. Although some studies found no association between PM2.5 and brain cancer incidence5,6,7, some components such as carbonaceous particles (components indicating a combustion source) in PM2.5 have been found to be positively associated with brain cancer incidence8. In addition, ultrafine particles (UFP, i.e., particles less than 100 nm in diameter) were associated with increased brain cancer incidence in a previous cohort study conducted in Toronto and Montreal, Canada5. Since there are few known modifiable risk factors for brain cancer, identifying and quantifying the effects of modifiable environmental exposures may be an important way to reduce brain cancer incidence.
While existing studies of outdoor air pollution and brain cancer generally focus on the most commonly measured pollutants such as mass concentrations of PM2.5, there is increased interest in novel air pollution exposure metrics that account for composition, toxicity, and/or size of particles. Specifically, measures that account for particle composition, toxicity, and size may vary at finer spatial scales than PM2.5 mass concentrations, which could make them more useful in epidemiologic studies of exposure variations within cities. One measure of interest is the magnetite nanoparticle content of outdoor PM2.5. Magnetite nanoparticles are small (< 100 nm in diameter), strongly magnetic iron oxide particles that are produced during high-temperature combustion and friction processes including both vehicle tailpipe emissions and brake-wear as well as industrial activity9,10,11. Moreover, existing evidence suggests that outdoor concentrations of magnetite nanoparticles measured in PM2.5 samples vary substantially within cities, much more so than traditional PM2.5 mass concentrations12.
The relationship between magnetite nanoparticles and brain cancer is of particular interest as these pollutants can enter the brain directly through the olfactory nerve, the neuroenteric system and via the circulation, and have been identified in human brains13,14,15. Once in the brain, magnetite may provoke redox activity that leads to oxidative stress13. In a study in Mexico City, neuroinflammatory markers were correlated with presence of metals in the frontal lobe in children and young adults13,16. The magnetite nanoparticles observed in human brains are co-associated with a range of other, potentially toxic, exogenous metal-bearing particles, including aluminum, titanium, nickel, and platinum13,14. Although urban populations are exposed to magnetite nanoparticles, and it is biologically plausible that such exposures could contribute to adverse health outcomes, to date there have been no epidemiologic studies of the health effects of exposure to magnetite nanoparticles in outdoor air pollution. The aim of this study was to estimate the association between within-city spatial variations in the concentration of magnetite nanoparticles, as represented by laboratory measurement of the anhysteretic remanent magnetization susceptibility (χARM) of outdoor PM2.5 (as described below) and incidence of brain cancer in Montreal and Toronto, Canada. As a secondary aim, we investigated whether associations between brain tumours and outdoor concentrations of nitrogen dioxide (a marker of the broader traffic-related air pollution mixture) and PM2.5 mass concentrations were modified by mass-normalized ARM susceptibility of PM2.5.
Methods
Cohort description
The Canadian Census Health and Environment Cohort (CanCHEC) is a population-based cohort that has been described previously17,18. The cohort includes multiple cycles of follow-up of Canadian Census records and includes non-institutionalized Canadians (aged 25 and older) who were among the approximately 20% of households selected for enumeration by the long-form Census questionnaire in one of the eligible census years19. These datasets were linked to postal code histories to obtain annual place of residence from Historical Tax Summary Files. CanCHEC includes information from Census questionnaires on individual-level and contextual variables including socioeconomic indicators, ethnicity, and place of residence, as well as environmental conditions17. Mortality data were linked from the Canadian Vital Statistics Death Database and cancer incidence data were linked from the Canadian Cancer Registry. The CanCHEC dataset was created under the authority of the Statistics Act and approved by the Executive Management Board at Statistics Canada (reference: 045-2015). This is equivalent to standard research ethics board approval. Informed consent was waived by the Executive Management Board at Statistics Canada because the database used in this study contains only deidentified individual records. All methods were carried out in accordance with relevant guidelines and regulations.
Our study population includes individuals in the 1991, 1996, 2001 or 2006 CanCHEC cohorts aged 25–90 years at baseline who lived in Toronto or Montreal for at least 2 years during follow-up. Since approximately 20% of households were randomly assigned to complete the long-form census in each cohort cycle, some individuals were enumerated on more than one long-form census. These individuals were assigned to the earliest cohort in which they appeared.
