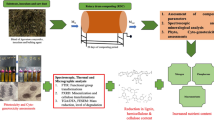
Abstract
Utilizing the organic fraction of agri-food industry waste for fertilization represents one approach to waste management, with composting emerging as a popular method. Composts derived from this waste may contain plant hormones alongside primary macronutrients. This study aimed to evaluate the content of plant hormones in composts crafted from the organic fraction of agri-food industry waste. The presence of these substances was ascertained using liquid chromatography–mass spectrometry (LC-MS) analysis, applied to extracted samples from three composts produced in a bioreactor and three obtained from companies. The results indicate the presence of 35 compounds, which belong to six types of plant hormones: auxins, cytokinins, gibberellins, brassinosteroids, abscisic acid, and salicylic acid, in composts for the first time. The highest amount of plant hormones was noted in buckwheat husk and biohumus extract (35 compounds), and the lowest in hemp chaff and apple pomace (14 compounds). Brassinosteroids (e.g., brassinolide, 28-homobrassinolide, 24-epicastasterone, 24-epibrassinolide, and 28-norbrassinolide) and auxins (e.g., indolilo-3-acetic acid) are dominant. The highest concentration of total phytohormones was reported in biohumus extract (2026.42 ng g−1 dry weight), and the lowest in organic compost (0.18 ng g−1 dry weight).
Similar content being viewed by others
Introduction
The management of organic waste and residues from the agri-food industry leads to a reduction in problems related to waste management and environmental pollution1. Additionally, the appropriate processing of these materials aligns with the fundamentals of the circular economy (CE). The agri-food industry generates substantial amounts of organic waste and by-products, presenting a severe challenge for many food producers2. One of the most effective ways to manage organic waste is to utilize it for agricultural purposes, primarily fertilization, with composting being one of the methods. Composting involves the biological decomposition of selectively collected organic waste under controlled conditions by micro- and macroorganisms. Compost, a natural fertilizer, enhances soil structure, water retention capacity, and air–water relations3. Furthermore, compost is a multi-ingredient fertilizer, supplying nutrients vital for plant growth and offering beneficial microorganisms that positively influence the condition of the roots and overall plant health4. Employing compost derived from organic waste can also mitigate the adverse impact of soil salinity on plant growth and development5 and inhibit the uptake of heavy metals from soil6. Bożym and Rajmund7 reported that the issue of heavy metal pollution is primarily related to compost created from municipal waste and sewage sludge used as fertilizer. Therefore, compost is increasingly used in agriculture, significantly reducing the need for synthetic fertilizers. Researchers have explored the use of composts made from various organic waste from industries such as bakeries8, white wine production9, food processing10, brewing11, and herbal pharmaceuticals12. It is crucial to note that composts, made from an organic fraction of waste, can contain active plant growth substances that influence plant growth and development13, in addition to primary macronutrients, i.e., nitrogen, phosphorus, and potassium. Phytohormones, considered natural plant growth stimulators, are increasingly utilized in agriculture, horticulture, and forestry. They influence numerous physiological processes in plants include auxins (AXs), cytokinins (CKs), gibberellins (GAs), brassinosteroids (BRs), abscisic acid (ABA), and salicylic acid (SA). The roles of these plant hormones, particularly in plant growth and developmental processes, have been presented in numerous publications. AXs are crucial as they are involved in cell division, cell elongation, root formation, and the differentiation of cellular tissues. CKs influence cell division, thereby affecting plant growth, and also stimulate lateral buds and induce flowering, fruiting, and seed set. GAs promote germination, interrupt plant dormancy, and stimulate cell division. BRs regulate root and shoot growth, vascular differentiation, fertility, flowering, and seed germination. ABA plays a pivotal role in seed development, germination, vegetative growth, and the modulation of root architecture. SA is vital for DNA damage/repair, fruit yield, and seed germination14,15,16,17,18,19,20,21. Additionally, these phytohormones have a positive effect on organisms (human and animals) because they show antitumor, antidepressant, antioxidant and anti-stress activities22.
