Abstract
Limited population-based studies discuss the association between fat mass index (FMI) and the risk of liver diseases. This investigation utilized data from the National Health and Nutrition Examination Survey (NHANES) to examine the linkage between the FMI and liver conditions, specifically steatosis and fibrosis. The study leveraged data from NHANES’s 2017–2018 cross-sectional study, employing an oversampling technique to deal with sample imbalance. Hepatic steatosis and fibrosis were identified by vibration-controlled transient elastography. Receiver operating curve was used to assess the relationship of anthropometric indicators, e.g., the FMI, body mass index (BMI), weight-adjusted-waist index (WWI), percentage of body fat (BF%), waist-to-hip ratio (WHR), and appendicular skeletal muscle index (ASMI), with hepatic steatosis and fibrosis. In this study, which included 2260 participants, multivariate logistic regression models, stratified analyses, restricted cubic spline (RCS), and sharp regression discontinuity analyses were utilized. The results indicated that the WHR and the FMI achieved the highest area under the curve for identifying hepatic steatosis and fibrosis, respectively (0.720 and 0.726). Notably, the FMI presented the highest adjusted odds ratio for both hepatic steatosis (6.40 [4.91–8.38], p = 2.34e−42) and fibrosis (6.06 [5.00, 7.37], p = 5.88e−74). Additionally, potential interaction effects were observed between the FMI and variables such as the family income-to-poverty ratio, smoking status, and hypertension, all of which correlated with the presence of liver fibrosis (p for interaction < 0.05). The RCS models further confirmed a significant positive correlation of the FMI with the controlled attenuation parameter and liver stiffness measurements. Overall, the findings underscore the strong link between the FMI and liver conditions, proposing the FMI as a potential straightforward marker for identifying liver diseases.
Similar content being viewed by others
Introduction
Hepatic steatosis is commonly observed in patients with chronic liver illnesses of various aetiologies, including alcoholic liver disease and viral hepatitis. Additionally, its incidence is on the rise in tandem with the global epidemic of nonalcoholic fatty liver disease (NAFLD)1,2,3,4. Recent research has brought attention to the influence of steatosis in accelerating the advancement of NAFLD to fibrosis and ultimately cirrhosis. Liver fibrosis is widely recognized as a risk factor for the development of liver cirrhosis and hepatocellular carcinoma5. Therefore, the prompt identification of hepatic steatosis and fibrosis is of great significance in the provision of efficient therapy for chronic liver disease before it progresses to an irreversible state.
The gold standard for assessing hepatic steatosis and fibrosis is liver biopsy for histology. Nevertheless, its limited usage can be attributed to its intrusive nature, poor repeatability, and potential for serious complications. Imaging techniques offer alternatives for the characterization of noninvasive steatosis and fibrosis by indicating changes in the physical properties of liver tissue with the development of fatty liver and liver fibrosis6,7. Vibration-controlled transient elastography (VCTE) represents a noninvasive tool with the ability to simultaneously assess hepatic steatosis and fibrosis8.
While body mass index (BMI) is widely utilized in clinical settings to evaluate body composition, its ability to accurately distinguish between muscle and adipose tissues is limited9. Due to its speed, simplicity, and minimal radiation exposure, dual-energy X-ray absorptiometry (DXA) is a largely acknowledged method for determining body composition10. However, there are few investigations on the correlation among the fat mass index (FMI), percentage of body fat (BF%), as measured by DXA, and the presence of hepatic steatosis and fibrosis.
The aim of this study was to evaluate the association of anthropometric indicators, including the FMI, BMI, weight-adjusted-waist index (WWI), BF%, waist-to-hip ratio (WHR), and appendicular skeletal muscle index (ASMI), with hepatic steatosis and fibrosis using National Health and Nutrition Examination Survey (NHANES) data.