Ascertainment of cancer diagnosis
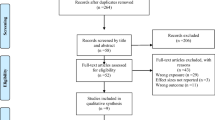
Cancer diagnoses in CanCHEC were identified using data linked to the CanCHEC cohorts from the Canadian Cancer Registry, a database that records incident primary cancers diagnosed for each person since 199220,21. Participants were followed for first incidence of primary malignant brain tumour (defined by International Classification of Diseases, 10th Revision (ICD-10) codes C71.0–C71.9; corresponding to International Classification of Diseases for Oncology, 3rd revision [ICD-O-3] histologic codes for glioma M-938-M-948). Follow-up time started on Census day 2001 for the 1991, 1996 and 2001 cohorts, and Census day 2006 for the 2006 cohort. This restricted follow-up period was implemented to reduce potential error caused by extrapolating ARM susceptibility of PM2.5 (i.e., magnetite nanoparticle concentrations) many years into the past. For members of the study population living in Montreal, cases were only identified in the period 2001–2010 as cancer diagnosis data were not available in the province of Quebec for diagnosis years from 2011 onward. Participants were excluded if they had any cancer diagnosis in the 3 years prior to the start of follow-up to reduce the possibility of confounding by potential exposure to ionizing radiation in cancer treatment. The study schema is illustrated in Fig. 1.
Spatial monitoring studies and estimation of magnetite nanoparticle exposures using anhysteretic remanent magnetization
PM2.5 samples were collected in outdoor monitoring campaigns conducted in 2018 in Montreal and Toronto, Canada. Monitoring sites were selected to capture important sources of ambient PM2.5 in each city while maximizing spatial coverage of the study area. A total of 124 sites in Montreal and 110 sites in Toronto were monitored. Mean daily temperatures over the sampled period ranged from 14.4 to 23.7 °C (57.9–74.7°F) in Montreal and 19.8–26.6 °C (67.6–79.9°F) in Toronto. Integrated 2-week PM2.5 samples were collected using Teflon filters and preset timers with a mix of Ultrasonic Personal Air Sample (UPAS) monitors (Access Sensor Technologies, Fort Collins, CO) at a flow rate of 1 L/min and cascade impactors at a flow rate of 5 L/min.
In order to quantify the content of magnetite particles in PM2.5 samples, anhysteretic remanent magnetization (ARM) was measured. ARM is roughly proportional to the concentration of ferrimagnetic minerals within a sample22 and specifically responds to the presence of magnetic nanoparticles with diameters between 30 and 50 nm23,24. First, PM2.5 samples (on PTFE filters) were exposed to four different direct current (DC) biasing fields of 0.06 mT, 0.08 mT, 1.0 mT and 1.2 mT. Subsequently, a 2G RAPID cryogenic magnetometer (2G Enterprises, Mountain View, CA) was used to measure the magnetic response of the samples. ARM measurements were also made of 20 blank PTFE filters and the mean of the ARM measurements taken on these blanks was subtracted from sampled filter values. ARM was expressed as a susceptibility of ARM normalized by the direct current (DC) field, calculated as the slope of the ARM(DC field) linear function. ARM susceptibility values were then normalized by air sampled volume (expressed as KARM, a dimensionless quantity) for the primary analysis and by particulate mass (expressed as χARM, in units of m3/kg) in secondary analyses.
Outdoor PM 2.5 and nitrogen dioxide concentrations
To evaluate the effects of spatial variations in other co-occurring pollutants, we assigned long-term estimates of outdoor PM2.5 and nitrogen dioxide (NO2) concentrations at residential address to cohort members in the same manner. Annual average outdoor PM2.5 mass concentrations were estimated using models described in detail previously25. Briefly, PM2.5 concentrations were estimated at a 1 × 1 km resolution using aerosol optical depth, a chemical transport model, and land-use data25,26. Annual average outdoor concentrations of NO2 were estimated using a land-use regression model27 in which estimates were derived from remote sensing and National Air Pollution Surveillance monitoring data; this model was developed from 2006 data and had a spatial resolution of 100 m2. PM2.5 and NO2 data indexed to DMTI Spatial Inc. postal codes were provided by CANUE (Canadian Urban Environmental Health Research Consortium).
Exposure assignment
PM2.5 ARM susceptibility values, as well as PM2.5 and NO2 concentrations, were assigned directly to residential 6-digit postal codes (an area equivalent to approximately one city block face in urban areas) from the value at the closest measured site. Postal codes were linked to monitored points using latitude and longitude from the master postal code list (CanMap Postal Suite, version v2015.3, DMTI Spatial Inc., Markham). In cases where a single postal code was represented by multiple points of latitude and longitude, an average estimate for the postal code was created by equally weighting the multiple pollutant values across points. Median distance from monitors to postal code centroids was 916.5 m in Montreal and 1338.3 m in Toronto. Time-varying exposures were estimated using residential postal code histories from annual income tax filings, allowing for movement within and between cities. Exposures were assigned to cohort members at their residential address as 3-year moving averages with a 1-year lag (e.g., an individual’s exposure for 2008 was the mean of their exposures for 2005, 2006, and 2007). This is consistent with the standard exposure assignment used in many studies using the CanCHEC cohort since ambient PM2.5 is regulated in Canada based on a 3-year time window28. In addition, longer time windows (e.g., 10-years) would have required extrapolation of exposure estimates further back in time.