The content of plant hormones in composts derived from agri-food industry waste is seldom studied; hence, this aspect is pivotal since composts containing these growth substances can substitute synthetic fertilizers. Consequently, the present study focuses on evaluating the content of plant hormones, such as: AXs, CKs, GAs, BRs, ABA, and SA, in composts made from the organic fraction of agri-food industry waste using liquid chromatography–mass spectrometry (LC–MS) analysis. Notably, this study reports on a large number of plant hormones (35 compounds) in composts for the first time. Determining the content of phytohormones should be helpful in horticulture and agriculture for utilizing compost as organic fertilizers and agents that improve soil properties, as well as natural plant growth stimulants.
Results and discussion
The research conducted revealed the presence of the following groups of phytohormones: auxins (AXs), brassinosteroids (BRs), cytokinins (CKs), gibberellins (GAs), abscisic acid (ABA), and salicylic acid (SA). Table 1 displays the content of phytohormones in composts. The average phytohormone content ranged from 0.13 to 2039.18 ng g−1 dry weight (dw). Brassinosteroids and AXs were the most prevalent, while GA3 was the least abundant.
Auxins (AXs) and brassinosteroids (BRs) were identified as the prevailing types of phytohormones, as evidenced in Fig. 1.
The utmost concentration of BRs was found in organic compost-pellets, at 99.87%, and in biohumus extract, at 96.01%. Additionally, considerable quantities of brassinosteroids were present in garden compost and compost with buckwheat husk, registering 69.37% and 51.97%, respectively. Notably, compost hemp chaff and apple pomace, or purely organic sources did not exhibit detectable levels of BRs. Nevertheless, BRs have proven to be effective even in scant. They are recognized for encouraging cell division and elongation, influencing the growth of stems and roots, and playing a role in the initiation of floral structures and fruit development21. Moreover, Divi and Krishna23 highlighted the ability of BRs to safeguard plants against various stresses, both abiotic and biotic. Conversely, AXs exhibited their highest concentration in compost with buckwheat husk, at 38.24%, and their lowest in organic compost-pellets, at a mere 0.001%. Both AXs and BRs are integral to numerous physiological processes within plants24,25. Aremu et al.26 found a notably high AXs concentration, between 0.55 and 0.77 pmol mL−1, in vermicompost derived from garden waste. Additionally, Façanha et al.27 observed that substances from earthworm compost could spur lateral root development in maize plants.
Abscisic acid and GA3 show the lowest content in the evaluated waste, both less than 0.20% (Fig. 1). A peak ABA level of 0.20% was found in compost with buckwheat husk, while four types of compost, i.e., garden, hemp chaff and apple pomace, organic compost, and organic compost-pellets, exhibited no detectable levels of ABA. A study by Stirk et al.28 also reported low ABA concentration. Regarding GA3, the highest level was present in organic compost (0.06%), whereas the smallest was in compost from hemp chaff and apple pomace (< 0.001%). GAs, vital throughout the plant life cycle, promote stages like germination, hypocotyl elongation, and the development of organs, flowers, and seeds29. Over 90% CKs were found in compost hemp chaff and apple pomace, and organic sources. Salicylic acid levels peaked in compost with buckwheat husk at 8.58%, and plumbed to their lowest in organic compost-pellets at 0.001%.
An analysis using liquid chromatography–mass spectrometry (LC–MS) quantified the presence of up to 35 phytohormones in the compost samples. The various composts displayed a range of 14–35 distinct phytohormone types, i.e., compost with buckwheat husk and biohumus extract contained 35 compounds; compost hemp chaff and apple pomace, 14; organic compost, 15; organic compost-pellets, 21; and garden compost, 25 (Table 2).
Biohumus extract exhibited the highest concentration of total phytohormones at 2026.42 ng g−1 dw, while organic compost had the lowest at 0.18 ng g−1 dw (Table 3). The compost with buckwheat husk showcased the highest content of SA (4.02 ng g−1 dw), whereas GA3 was the least concentrated hormones across all composts (Fig. 2).
The content of abscisic acid (ABA), auxins (AXs), gibberellic acid (GA3) and salicylic acid (SA) in different type of compost. Data represent the mean (n = 4) ± standard deviation. Means with the same letters are not significantly different (p ≥ 0.05) according to Tukey’s post hoc test. Abbreviations of composts: GC, garden compost; CHCAP, compost hemp chaff and apple pomace; CBH, compost with buckwheat husk; OC, organic compost; OC-P, organic compost-pellets; BE, biohumus extract.