Results
Baseline characteristics
The demographic characteristics of the 2260 participants (non-steatosis: 1081 vs. steatosis: 1179; non-fibrosis: 2090 vs. fibrosis: 170) are summarized in Table 1. The individuals involved in this study had an average age of 38.20 ± 12.57 years; 47.74% were men, and 52.26% were women. Among patients diagnosed with steatosis, it was found that 11% had a BMI within the normal range, 32% were classified as overweight, and the majority, 57%, were categorized as obese. In contrast to participants without hepatic steatosis and fibrosis, those with fatty liver disease had a greater propensity towards being male, being older, and having elevated ALT, AST, LDH, and TG levels. Statistical differences were observed in the distribution of age, history of hypertension and diabetes, and HDL, ALB, GLB, ALT, AST, LDH, and TG levels (p values < 0.05). In addition, the balanced fibrosis dataset (non-fibrosis: 2090 vs. fibrosis: 2136) was used in the investigation of fibrosis in this study (Figure S1).
ROC analysis of indicators in the identification of hepatic steatosis/fibrosis patients
An individual is classified as overweight if their BMI is ≥ 25 kg/m2 according to the World Health Organization (WHO). However, there is currently no universally accepted standard or definitive cut-off criterion for assessing factors such as the FMI1, FMI2, WWI, BF%, WHR, and ASMI. Given the binary classification needs, we employed ROC analysis to ascertain the optimal threshold FMI1, FMI2, WWI, BF%, WHR, and ASMI values. Tables S1 and S2 demonstrate the optimal cut-off values of the indicators identified by maximizing Youden’s index in the identification of hepatic steatosis and fibrosis patients.
The continuous variables of the seven body composition indices were transformed into dichotomous variables using cut-off values in this research. Table 2 displays the values for the areas under the curve (AUCs), as well as the sensitivity and specificity of the seven categorical anthropometric indicators for identifying hepatic steatosis and fibrosis patients. As shown in Table 2, the WHR and FMI1 achieved the highest AUCs in identifying hepatic steatosis and fibrosis patients, respectively (0.720 and 0.726), which demonstrates the optimal anthropometric indicators among the parameters.
Association among the seven anthropometric indicators, hepatic steatosis, and fibrosis
As shown in Table 3, higher FMI1, FMI2, WWI, BMI, BF%, WHR, and ASMI values were associated with a higher risk of hepatic steatosis and fibrosis (unadjusted p < 0.001). After adjustment for age, sex, race, hypertension, diabetes, education level, smoking, alcohol consumption, the family income to poverty ratio, physical activity, hepatitis B or C and HDL, ALB, GLB, ALT, AST, LDH, TBIL, and TG values, the FMI1 value had the significantly highest OR for both hepatic steatosis (6.40 [4.91–8.38]) and fibrosis (6.06 [5.00, 7.37]) among the seven indicators (Table 3). In Tables S3 and S4., we conducted a detailed stratification of steatosis and fibrosis severity using FibroScan values. The FMI demonstrated the highest OR across various stages and was notably significant in subsets of mild steatosis (≤ S1), advanced steatosis (S2-S3), as well as across the fibrosis spectrum, including significant and severe fibrosis (F2-F3), and cirrhosis (F4).
Subgroup analysis
To clarify the stability of the outcomes obtained from multivariate regression analysis, we conducted a stratified multivariate regression analysis to study the relationship between the FMI1 value and the likelihood of developing hepatic steatosis and fibrosis in various subgroups within the population. The analysis was stratified based on demographic factors, including age, sex, race, education level, the family income to poverty ratio, and health-related factors, such as smoking, physical activity, hepatitis B or C, hypertension, and diabetes.
As illustrated in Fig. 1, the risk of developing hepatic steatosis among individuals with high FMI1 values was consistent in the abovementioned subgroups. Additionally, Fig. 2 reveals that among individuals with a high family income to poverty ratio, individuals with no smoking history, and nonhypertensive individuals, those with high FMI1 values exhibited increased susceptibility to liver fibrosis. Significant disparities were identified in the interaction test. There may be potential interaction effects between the family income to poverty ratio, smoking, hypertension, and FMI1 value that are associated with the presence of liver fibrosis (p for interaction < 0.05).