Statistical analyses
Stratified Cox proportional hazards models were used to estimate hazard ratios describing the relationship between PM2.5 ARM susceptibility (volume-normalized, i.e., KARM) and incidence of brain tumours. Follow-up time started with time of entry into the CanCHEC cohort (e.g., Census day 2001 for the 2001 cohort). Subjects were censored if they moved outside the cities of Montreal or Toronto, if they were lost to follow-up, at the end of study period, or at time of death, whichever came first. Data were accessed and analyzed in the secure facilities of the McGill-Concordia Research Data Centre located at McGill University. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA) and R 4.2.2 (R Foundation for Statistical Computing, Vienna, Austria).
Variables used for regression adjustment were chosen based on a Directed Acyclic Graph (DAG) (Fig. S1). There are few well-established risk factors for primary brain tumours except for exposure to ionizing radiation and family history29. Nonetheless, we adjusted for a number of demographic and socioeconomic status variables which could confound the relationship through chance associations with the outcome. Specifically, we adjusted for age (5-year age groups as a strata variable), sex (male/female strata), immigration status (immigrant/nonimmigrant strata), Census cohort year (four categories as strata: 1991, 1996, 2001, and 2006), visible minority status, occupational level, educational attainment, marital status, and income quintile. Additionally, models were adjusted for PM2.5 mass concentrations and NO2 to evaluate the sensitivity of effect estimates to spatial variations in long-term exposures to these pollutants.
As an additional analysis, we examined the effects of PM2.5 and NO2 stratified by mass-normalized ARM susceptibility (χARM). When stratifying by ARM susceptibility, our goal in the estimation of NO2 effects on brain cancer incidence was to investigate whether the mixture of traffic-related air pollutants (of which NO2 is a marker) is more harmful in areas with greater ARM susceptibility; in the PM2.5 analysis, our goal was to evaluate whether the effect of exposure to fine particles was greater in areas where PM2.5 ARM susceptibility is higher. We performed Cox proportional hazards regression as described above and below the median of χARM values.
Sensitivity analyses
Some individual-level risk factor variables, notably cigarette smoking and body mass index (BMI), are not available in the CanCHEC database. Although the evidence linking smoking to brain cancer incidence is inconsistent, and some studies suggest no relationship30, cigarette smoking is an important cause of human cancer and meta-analysis suggests a possible association with brain cancer incidence31. Similarly, evidence suggests a possible assocation of obesity with some types of brain cancer32. Smoking and BMI are not causes of outdoor air pollutant concentrations, so they are not confounders in the standard definition33. Nonetheless, chance associations could confound the relationship between outdoor PM2.5, NO2 or ARM susceptibility and brain tumour incidence, and the indirect adjustment method was applied to address this possibility. The indirect adjustment method has been described in detail previously34; briefly, this method uses data on the correlation between measured covariates and unmeasured risk factors from a secondary data source, as well as estimates of the relationship between the missing risk factors and incidence of the outcome. We used data from multiple cycles of the Canadian Community Health Survey, a biannual national health survey that has the same target population as the Canadian census (i.e., the Canadian population) and collects data on health and lifestyle characteristics including smoking and BMI. The relationships between smoking and brain cancer, as well as BMI and brain cancer, were estimated from the literature based on systematic reviews and meta-analyses of the existing evidence31,32.
Results
Cohort characteristics are presented in Table 1. In total, we identified approximately 1300 eligible cases of malignant primary brain tumours over 13.6 million person-years of follow-up in 1.29 million individuals (all numbers rounded to the nearest 100 to satisfy institutional confidentiality requirements). Incident brain tumours were identified at a higher rate in people of increased age, in men relative to women, and in people who identified as white relative to those who identified as visible minorities (Table 1).