Every type of phytohormone was detectable in both compost with buckwheat husk and biohumus extract. Dominant phytohormones in composts derived from agri-food industry waste included IAA (Fig. 2), BL, HBL, epiCS, epiBL, and norBL (Fig. 3).
The content of brassinosteroids (BRs) in different type of compost. Data represent the mean (n = 4) ± standard deviation. Means with the same letters are not significantly different (p ≥ 0.05) according to Tukey’s post hoc test. Abbreviations of composts: GC, garden compost; CHCAP, compost hemp chaff and apple pomace; CBH, compost with buckwheat husk; OC, organic compost; OC-P, organic compost-pellets; BE, biohumus extract.
cZ-type of CKs were not detected in organic compost and aromatic CKs were not noted in organic compost-pellets (Table 4). Dominant type of CK group in composts was cZ-type (Fig. 4). Schäfer et al.30 summarized studies that investigate the role of this type of CK in regulating plant development and defense responses to pathogen and herbivore attack. It can be concluded that the diversity in phytohormone content across composts is influenced by the type of substrates utilized during the composting process13,31.
The content of cytokinins (CKs) in different type of compost. Data represent the mean (n = 4) ± standard deviation. Means with the same letters are not significantly different (p ≥ 0.05) according to Tukey’s post hoc test. Abbreviations of composts: GC, Garden compost; CHCAP, Compost hemp chaff and apple pomace; CBH, Compost with buckwheat husk; OC, Organic compost; OC-P, Organic compost-pellets; BE, Biohumus extract.
Our findings suggest a notably elevated content of plant hormones in the leachate derived from composted hemp chaff and grass. The overall content of the plant hormones analyzed spanned from 1456.32 to 4094.34 ng g−1 dw, averaging at 462.56 ng g−1 dw. This leachate demonstrated the highest concentrations of BRs (ranging from 661.66 to 3368.31 ng g−1 dw), AXs (324.75–336.73 ng g−1 dw), and SA (300.31–350.55 ng g−1 dw). Arthur et al.32 and Aremu et al.25 have noted that leachate from thoroughly decomposed compost contains CK-like substances, originating from the hydrolysis of CK glucosides by β-glucosidase, an enzyme produced by microbes. Subsequent research should, therefore, prioritize evaluating the content of plant hormones in leachates, specifically those derived from composts produced from the organic fraction of agri-food industry waste33.
Hierarchical Cluster Analysis effectively delineated the assessed composts into two discrete clusters, denominated as A and B, based on the constituent phytohormone content (Fig. 5). Cluster A, encompassing garden compost, compost derived from hemp chaff and apple pomace, compost with an inclusion of buckwheat husk, organic compost, and analogous organic compost in a pelletized configuration, exhibits a phytohormone composition that is discernibly lower in concentrations of ABA, AXs, BRs, CKs, and GA3 relative to the mean of the group; notwithstanding, it is punctuated by the pinnacle of SA concentration within this cluster. Such a phytohormone profile implicates potential limitations of these compost variants for agricultural applications. Conversely, cluster B, exclusively represented by biohumus extract, albeit exhibiting diminished SA content, is distinctly characterized by superlative concentrations of ABA, AXs, BRs (i.e., BL, HBL, epiCS, epiBL, and norBL), CKs, and GA3, thereby positing it as a potentially preeminent stimulator of plant growth.
The analysis of plant hormone content in the examined composts revealed two distinctly dissimilar groups of objects when objects and features were simultaneously grouped (Fig. 6). In the upper part of the map, an area was obtained where the garden compost, organic compost, organic compost in the form of pellets, compost from hemp chaff and apple pomace, and compost with buckwheat husk were grouped (green color). Conversely, in the lower part of the map, an area was designated where the biohumus extract was grouped (red color).
PCA analysis enabled the categorization of analyzed composts, maintaining a significant degree of explained variance. During this analysis, the variable count was condensed to two principal components (designated as PC1 and PC2), which intimates that the initial dataset of 35 plant hormones is notably correlated and therefore reducible (Fig. 7). All variables, with the exception of DHZ7G and SA, presented high negative loadings, ranging from − 0.9996 to − 0.8995, in association with the first component. Contrastingly, DHZ7G was associated with a high positive loading (0.6334), whereas SA exhibited a high negative loading (− 0.8768) with the second component. The values of factor loadings for most variables were proximate, resulting in a superimposition of points on a singular graph.