Stratified associations between the FMI1 value and hepatic steatosis. All ORs were calculated by adjusting for age, sex, race, hypertension, diabetes, education level, smoking at least 100 cigarettes in one’s lifetime, alcohol consumption, the family income to poverty ratio, physical activity, hepatitis B or C, HDL cholesterol, albumin, globulin, ALT, AST, LDH, total bilirubin, and triglyceride levels.
Stratified associations between the FMI1 value and liver fibrosis. All ORs were calculated by adjusting for age, sex, race, hypertension, diabetes, education level, smoking at least 100 cigarettes in one’s lifetime, alcohol consumption, the family income to poverty ratio, physical activity, hepatitis B or C, HDL cholesterol, albumin, globulin, ALT, AST, LDH, total bilirubin, and triglyceride levels.
To investigate the impact of seven anthropometric indicators on nonobese steatosis, multivariate analyses were carried out among nonobese participants (n = 1546) (Table S5.) The FMI1 had the significantly highest OR for nonobese steatosis (4.80 [2.70–8.80]) among the seven indicators in Model 4.
The association of the FMI1 value with the CAP and LSM values
Both the CAP and LSM values showed a nonlinear connection with the FMI1 value (p for nonlinearity < 0.001). In Fig. 3, restricted cubic spline (RCS) models were employed to flexibly model and visually represent the nonlinear relationship between the FMI1 value and the CAP and LSM values after adjusting for all variables in Model 4. The RCS models show a significant positive correlation of the FMI1 value with the CAP and LSM values (Fig. 3).
Nonlinear association of the FMI1 value with the CAP (a) and LSM (b) values according to the restricted cubic spline model adjusted for age, sex, race, hypertension, diabetes, education level, smoking at least 100 cigarettes in one’s lifetime, alcohol consumption, the family income to poverty ratio, physical activity, hepatitis B or C, HDL cholesterol, albumin, globulin, ALT, AST, LDH, total bilirubin, and triglyceride levels. The red dashed line represents the upper and lower bounds of the 95% confidence intervals.
Sharp regression discontinuity plot of the FMI1 value versus hepatic steatosis and fibrosis
Figure 4 shows a noticeable increase in hepatic steatosis at the FMI1 cut-off value of 7.61, with noticeable discontinuities. Similarly, there was a discontinuous decrease in liver fibrosis at the FMI1 threshold value of 11.97. The ‘jump’ observed in the regression line at the threshold signifies the impact of the FMI1 cut-off value on the diagnosis of hepatic steatosis and fibrosis and the intention-to-treat effect at the threshold.
The graphical depiction of the effect observed at the threshold in hepatic steatosis (a) and fibrosis (b). The ‘jump’ observed in the regression line at the threshold signifies the impact of the FMI1 cut-off value on the diagnosis of hepatic steatosis and fibrosis and the intention-to-treat effect at the threshold.
Discussion
This study evaluated the correlation between seven anthropometric indicators and the presence of hepatic steatosis and fibrosis. Given the cross-sectional population-based design, we observed a significant correlation between the FMI value and the presence of hepatic steatosis and fibrosis.
Obesity is well established to be linked to lipid metabolism disorders, which not only play a role in the occurrence and progression of hepatic steatosis but also pose a risk of metabolic syndromes11. BMI is extensively employed as a means of assessing the degree of obesity. While NAFLD is commonly observed in individuals who are obese or have diabetes mellitus, up to 7% to 20% of cases of NAFLD are diagnosed in individuals with a BMI < 25 kg/m212. Recent research indicates that individuals diagnosed with lean NAFLD have a similar or higher incidence of liver fibrosis than people with nonlean NAFLD13. Body weight measurement is not a precise substitute for assessing total body fat mass by anthropometric means14. Additionally, the discriminative ability of BMI is restricted in regard to differentiating between muscle and adipose tissue components9. This suggests that BMI may not be the most reliable anthropometric indicator for screening hepatic steatosis and fibrosis.