The mean volume-normalized ARM susceptibility (KARM) across all eligible person-years was 5.8 × 10−14 (SD = 3.4 × 10−14) and mean mass-normalized ARM susceptibility (χARM) was 9.3 × 10−6 m3/kg (SD = 6.7 × 10−6 m3/kg). Spatial variations in ARM susceptibility were much greater than spatial variations in PM2.5 mass concentrations. The mean PM2.5 concentration was 9.4 μg/m3 (SD = 1.3 μg/m3) and the mean NO2 concentration was 21.2 ppb (SD = 5.5 ppb) (Table 2). ARM susceptibility parameters showed little correlation with PM2.5 mass concentration or NO2 concentration. Spatial variations in KARM (volume-normalized) were very weakly correlated with PM2.5 mass concentration (r = − 0.0007) and NO2 (r = 0.0496), and similarly spatial variations in χARM (mass-normalized) were very weakly correlated with PM2.5 (r = 0.023) and NO2 (r = − 0.028). Distributions of PM2.5 and NO2 at measured sites were similar to values across the entire study area (Table S1).
Cox regression model results are presented in Table 3. Models showed no association between volume-normalized ARM susceptibility (KARM) and brain cancer incidence (HR per 3.0 × 10−14: 0.998, 95% CI 0.988, 1.009). Long-term exposures to PM2.5 were inversely though nonsignificantly associated with brain cancer incidence (HR per 3 µg/m3: 0.833, 95% CI 0.681, 1.021). When stratified by mass-normalized ARM susceptibility (χARM), the effect of PM2.5 was closer to the null (i.e., indicating a less strong protective effect) above the median XARM (HR: 0.899, 95% CI 0.774, 1.043) relative to below the median (HR: 0.711, 95% CI 0.374, 1.350), but estimates were imprecise. Similarly, long-term average exposures to NO2 were not associated with brain cancer incidence (HR per 10 ppb: 0.963, 95% CI 0.876, 1.058). In stratified analyses, the point estimate of the effect of NO2 above the median of χARM (HR: 0.945, 95% CI 0.848, 1.053) was protective whereas below the median of χARM the effect was deleterious (HR: 1.020, 95% CI 0.877, 1.195); however, confidence intervals were wide and overlapping. For all pollutants, indirect adjustment for smoking and body mass index had little effect on hazard ratios (Table 3).
Discussion
We conducted a population-based cohort study examining the relationship between within-city spatial variations in fine particle (PM2.5) ARM susceptibility on malignant brain tumour incidence in two Canadian cities. We found no relationship between exposures to volume-normalized ARM susceptibility of PM2.5 and brain tumour incidence; moreover, we found no evidence that the effects of other long-term outdoor pollutant exposures (i.e., PM2.5 mass concentration and NO2 as a marker for traffic-related air pollution) were modified by the mass-normalized ARM susceptibility of fine particles. In general, our findings do not support a relationship between spatial variations in ARM susceptibility of PM2.5, a measure which corresponds to the presence of magnetite nanoparticles (~ 30–50 nm diameter), and incidence of brain cancer. We identified a non-significant inverse effect of PM2.5 on brain cancer incidence which we cannot explain. However, this result is similar to protective effects previously observed for the effect of PM2.5 on brain cancer incidence, such as by Jorgenson et al. (HR per 3 µg/m3: 0.985, 95% CI 0.635, 1.54)35, by Harbo Poulsen et al. (OR per 3 µg/m3: 0.992, 95% CI 0.946, 1.039)8, or by Weichenthal et al. (HR per 3 µg/m3: 0.907, 95% CI 0.762, 1.079)5.
Although we found no effect of PM2.5 ARM susceptibility on brain cancer incidence, nonetheless the health effects of exposures to magnetite nanoparticles merit further study with different exposure metrics. Exposure to magnetite particles in human cells in vitro can induce reactive oxygen species generation36, which contributes to the oxidative stress pathway that may be responsible for many of the observed adverse health effects of PM exposure. Further, experiments in rat cortical neurons suggest that exposure to magnetite may play a role in the development of neurodegenerative diseases such as Alzheimer’s disease37. Magnetite nanoparticles observed in the human brain are co-associated with other exogenous metal-bearing nanoparticles, including titanium, aluminum, platinum, nickel, and cobalt13,14. Toxicological studies have suggested that metal-rich ultrafine particles are able to access all major organs38,39,40,41, suggesting their relevance to health outcomes including those affecting the brain42. Given the toxicological evidence, there remains a need for future epidemiologic studies assessing the effects on health of magnetite nanoparticle exposures.