Biplot of plant hormones content for each repetition (n = 4) in six types of composts, showing the first two principal components (PC1 and PC2) of the PCA model that together explain 96.9% of the total variance, i.e., 92.45% and 4.40% for PC1 and PC2, respectively. Blue biplot vectors indicate the strength and direction of factor loading for all analyzed variables: 1, ABA; 2, IAA; 3, IBA; 4, IPA; 5, PAA; 6, BL; 7, HBL; 8, epiBL; 9, norBL; 10, epiCS; 11, CT; 12, 6dTY; 13, cZ; 14, cZR; 15, cZ9G; 16, cZOGR; 17, tZ; 18, tZR; 19, tZOG; 20, tZOGR; 21, tZ9G; 22, DHZ; 23, DHZR; 24, DHZOG; 25, DHZOGR; 26, DHZ7G; 27, DHZ9G; 28, IP; 29, IPR7G; 30, oT; 31, mT; 32, pT; 33, BA; 34, GA3; 35, SA. Composts are marked in red: BE, biohumus extract; CHCAP, compost hemp chaff and apple pomace; GC, garden compost; OC, organic compost; OC-P, organic compost-pellets; CBH, compost with buckwheat husk.
Comparison of case positions on the graph, considering component forms and factor loadings, revealed distinct characteristics among them. Specifically, Case 1, exhibiting negative coordinate values on the first axis, was identified as having a higher content of plant hormones, excluding DHZ7G and SA, according to the relevant factor loadings. Conversely, Case 2, with a positive coordinate value for the second axis, was associated with a higher DHZ7G content, based on the factor loading with the second axis. In contrast, Case 3, showing a negative coordinate value on the second axis, was characterized by a higher SA content, based on its factor loading with the second axis (Fig. 7). The interplay between the content of plant hormones and the two principal components (PC1 and PC2) is visually represented in a three-dimensional surface plot (Fig. 8).
Conclusions
The variation in plant hormone content within the tested organic composts was influenced by the choice of substrates utilized during the composting process. Brassinosteroids, identified with notable prevalence in the tested composts, could potentially expedite plant growth, as well as trigger the onset of flowering and fruit development. The compost comprising buckwheat husk and biohumus extract demonstrated the most significant phytohormone content variation and was established to contain all recognized groups of plant hormones. Characterized by the highest total phytohormone concentration, biohumus extract is posited as the optimal natural stimulant for plant growth. It has been ascertained that the pelleting process can modify phytohormone content, evidenced by an elevated concentration of brassinosteroids in compost post-pelletization relative to its pre-pelletization state.
Materials and methods
Characteristics of composts
The composted wastes were collected from facilities located in the Podlasie Voivodeship (Poland), an area where the predominant sectors of the agri-food industry include milk, meat, fruit and vegetable processing plants. The composts were formulated using the organic fraction of agri-food industry waste and residues from fruit and vegetable processing, all within a laboratory bioreactor with engineered aeration (Fig. 9, Table 5). The study entailed crafting experimental compost mixtures through a two-stage composting process. Initially, the composting process involved a phase of notably intense decomposition of the organic fraction at temperatures ranging from 60 to 75 °C. Subsequently, an intense yet diminishing decomposition of the organic fraction occurred over time, leading to a gradual temperature decrease to between 30 and 40 °C. The bioreactor composting process spanned 14 days. In Table 5 the composition of tested composts obtained from companies was also presented.
The compost blends were formulated considering the fundamental characteristics such as pH, dry matter content, organic matter, and elements like nitrogen, phosphorus, potassium, and carbon from the processing waste used. Details on the physical and chemical parameters of specific agri-food processing residues were previously documented34. The composting process yielded fully matured composts, distinguishable by their dark brown hue, consistent texture, and a distinctive fresh soil aroma (Fig. 10a,b). The organic solid compost was additionally subjected to a granulation process under laboratory conditions to evaluate the impact of thermal processing (Fig. 10c).