The key factor driving the development of nonalcoholic steatohepatitis, insulin resistance, and cardiovascular disease has been identified as central obesity, more precisely the accumulation of adipose tissue in deep subcutaneous tissue15. Moreover, previous research has indicated that adipose tissue distribution, including abdominal fat distribution, is increasingly related to hepatic steatosis and metabolic syndrome16,17,18. The WHR can serve as a reliable clinical marker of the distribution of abdominal fat and central obesity19. In this context, we found that the WHR showed a more remarkable association with hepatic steatosis than BMI.
Recent studies found that an increased fat mass is linked to increased hepatic steatosis risk20,21. Furthermore, another analysis revealed that adipose tissue accumulation is a significant contributing factor in the development of NAFLD among male Japanese young adults, even among nonobese persons22. In our research, multivariable logistic analysis indicated that high-fat accumulation assessed by higher FMI values showed positively and independently association with the development of hepatic steatosis. The findings were further confirmed by a study of physical examination data from 56,639 Japanese participants that evaluated subjects with FMI-predominant and fat-free mass index-dominant body compositions and showed that there was a significant independent association between the predominance of fat mass in qualitative body composition changes and the presence of fatty liver in both men and women23. According to Burton, the utilization of the dimensionless quantity (fat mass)/height3 is a more appropriate method for determining the FMI in children and adolescents compared to the use of (fat mass)/height2 due to their closer resemblance to BF% curves24. However, BF% fails to account for the distinct contributions of fat and lean body mass, which could lead to the inability to recognize individuals with high fat and lean body mass25. Furthermore, the connection between height and (fat mass)/height3 was found to be lower than the correlation between height and (fat mass)/height2. The question of whether the best indicator of adiposity should be independent of height remains ambiguous because this may obscure biological relationships between adiposity and height26.
An estimate of the degree of liver fibrosis can be assessed by the LSM value, while hepatic steatosis can be quantified by using the CAP value27,28. For this study, we found a significant positive correlation among FMI, CAP, and LSM values. The connection among fat accumulation, hepatic steatosis, and fibrosis has several explanations. The overflow hypothesis claims that subcutaneous tissue has a limited capacity to increase the size and quantity of adipocytes and that each person’s ability to store lipids in adipose tissues is restricted29,30. Under such conditions, lipids tend to mainly concentrate in the liver tissue, resulting in the development of hepatic steatosis29,30. Adipose tissue has the potential to continuously release proinflammatory stimuli, hence contributing to the development of systemic inflammation, metabolic problems, and histological changes in the liver31. In addition, a proposal revealed that an imbalance in lipid metabolism contributes to the creation of lipotoxic intermediates, which in turn lead to oxidative stress and endoplasmic reticulum stress, trigger inflammasome activation, and initiate apoptotic cell death, followed by inflammation, tissue regeneration, and fibrogenesis32.
We reported that the FMI is an independent risk factor for the development of liver fibrosis. The FMI has been identified as an effective tool for the identification of patients with metabolic syndrome33,34, which is a cause and a consequence of NAFLD35. Certain subtypes of ceramidases produced from adipose tissue have the potential to induce insulin desensitization and hence contribute to the development of insulin resistance36. Insulin resistance leads to the advancement of fibrosis in nonalcoholic steatohepatitis through mechanisms such as hepatocyte cell death, the increased generation of reactive oxygen species, and disturbances in the balance of adipokines and cytokines37. The presence of liver fibrosis in individuals with fatty liver is commonly linked to metabolic disorders, including diabetes mellitus and dyslipidaemia, rather than BMI37. In our study, the FMI exhibited superior efficacy as a screening tool for identifying the presence of liver fibrosis compared with the WWI, BMI, BF%, WHR, and ASMI. We further validated the effect of the FMI threshold on the diagnosis of hepatic steatosis and fibrosis through sharp regression discontinuity.