Future studies may benefit from exploring different measures of magnetite nanoparticles. We used the room temperature ARM of PM2.5 as a surrogate measure of magnetic nanoparticle content as it reflects the concentration of particles of approximately 30–50 nm in diameter23,24. However, it is possible that the ARM in PM2.5 samples may not be a good proxy for sampling the actual nanoparticle size range. We are most interested in the smallest particles (e.g., UFPs, which have diameter less than 0.1 µm) as there is evidence that they may be relevant to the development of brain cancer5. Given the potential relevance of UFPs to brain health, a possible direction for future research could be the measurement of magnetic parameters on the ultrafine fraction of particulate matter: for example, Gonet et al. analyzed isothermal remanent magnetization (IRM, a measure of magnetic remanence which results from short-term exposure to strong magnetizing fields) on size-fractionated particles sampled from brake-wear emissions (using 14 size fractions ranging from 0.016 to 10 µm)43. Although an increasing body of literature exists describing magnetic parameters of particles collected from air samples44,45 or from tree leaf surfaces near roadways9,46,47,48, it remains to be determined which particle magnetic properties and which particle size fractions are most relevant in health studies; future studies may consider size-resolved evaluation of magnetic characteristics of particles as a step towards assessment of their potential health impact. Additionally, a focus on low-temperature (LT) magnetic measurements may better characterize the UFP fraction. Muxworthy44 performed LT magnetic remanence measurements and found significantly higher concentrations of magnetite nanoparticles than previously estimated using room-temperature (RT) measures. In addition, Sheikh et al.49 collected air samples from the London Underground and analyzed them using ARM as well as both room temperature saturation isothermal remanence (RT-SIRM) and low temperature SIRM (LT-SIRM). Given evidence that the predominant size range of magnetite nanoparticles identified in the brain is 5–20 nm50, and that magnetite nanoparticles in this size range are more accurately quantified using LT methods44, future studies of PM may be better served by LT magnetic measurements rather than the RT measures performed here.
This study had several notable strengths, including high-resolution estimates of spatial variations in the ARM susceptibility of PM, the availability of updated exposure information for subjects moving within and between cities, and time-varying estimates of NO2 and PM2.5 exposures, as well as detailed individual-level data on potential confounders. A further advantage is the availability of data on incident, rather than prevalent brain tumour diagnoses. However, our study also had a number of limitations. First, the PM2.5 ARM susceptibility values were based on measurements of air filters collected during 2-week monitoring periods in 2018 (i.e., after the end of the follow-up period), and due to the absence of historical measurements it was not possible for us to extrapolate ARM susceptibility estimates backward in time. This is a source of possible exposure measurement error; however, a systematic difference in the degree of exposure error between brain cancer cases and non-cases is not expected and therefore bias would tend toward the null. Additionally, since major changes in spatial patterns of roadway infrastructure have not occurred during the study period, we did not expect major changes in the spatial patterns of tailpipe and brake wear emissions. Next, measurements of PM2.5 ARM susceptibility were made at room temperature, rather than at low temperature (e.g., liquid nitrogen, 77 K, or helium, 4.2 K, temperatures). Recent, low temperature-based studies show that both the total magnetite content and the numbers of ultrafine particles < 10 nm in size (magnetically ‘invisible’ at room temperature) are being routinely under-estimated in magnetic characterisation of particulate air pollution49. Further, we assume that the use of 2-week monitoring periods represents a sufficient approximation to long-term average spatial variations in PM2.5 ARM susceptibility. We based this assumption on existing evidence that suggests that the spatial pattern of pollutant concentrations derived from short-term monitoring campaigns remains relatively stable over time51,52. Although the ARM susceptibility measurements were collected after the end of follow-up, spatial contrasts are assumed to be representative of earlier spatial contrasts within each city during the follow-up period.
A second limitation was the absence of individual-level data on potential confounders such as smoking and body mass index. However, as described in the conceptual directed acyclic graph (Fig. S1), these individual-level variables are not likely causes of long-term air pollution exposures including PM2.5 ARM susceptibility, so they are not strictly confounders. Nonetheless, we performed an indirect adjustment method to account for confounding that could occur by chance associations between PM2.5 ARM susceptibility and individual-level variables. Similarly, we lacked individual-level data on other potential causes of brain cancer (e.g., family history of brain cancer or exposures to ionizing radiation). Since these factors were not present in the ancillary database we used to perform the indirect adjustment, we were unable to adjust for them. It is possible that potential confounding by these or other unmeasured confounders remains, if there exists a systematic relationship between the potential confounders and spatial variations in outdoor PM2.5, NO2 or ARM susceptibility. Lastly, the definition of the study outcome as all primary malignant brain tumours could potentially obscure any effect of ARM on individual tumour types as some tumour types may be more strongly related to ARM susceptibility than others. However, there is little evidence from previous studies to suggest which types of tumours may be more or less susceptible to the effects of inhaled pollutants. Although an examination of specific tumour subtypes may be an important opportunity for future research, given the relatively small number of events, we focused on the combined outcome definition to maximize precision.