In the analyzed composts, nitrogen was determined by acid digestion using a catalyst, phosphorus was determined by mineralization to phosphorus (V) followed by a spectrophotometric method, and potassium was evaluated by digestion with an acid mixture, and subsequently determined by atomic spectrometry35. The values of the total solids (TS) content were determined using the standard PN-EN14346:201136, while the values of the volatile solids (VS) content were determined using the standard PN-Z-15011-3:200135. Carbon was determined throught catalytic oxidation. Table 6 shows the results from evaluating the fertilizing properties of the finalized composts, obtained under laboratory conditions.
Chemicals
All chemicals used in phytohormone extraction and salicylic acid (SA) were purchased from Merck KGaA (Darmstadt, Germany). The solvents used for liquid chromatography–mass spectrometry (LC–MS) analyses were of high-performance grade. Phytohormone standards, i.e., abscisic acid (ABA), indole-3-acetic acid (IAA), indole-3-butyric acid (IBA), indole-3-pyruvic acid (IPA), phenylacetic acid (PAA), brassinolide (BL), 28-homobrassinolide (HBL), 24-epibrassinolide (epiBL), 28-norbrassinolide (norBL), 24-epicastasterone (epiCS), cathasterone (CT), 6-deoxytyphasterol (6dTY), cis-zeatin (cZ), cis-zeatin-riboside (cZR), cis‐zeatin‐9‐glucoside (cZ9G), cis-zeatin-O-glucoside riboside (cZOGR), trans-zeatin (tZ), trans-zeatin-riboside (tZR), trans-zeatin-O-glucoside (tZOG), trans-zeatin-O-glucoside riboside (tZOGR), trans-zeatin-9-glucoside (tZ9G), trans-zeatin-9-glucoside-O-glucoside (tZ9GOG), dihydrozeatin (DHZ), dihydrozeatin riboside (DHZR), dihydrozeatin-O-glucoside (DHZOG), dihydrozeatin-O-glucoside riboside (DHZOGR), dihydrozeatin-7-glucoside (DHZ7G), dihydrozeatin-9-glucoside (DHZ9G), N6-isopentenyladenine (IP), N6-isopentenyladenosine-7-glucoside (IPR7G), o-topolin (oT), m-topolin (mT), p-topolin (pT), benzyladenine (BA), and gibberellic acid (GA3) were purchased from OlChemIm (Olomouc, Czech Republic).
Phytohormone extraction
For the measurement of phytohormones, 500 mg of composts and 20 mg of biohumus (after evaporation) were placed into the 2 mL Eppendorf tubes, suspended in 1 mL 50% (v/v) acetonitrile (ACN) and homogenized in a bead mill homogenizer (3 cycles / 3 min, speed 3.10 m s−1; OMNI International a PerkinElmer company, Kennesaw, GA, USA) using two 3 mm tungsten balls. Then, samples were homogenized using the ultrasound processor VCX 130 (power 130 W, frequency 20 kHz, 5 min) equipped with a titanium probe (Sonics & Materials Inc., Newtown, CT, USA) and mixed in a laboratory shaker (90 rpm, dark, 5 °C, 30 min; LC-350, Pol-Eko-Aparatura, Poland). Samples were centrifuged (9000 × g, 5 min; MPW-55, Med. Instruments, Gliwice, Poland) and collected in a glass tube. For quantification of phytohormones, stable isotope-labeled standards of [2H6] ( +)-cis, trans-ABA (50 ng), [2H5] IAA (30 ng), [2H6] IP (50 ng), [2H5] tZ (30 ng), [2H5] tZOG (30 ng), [2H3] DHZR (30 ng), [2H2] GA3 (30 ng), [2H3] BL (20 ng) and [2H3] CS (20 ng) were added to samples as internal standards. Prepared extracts were purged using Waters SPE Oasis® HLB cartridge (Waters Corporation, Milford, MA, USA), previously activated and equilibrated using 1 mL 100% methanol (MeOH), 1 mL H2O, and 1 mL 50% (v/v) ACN. Then, extracts were loaded and collected to the Eppendorf tubes and eluted with 1 mL 30% (v/v) ACN. Samples were evaporated to dryness by centrifugal vacuum concentrator (Labconco CentriVap micro IR; Labconco Corp. Kansas City, MO, USA), dissolved in 50 μL 30% (v/v) ACN, and transferred into the insert vials.