Based on subgroup analysis, we observed that the family income to poverty ratio, smoking, and hypertension affected the association between the FMI value and liver fibrosis risk. The interaction can be further investigated and verified by RCT studies with a larger population in the future. Moreover, the mechanism of the interaction remains to be determined and could be investigated in the future through basic experiments.
This study had some strengths. We employed an oversampling technique to address the imbalance in hepatic fibrosis sample sizes. We conducted a comparative analysis to assess the ability of seven anthropometric indicators to identify hepatic steatosis and fibrosis patients, and we found a strong association between the FMI value and both hepatic steatosis and fibrosis. Moreover, to account for potential confounding variables, we distinguished the likely protected population with higher FMI values by stratified analysis. Finally, the rationale and validity of the cut-off values of several anthropometric indicators for identifying hepatic steatosis and fibrosis were further confirmed using SRD analysis.
Nonetheless, several limitations to this study should be mentioned. First, we are unable to draw any conclusions on the cause-and-effect relationship between the FMI value and hepatic steatosis or fibrosis due to the limitations of the cross-sectional study design. Second, VCTE was used to diagnose hepatic steatosis and fibrosis in this investigation; however, additional validation is required in a population of patients with confirmed biopsy results. Third, it should be noted that while the NHANES was carried out in the United States and included a multi-ethnic adult population, the generalizability of the findings to other geographical regions or ethnicities requires further validation. Then, it is necessary to conduct a larger cohort to further investigate the associations between the FMI and different stages of liver disease, specifically mild or advanced steatosis and mild (F1), significant and severe fibrosis (F2–F3), and cirrhosis (F4) in the future. Last, given that it is unclear how the FMI and each subgroup interact with each other in patients with liver fibrosis, future research should be conducted to further investigate the precise mechanism underlying our findings.
Materials and methods
Data and sample sources
The NHANES, which is conducted biennially by the National Center for Health Statistics (NCHS), is a comprehensive study designed to obtain information about the nutritional and physical health status of the general population in the United States through interviews, physical examinations, and laboratory tests. The survey received approval from the Centers for Disease Control and Prevention Research Ethics Review Board, and the informed permission of all participants was documented.
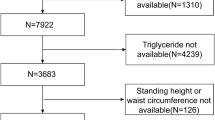
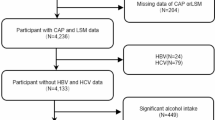
The present research utilized 2017–2018 data obtained from the NHANES database. Among the total sample of 9254 participants, we excluded those with incomplete FMI1 ([fat mass]/height2), FMI2 ([fat mass]/height3), WWI, BMI, BF%, WHR, and ASMI data (n = 6202), those with incomplete or ineligible (a liver stiffness interquartile range/median value ≥ 30%) controlled attenuation parameter (CAP)/liver stiffness measurement (LSM) data (n = 42), and those without standard biochemistry data (n = 197). Additionally, individuals who were younger than 18 years old (n = 553) were also excluded from the analysis. The final analysis was conducted with a sample size of 2260 (Fig. 5).
Flowchart of the inclusion of study participants. Abbreviations: NHANES, National Health and Nutrition Examination Survey; FMI1, fat mass index ([fat mass]/height2); FMI2, fat mass index ([fat mass]/height3); WWI, weight-adjusted-waist index; BMI, body mass index; BF%, percentage of body fat; WHR, waist-to-hip ratio; ASMI, appendicular skeletal muscle index; CAP, controlled attenuation parameter; LSM, liver stiffness measurement.
Definition and measurement of the seven anthropometric indicators
The fat mass, appendicular skeletal muscle mass and BF% values were measured by DXA. The waist circumference, hip circumference, weight, and height measurements were conducted within a mobile examination center, where tests were performed in controlled conditions. Muscle mass was quantified through the employment of the ASMI which is a standardized measure. The FMI1, FMI2, WWI, BMI, BF%, WHR, and ASMI were incorporated as exposure variables in our research, and the computation was performed in the following manner:
Assessment of hepatic steatosis and fibrosis
The assessment of liver fibrosis was carried out by FibroScan®, which employs ultrasound and VCTE to record the liver stiffness measurement (LSM) value. The instrument concurrently calculates the ultrasonic attenuation of the echo wave linked to hepatic steatosis and derives the controlled attenuation parameter (CAP) as an index for hepatic steatosis38. The elastography measurements were captured using the FibroScan model 502 V2 Touch, which was equipped with either a medium (M) or extra-large (XL) probe, as documented in the NHANES database. Individuals were classified as having hepatic steatosis (≤ S1) if the CAP value was ≥ 248 dB/m39. A median LSM value ≥ 8 kPa was regarded as an indication of mild (≥ F1) liver fibrosis40.