In conclusion, we performed the first cohort study of spatial variations in ARM susceptibility of outdoor PM2.5 and incident brain tumours. We did not find an association between ARM susceptibility, a measure which is proportional to the concentration of magnetite nanoparticles, and brain cancer incidence. Nonetheless, future studies should continue to exposure explore the potential health impacts of magnetite nanoparticles using alternative exposure metrics due to the prevalence of exposure to these pollutants in urban areas.
Data availability
CanCHEC cohort data are held in secure Research Data Centres facilities managed by Statistics Canada. These can be accessed through the microdata access portal application process. The application process and procedures are available online here: www.statcan.gc.ca/en/microdata/data-centres/access.
References
Turner, M. C. et al. Outdoor air pollution and cancer: An overview of the current evidence and public health recommendations. CA Cancer J. Clin. https://doi.org/10.3322/caac.21632 (2020).
Kelly, F. J. Oxidative stress: Its role in air pollution and adverse health effects. Occup. Environ. Med. 60, 612–616 (2003).
Sowers, J. L., Johnson, K. M., Conrad, C., Patterson, J. T. & Sowers, L. C. The role of inflammation in brain cancer. Adv. Exp. Med. Biol. 816, 75–105. https://doi.org/10.1007/978-3-0348-0837-8_4 (2014).
Alghamri, M. S. et al. Targeting neuroinflammation in brain cancer: Uncovering mechanisms, pharmacological targets, and neuropharmaceutical developments. Front. Pharmacol. 12, 680021. https://doi.org/10.3389/fphar.2021.680021 (2021).
Weichenthal, S. et al. Within-city spatial variations in ambient ultrafine particle concentrations and incident brain tumors in adults. Epidemiology 31, 177–183. https://doi.org/10.1097/EDE.0000000000001137 (2020).
McKean-Cowdin, R. et al. Ambient air pollution and brain cancer mortality. Cancer Causes Control 20, 1645–1651. https://doi.org/10.1007/s10552-009-9412-1 (2009).
Coleman, N. C. et al. Fine particulate matter exposure and cancer incidence: Analysis of SEER cancer registry data from 1992–2016. Environ. Health Perspect. 128, 107004. https://doi.org/10.1289/EHP7246 (2020).
Harbo Poulsen, A. et al. Components of particulate matter air-pollution and brain tumors. Environ. Int. 144, 106046. https://doi.org/10.1016/j.envint.2020.106046 (2020).
Mitchell, R. & Maher, B. A. Evaluation and application of biomagnetic monitoring of traffic-derived particulate pollution. Atmos. Environ. 43, 2095–2103. https://doi.org/10.1016/j.atmosenv.2009.01.042 (2009).
Gonet, T., Maher, B. A. & Kukutschova, J. Source apportionment of magnetite particles in roadside airborne particulate matter. Sci. Total Environ. 752, 141828. https://doi.org/10.1016/j.scitotenv.2020.141828 (2020).
Magiera, T., Goluchowska, B. & Jablonska, M. Technogenic magnetic particles in alkaline dusts from power and cement plants. Water Air Soil Pollut. 224, 1389. https://doi.org/10.1007/s11270-012-1389-9 (2013).
Ripley, S. et al. Predicting spatial variations in multiple measures of PM2.5 oxidative potential and magnetite nanoparticles in Toronto and Montreal. Canada. Environ. Sci. Technol. 56, 7256–7265. https://doi.org/10.1021/acs.est.1c05364 (2022).
Maher, B. A. et al. Magnetite pollution nanoparticles in the human brain. Proc. Natl. Acad. Sci. USA 113, 10797–10801. https://doi.org/10.1073/pnas.1605941113 (2016).
Calderon-Garciduenas, L. et al. Quadruple abnormal protein aggregates in brainstem pathology and exogenous metal-rich magnetic nanoparticles (and engineered Ti-rich nanorods). The substantia nigrae is a very early target in young urbanites and the gastrointestinal tract a key brainstem portal. Environ. Res. 191, 110139. https://doi.org/10.1016/j.envres.2020.110139 (2020).
Hammond, J., Maher, B. A., Ahmed, I. A. M. & Allsop, D. Variation in the concentration and regional distribution of magnetic nanoparticles in human brains, with and without Alzheimer’s disease, from the UK. Sci. Rep. 11, 9363. https://doi.org/10.1038/s41598-021-88725-3 (2021).