LC–MS analysis of phytohormones
Targeted compounds were analyzed using a LC–MS 8050 system consisting of a pump, degasser, autosampler, column oven, and mass spectrometer with triple quadrupole (Shimadzu Corporation, Kyoto, Japan). 10 μL of each sample was injected into the Waters XSelect C18 column (250 mm × 3.0 mm, 5 μm) (Waters Corporation, Milford, MA, USA), heated up to 50 °C. Mobile phase A was 0.01% (v/v) formic acid (FA) in ACN and phase B 0.01% (v/v) FA in H2O; the flow was 0.5 mL min−1. Separation of the above hormones was done in ESI positive mode with the following gradient: 0–8 min flowing increased linearly from 5 to 30% A, 8–25 min 80% A, 25–28 min 100% A, 28–30 min 5% A. The mobile LC phase consisted of binary gradients of ACN with 0.01% (v/v) formic acid (FA) (A) and 0.01% (v/v) aqueous FA (B), flowing at 0.5 mL min−1, which depended on the ESI mode, as described below. Analytical data were analyzed using Shimadzu BrowserWorkstation Software for LC–MS (Shimadzu Corporation, Kyoto, Japan).
Statistical analysis
All the results are presented as mean values ± standard deviation (SD) of four biological replicates. Before selecting the appropriate statistical analysis method, the data were tested for normality (Shapiro–Wilk test) and homogeneity of variances (Levene’s test). The normality of data and homogeneity of variances were reported. Therefore, the data were analyzed using one-way ANOVA and the F-test established that there are statistically significant differences between calculated means. The means were grouped using Tukey’s post hoc test. The level of significance in all statistical tests was p ≤ 0.05. Hierarchical cluster analysis was applied to build a dendrogram which grouped data into a tree of clusters based on distances between all pairs of objects. A dendrogram of obtained clusters was created with Euclidean distance, while the agglomerative criterion was set to Ward’s method. After that, principal component analysis (PCA) was performed to build the relationship model between variables. The first twenty-three factors were preserved in a biplot for further analysis. The final biplot was created using two main components (PC1, PC2), which together explain 96.9% of the total variance. Statistical analyses were performed in Statistica 13.3 software (TIBCO Software Inc., Palo Alto, CA, USA)37.
Data availability
The datasets used and analysed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- ABA:
-
Abscisic acid
- BA:
-
Benzyladenine
- BE:
-
Biohumus extract
- BL:
-
Brassinolide
- CBH:
-
Compost with buckwheat husk
- CHCAP:
-
Compost hemp chaff and apple pomace
- CT:
-
Cathasterone
- cZ:
-
cis-Zeatin
- cZ9G:
-
cis-Zeatin-9-glucoside
- cZOGR:
-
cis-Zeatin-O-glucoside riboside
- cZR:
-
cis-Zeatin-riboside
- 6dTY:
-
6-Deoxytyphasterol
- DHZ:
-
Dihydrozeatin
- DHZ7G:
-
Dihydrozeatin-7-glucoside
- DHZ9G :
-
Dihydrozeatin-9-glucoside
- DHZOG :
-
Dihydrozeatin-O-glucoside
- DHZOGR :
-
Dihydrozeatin-O-glucoside riboside
- DHZR :
-
Dihydrozeatin riboside
- epiBL:
-
24-Epibrassinolide
- epiCS:
-
24-Epicastasterone
- GA3 :
-
Gibberellic acid
- GC:
-
Garden compost
- HBL:
-
28-Homobrassinolide
- IAA:
-
Indole-3-acetic acid
- IBA:
-
Indole-3-butyric acid
- IP:
-
N6-Isopentenyladenine
- IPA:
-
Indole-3-pyruvic acid
- IPR7G:
-
N6-Isopentenyladenosine-7-glucoside
- mT:
-
m-Topolin
- norBL:
-
28-Norbrassinolide
- OC:
-
Organic compost
- OC-P:
-
Organic compost-pellets
- oT:
-
o-Topolin
- PAA :
-
Phenylacetic acid
- pT:
-
p-Topolin
- SA :
-
Salicylic acid
- tZ:
-
trans-Zeatin
- tZ9G :
-
trans-Zeatin-9-glucoside
- tZ9GOG :
-
trans-Zeatin-9-glucoside-O-glucoside
- tZOG:
-
trans-Zeatin-O-glucoside
- tZOGR:
-
trans-Zeatin-O-glucoside riboside
- tZR:
-
trans-Zeatin-riboside
References
Tiwari, A. & Khawas, R. in Innovation in the Food Sector Through the Valorization of Food and Agro-Food by-Products (eds. Barros, A. N. & Gouvinhas, I.) 1–14 (IntechOpen, 2021).