Covariates
The selection of covariates was informed by previous research. Demographic data comprised sex, age, race, educational level, and the family income to poverty ratio. Additionally, our study included personal life history data obtained from self-report questionnaires regarding smoking status (whether they smoked at least 100 cigarettes in their lifetime), alcohol status (whether they drank an average of ≥ 20 g/day and ≥ 30 g/day for women and men, respectively), physical activity status, hepatitis status (whether they had hepatitis B or hepatitis C), hypertension history, and diabetes history. The variables encompassed in the laboratory data were direct high-density lipoprotein cholesterol (HDL, mmol/L), albumin (ALB, g/L), globulin (GLB, g/L), alanine aminotransferase (ALT, U/L), aspartate aminotransferase (AST, U/L), lactate dehydrogenase (LDH, IU/L), total bilirubin (TBIL, µmol/L), and triglyceride (TG, mmol/L) levels. More thorough details of all variables can be acquired from the NHANES database website (https://www.cdc.gov/nchs/nhanes/index.htm).
Statistical analysis
We imputed and interpolated missing covariate values with the random forest algorithm using the mice (version 3.16.0) package in the R program (version 4.3.1)41. We used the oversampling method in the ROSE (version 0.0.4) package to address liver fibrosis sample imbalance. The median and interquartile ranges are used to report quantitative variables. Qualitative data are expressed in terms of patient counts and percentages (%). The Wilcoxon rank test was used to evaluate differences across several groups for continuous data, while the chi-squared test was utilized for categorical variables.
The optimal cut-off values of different indices were selected by maximizing Youden’s index. The continuous variables of the seven body composition indices were transformed into dichotomous variables using cut-off values in the following research. Using receiver operating characteristic curves, the discriminative ability of each body composition categorical variable was determined by calculating the area under the curve (AUC) with a 95% confidence interval (CI). To further investigate the relationship between the FMI1, FMI2, WWI, BMI, WHR, ASMI, and BF% values and hepatic steatosis and fibrosis, we constructed four types of separate multivariable logistic regression models that adjusted for different variables (Models I–IV). The odds ratios (ORs) and 95% CIs of the results are presented. Furthermore, stratified analysis was conducted to identify the stratified connection between the FMI1 value and hepatic steatosis as well as fibrosis.
Using restricted cubic splines with five knots at the 5th, 27.5th, 50th, 72.5th, and 95th percentage points, the relationship among the FMI, CAP, and LSM values was investigated. A sharp regression discontinuity (SRD) analysis was used to explore the impact of FMI1 cut-off values on the diagnosis of hepatic steatosis and fibrosis. All studies were carried out using R version 4.3.1 and Python version 3.11.3. A two-sided p value less than 0.05 was indicative of statistical significance in our study.
Institutional review board statement
NHANES was conducted in accordance with the Declaration of Helsinki, and approved by the NCHS Research Ethics Review Board (Continuation of Protocol #2011–17 and Protocol #2018–01, 26 October 2017). We used publicly available datasets from the official NHANES website (https://wwwn.cdc.gov/nchs/nhanes). Therefore, the need for ethical approval was not applicable.
Conclusions
This study found a strong association of the FMI value with hepatic steatosis and fibrosis and suggested that the FMI value may be a potential anthropometric indicator to identify patients with liver diseases.
Data availability
All NHANES data for this study are publicly available and can be found here: https://wwwn.cdc.gov/nchs/nhanes.