Calderon-Garciduenas, L. et al. The impact of environmental metals in young urbanites’ brains. Exp. Toxicol. Pathol. 65, 503–511. https://doi.org/10.1016/j.etp.2012.02.006 (2013).
Peters, P. A. et al. Data resource profile: 1991 Canadian Census Cohort. Int. J. Epidemiol. 42, 1319–1326. https://doi.org/10.1093/ije/dyt147 (2013).
Crouse, D. L. et al. Risk of nonaccidental and cardiovascular mortality in relation to long-term exposure to low concentrations of fine particulate matter: A Canadian national-level cohort study. Environ. Health Perspect. 120, 708–714. https://doi.org/10.1289/ehp.1104049 (2012).
Christidis, T., Labrecque-Synnott, F., Pinault, L., Saidi, A. & Tjepkema, M. Analytical Studies: Methods and References (ed. Health Analysis Division and Household Survey Methods Division) (Statistics Canada, Ottawa, 2018).
Canadian Cancer Registry (CCR), <https://www23.statcan.gc.ca/imdb/p2SV.pl?Function=getSurvey&SDDS=3207> (
Carpenter, M., Fair, M. & Polliquin, C. History and development of the 1969–1991 Canadian Cancer Data Base. Report No. OEHRS–16, (Statistics Canada, Health Statistics Division, Occupational and Environmental Health Research Section, Ottawa, 2006).
Maher, B. A. Characterisation of soils by mineral magnetic measurements. Phys. Earth Planet Inter. 42, 76–92 (1986).
Özdemir, O. & Banerjee, S. K. A preliminary magnetic study of soil samples from west-central Minnesota. Earth Planet Sci. Lett. 59, 393–403 (1982).
Maher, B. A. Magnetic properties of some synthetic sub-micron magnetites. Geophys. J. R Astron. 94, 83–96 (1988).
Hammer, M. S. et al. Global estimates and long-term trends of fine particulate matter concentrations (1998–2018). Environ. Sci. Technol. 54, 7879–7890. https://doi.org/10.1021/acs.est.0c01764 (2020).
CanMap Postal Code Suite v. 2015.3 (DMTI Spatial Inc., Markham, 2015).
Hystad, P. et al. Creating national air pollution models for population exposure assessment in Canada. Environ. Health Perspect. 119, 1123–1129. https://doi.org/10.1289/ehp.1002976 (2011).
CCME. Guidance document on achievement determination for Canadian Ambient Air Quality Standards for fine particulate matter and ozone (Canadian Council of Ministers of the Environment, 2012).
Bondy, M. L. et al. Brain tumor epidemiology: Consensus from the Brain Tumor Epidemiology Consortium. Cancer 113, 1953–1968. https://doi.org/10.1002/cncr.23741 (2008).
Vida, S. et al. Brain tumours and cigarette smoking: Analysis of the INTERPHONE Canada case–control study. Environ. Health. 13, 55. https://doi.org/10.1186/1476-069X-13-55 (2014).
Li, H. X. et al. Cigarette smoking and risk of adult glioma: A meta-analysis of 24 observational studies involving more than 2.3 million individuals. Onco. Targets Ther. 9, 3511–3523. https://doi.org/10.2147/OTT.S99713 (2016).
Niedermaier, T. et al. Body mass index, physical activity, and risk of adult meningioma and glioma: A meta-analysis. Neurology 85, 1342–1350 (2015).
Pearl, J. Causal diagrams for empirical research. Biometrika 82, 669–688 (1995).
Shin, H. H. et al. Indirect adjustment for multiple missing variables applicable to environmental epidemiology. Environ. Res. 134, 482–487. https://doi.org/10.1016/j.envres.2014.05.016 (2014).
Jorgensen, J. T. et al. Long-term exposure to ambient air pollution and incidence of brain tumours: The Danish Nurse Cohort. Neurotoxicology 55, 122–130. https://doi.org/10.1016/j.neuro.2016.06.003 (2016).
Konczol, M. et al. Cytotoxicity and genotoxicity of size-fractionated iron oxide (magnetite) in A549 human lung epithelial cells: Role of ROS, JNK, and NF-kappaB. Chem. Res. Toxicol. 24, 1460–1475. https://doi.org/10.1021/tx200051s (2011).
Teller, S., Tahirbegi, I. B., Mir, M., Samitier, J. & Soriano, J. Magnetite-Amyloid-beta deteriorates activity and functional organization in an in vitro model for Alzheimer’s disease. Sci. Rep. 5, 17261. https://doi.org/10.1038/srep17261 (2015).