Klein, O., Nier, S. & Tamásy, C. Circular agri-food economies: Business models and practices in the potato industry. Sustain. Sci. 17, 2237–2252 (2022).
Azim, K. et al. Composting parameters and compost quality: A literature review. Org. Agric. 8, 141–158 (2017).
Donn, S., Wheatley, R. E., McKenzie, B. M., Loades, K. W. & Hallett, P. D. Improved soil fertility from compost amendment increases root growth and reinforcement of surface soil on slopes. Ecol. Eng. 71, 458–465 (2014).
Meena, M. D. et al. Municipal solid waste (MSW): Strategies to improve salt affected soil sustainability: A review. Waste Manag. 84, 38–53 (2019).
Alam, M. et al. The effects of organic amendments on heavy metals bioavailability in mine impacted soil and associated human health risk. Sci. Hortic. 262, 109067 (2020).
Bożym, M. & Rajmund, A. The study of cobalt leaching from soils, sewage sludges and composts using a one-step extraction. Ochrona Środowiska i Zasobów Naturalnych 26, 1–6 (2015).
Govindaraju, M., Sathasivam, K. V. & Marimuthu, K. Waste to wealth: Value recovery from bakery wastes. Sustainability 13, 2835 (2021).
Pinto, R., Correia, C., Mourão, I., Moura, L. & Brito, L. M. Composting waste from the white wine industry. Sustainability 15, 3454 (2023).
Hung, Y.-T., Holloman, K., Paul, H. H. & Huhnke, C. R. Composting processes for food processing wastes: A review. GSC Adv. Res. Rev. 8, 183–186 (2021).
Assandri, D., Pampuro, N., Zara, G., Cavallo, E. & Budroni, M. Suitability of composting process for the disposal and valorization of brewer’s spent grain. Agriculture 11, 2 (2020).
Ali, M. et al. High rate composting of herbal pharmaceutical industry solid waste. Water Sci. Technol. 65, 1817–1825 (2012).
Klimas, E., Szymańska-Pulikowska, A., Górka, B. & Wieczorek, P. Presence of plant hormones in composts made from organic fraction of municipal solid waste. J. Elem., 1043–1053 (2016).
Aremu, A. O. et al. Applications of cytokinins in horticultural fruit crops: Trends and future prospects. Biomolecules 10, 1222 (2020).
Castro-Camba, R., Sánchez, C., Vidal, N. & Vielba, J. M. Plant development and crop yield: The role of gibberellins. Plants 11, 2650 (2022).
Iqbal, N. et al. Ethylene role in plant growth, development and senescence: Interaction with other phytohormones. Front. Plant Sci. 8, 475 (2017).
Sah, S. K., Reddy, K. R. & Li, J. Abscisic acid and abiotic stress tolerance in crop plants. Front. Plant Sci. 7, 571 (2016).
Zhang, Q., Gong, M., Xu, X., Li, H. & Deng, W. Roles of auxin in the growth, development, and stress tolerance of horticultural plants. Cells 11, 2761 (2022).
Wybouw, B. & De Rybel, B. Cytokinin-A developing story. Trends Plant Sci. 24, 177–185 (2019).
Arif, Y., Sami, F., Siddiqui, H., Bajguz, A. & Hayat, S. Salicylic acid in relation to other phytohormones in plant: A study towards physiology and signal transduction under challenging environment. Environ. Exp. Bot. 175, 104040 (2020).
Ahanger, M. A., Ashraf, M., Bajguz, A. & Ahmad, P. Brassinosteroids regulate growth in plants under stressful environments and crosstalk with other potential phytohormones. J. Plant Growth Regul. 37, 1007–1024 (2018).
Mukherjee, A. et al. The bioactive potential of phytohormones: A review. Biotechnol. Rep. 35, 1–9 (2022).
Divi, U. K. & Krishna, P. Brassinosteroid: A biotechnological target for enhancing crop yield and stress tolerance. New Biotechnol. 26, 131–136 (2009).
Pant, A. P., Radovich, T. J. K., Hue, N. V. & Paull, R. E. Biochemical properties of compost tea associated with compost quality and effects on pak choi growth. Sci. Hortic. 148, 138–146 (2012).
Aremu, A. O., Kulkarni, M. G., Bairu, M. W., Finnie, J. F. & Van Staden, J. Growth stimulation effects of smoke-water and vermicompost leachate on greenhouse grown-tissue-cultured ‘Williams’ bananas. Plant Growth Regul. 66, 111–118 (2012).
Aremu, A. O., Masondo, N. A. & Van Staden, J. Physiological and phytochemical responses of three nutrient-stressed bulbous plants subjected to vermicompost leachate treatment. Acta Physiol. Plant. 36, 721–731 (2014).
Façanha, A. R. et al. Bioatividade de ácidos húmicos: Efeitos sobre o desenvolvimento radicular e sobre a bomba de prótons da membrana plasmática. Pesqui. Agropecu. Bras. 37, 1301–1310 (2002).
Stirk, W. A., Tarkowska, D., Turecova, V., Strnad, M. & van Staden, J. Abscisic acid, gibberellins and brassinosteroids in Kelpak®, a commercial seaweed extract made from Ecklonia maxima. J. Appl. Phycol. 26, 561–567 (2014).
Gao, X., Zhang, Y., He, Z. & Fu, X. in Hormone Metabolism and Signaling in Plants (eds. Li, J., Li, C. & Smith, S. M.) 107–160 (Academic Press, 2017).
Schäfer, M. et al. The role of cis-zeatin-type cytokinins in plant growth regulation and mediating responses to environmental interactions. J. Exp. Bot. 66, 4873–4884 (2015).
Weerasinghe, T. K. & De, S. I. H. W. K. Effect of applying different ratios of compost made of municipal solid waste on the growth of Zea mays L. (Corn). J. Soil Sci. Environ. Manag. 8, 52–60 (2017).
Arthur, G. D., Jäger, A. K. & Van Staden, J. The release of cytokinin-like compounds from Gingko biloba leaf material during composting. Environ. Exp. Bot. 45, 55–61 (2001).
Cwalina, P., Obidziński, S., Kowczyk-Sadowy, M., Krasowska, M. & Siegień, G. Management of waste from the dairy industry for energy purposes. Environ. Sci. Proc. 18, 4 (2022).
Krasowska, M. & Kowczyk-Sadowy, M. Assessment of the possibility of using waste from agri-food processing for fertilization purposes. Studia Quaternaria 39, 41–47 (2022).
PN-Z-15011-3:2001—Polish Version (The Polish Committee for Standardization, Warsaw, 2001).
PN-EN14346:2011—Polish Version (The Polish Committee for Standardization, Warsaw, 2011).
Stanisz, A. Accessible Statistics Course Using STATISTICA PL on Examples from Medicine Vol. 1 (StatSoft, Kraków, 2007).
Acknowledgements
The work was carried out as part of a team project no. WZ/WB-IIŚ/5/2023 and financed by the Ministry of Education and Science as part of a grant for maintaining research potential awarded to the Faculty of Civil Engineering and Environmental Sciences, Bialystok University of Technology and the Faculty of Biology, University of Bialystok, Poland.
Author information
Authors and Affiliations
Contributions
A.S. is responsible for the conceptualization; A.S. and A.P.-N. are responsible for the methodology; A.B. is responsible for the software; A.P.-N. for the validation; A.B. for the formal analysis; A.S., M.K., M.K.-S. and A.P.-N. are responsible for the investigation; A.S. and A.P.-N. for the resources; A.P.-N. is responsible for the data curation; A.S., M.K., M.K.-S. and A.P.-N. are responsible for the writing—original draft preparation; S.O. and A.B. for the writing—review and editing; A.B. is responsible for the visualization; S.O. and A.B. are responsible for the supervision; A.S. is responsible for the project administration; S.O. and A.B. are responsible for the funding acquisition. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sienkiewicz, A., Krasowska, M., Kowczyk-Sadowy, M. et al. Occurrence of plant hormones in composts made from organic fraction of agri-food industry waste. Sci Rep 14, 6808 (2024). https://doi.org/10.1038/s41598-024-57524-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-57524-x
- Springer Nature Limited