Calderón-Garcidueñas, L. et al. Combustion- and friction-derived magnetic air pollution nanoparticles in human hearts. Environ. Res. 176, 108567. https://doi.org/10.1016/j.envres.2019.108567 (2019).
Liu, N. M. et al. Evidence for the presence of air pollution nanoparticles in placental tissue cells. Sci. Total. Environ. 751, 142235. https://doi.org/10.1016/j.scitotenv.2020.142235 (2021).
Lu, D. et al. Chemical multi-fingerprinting of exogenous ultrafine particles in human serum and pleural effusion. Nat. Commun. 11, 2567. https://doi.org/10.1038/s41467-020-16427-x (2020).
Qi, Y. et al. Passage of exogeneous fine particles from the lung into the brain in humans and animals. Proc. Natl. Acad. Sci. USA 119, e2117083119. https://doi.org/10.1073/pnas.2117083119 (2022).
Nel, A., Xia, T., Madler, L. & Li, N. Toxic potential of materials at the nanolevel. Science 311, 622–627. https://doi.org/10.1126/science.1114397 (2006).
Gonet, T. et al. Size-resolved, quantitative evaluation of the magnetic mineralogy of airborne brake-wear particulate emissions. Environ. Pollut. 288, 117808. https://doi.org/10.1016/j.envpol.2021.117808 (2021).
Muxworthy, A. R. et al. Magnetic characterisation of London’s airborne nanoparticulate matter. Atmos. Environ. https://doi.org/10.1016/j.atmosenv.2022.119292 (2022).
Revuelta, M. A. et al. Partitioning of magnetic particles in PM10, PM2.5 and PM1 aerosols in the urban atmosphere of Barcelona (Spain). Environ. Pollut. 188, 109–117. https://doi.org/10.1016/j.envpol.2014.01.025 (2014).
Maher, B. A., Gonet, T., Karloukovski, V. V., Wang, H. & Bannan, T. J. Protecting playgrounds: Local-scale reduction of airborne particulate matter concentrations through particulate deposition on roadside “tredges” (green infrastructure). Sci. Rep. 12, 14236. https://doi.org/10.1038/s41598-022-18509-w (2022).
Matzka, J. & Maher, B. A. Magnetic biomonitoring of roadside tree leaves: Identification of spatial and temporal variations in vehicle-derived particulates. Atmos. Environ. 33, 4565–4569 (1999).
Sheikh, H. A., Maher, B. A., Karloukovski, V., Lampronti, G. I. & Harrison, R. J. Biomagnetic characterization of air pollution particulates in Lahore. Pakistan. Geochem. Geophys. https://doi.org/10.1029/2021gc010293 (2022).
Sheikh, H. A., Tung, P.-Y., Ringe, E. & Harrison, R. J. London Underground air pollution particles are finer than you think. Res. Square. https://doi.org/10.21203/rs.3.rs-2139550/v1 (2022).
Maher, B. A. Airborne magnetite- and iron-rich pollution nanoparticles: Potential neurotoxicants and environmental risk factors for neurodegenerative disease, including Alzheimer’s disease. J. Alzheimers Dis. 71, 361–375 (2019).
Lebret, E. et al. Small area variations in ambient NO2 concentrations in four European areas. Atmos. Environ. 34, 177–185 (2000).
Sahsuvaroglu, T. et al. A land use regression model for predicting ambient concentrations of nitrogen dioxide in Hamilton, Ontario, Canada. J. Air Waste Manag. Assoc. 56, 1059–1069. https://doi.org/10.1080/10473289.2006.10464542 (2006).
Funding
This study was funded by Canadian Institutes of Health Research, CIHR Doctoral Award, Foundation Grant, Natural Sciences and Engineering Research Council of Canada, Discovery Grant.
Author information
Authors and Affiliations
Contributions
SR refined the research question, prepared the data, performed all statistical analyses and wrote the first draft of the manuscript. BAM supervised magnetic analyses and contributed to interpretation of the magnetite results. MH assisted with design and coordination of field work. SW conceptualized and funded the study, oversaw statistical analyses, and provided critical revisions of the manuscript. All authors revised the final draft.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ripley, S., Maher, B.A., Hatzopoulou, M. et al. Within-city spatial variations in PM2.5 magnetite nanoparticles and brain cancer incidence in Toronto and Montreal, Canada. Sci Rep 14, 12136 (2024). https://doi.org/10.1038/s41598-024-58119-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-58119-2
- Springer Nature Limited