References
Chalasani, N. et al. The diagnosis and management of non-alcoholic fatty liver disease: Practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology 142, 1592–1609. https://doi.org/10.1053/j.gastro.2012.04.001 (2012).
Panel, A. I. H. G. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology 62, 932–954. https://doi.org/10.1002/hep.27950 (2015).
Singal, A. K., Bataller, R., Ahn, J., Kamath, P. S. & Shah, V. H. ACG clinical guideline: Alcoholic liver disease. Am. J. Gastroenterol. 113, 175–194. https://doi.org/10.1038/ajg.2017.469 (2018).
Terrault, N. A. et al. AASLD guidelines for treatment of chronic hepatitis B. Hepatology 63, 261–283. https://doi.org/10.1002/hep.28156 (2016).
Tsochatzis, E. A., Bosch, J. & Burroughs, A. K. Liver cirrhosis. Lancet 383, 1749–1761. https://doi.org/10.1016/S0140-6736(14)60121-5 (2014).
Berzigotti, A. Getting closer to a point-of-care diagnostic assessment in patients with chronic liver disease: Controlled attenuation parameter for steatosis. J. Hepatol. 60, 910–912. https://doi.org/10.1016/j.jhep.2014.01.017 (2014).
Xu, Y. et al. Comparison of FibroTouch and FibroScan for staging fibrosis in chronic liver disease: Single-center prospective study. Dig. Liver Dis. 51, 1323–1329. https://doi.org/10.1016/j.dld.2019.02.009 (2019).
Selvaraj, E. A. et al. Diagnostic accuracy of elastography and magnetic resonance imaging in patients with NAFLD: A systematic review and meta-analysis. J. Hepatol. 75, 770–785. https://doi.org/10.1016/j.jhep.2021.04.044 (2021).
Ebadi, M. et al. Review article: Prognostic significance of body composition abnormalities in patients with cirrhosis. Aliment. Pharmacol. Ther. 52, 600–618. https://doi.org/10.1111/apt.15927 (2020).
Genant, H. K. et al. Noninvasive assessment of bone mineral and structure: State of the art. J. Bone Miner. Res. 11, 707–730. https://doi.org/10.1002/jbmr.5650110602 (1996).
Polyzos, S. A., Kountouras, J. & Mantzoros, C. S. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metabolism 92, 82–97. https://doi.org/10.1016/j.metabol.2018.11.014 (2019).
Long, M. T., Noureddin, M. & Lim, J. K. AGA clinical practice update: Diagnosis and management of nonalcoholic fatty liver disease in lean individuals: Expert review. Gastroenterology 163, 764–774 e761 (2022). https://doi.org/10.1053/j.gastro.2022.06.023
Nabi, O. et al. Lean individuals with NAFLD have more severe liver disease and poorer clinical outcomes (NASH-CO Study). Hepatology 78, 272–283. https://doi.org/10.1097/HEP.0000000000000329 (2023).
Do, A. & Lim, J. K. Lean NAFLD is associated with adverse liver events and mortality: Moving beyond BMI. Hepatology 78, 6–7. https://doi.org/10.1097/HEP.0000000000000360 (2023).
Shen, W. et al. Changes in abdominal adipose tissue depots assessed by MRI correlate with hepatic histologic improvement in non-alcoholic steatohepatitis. J. Hepatol. 78, 238–246. https://doi.org/10.1016/j.jhep.2022.10.027 (2023).
Monteiro, P. A. et al. Body composition variables as predictors of NAFLD by ultrasound in obese children and adolescents. BMC Pediatr. 14, 25. https://doi.org/10.1186/1471-2431-14-25 (2014).
Yoo, H. J. et al. Cutoff points of abdominal obesity indices in screening for non-alcoholic fatty liver disease in Asians. Liver Int. 30, 1189–1196. https://doi.org/10.1111/j.1478-3231.2010.02300.x (2010).
Stranges, S. et al. Body fat distribution, relative weight, and liver enzyme levels: A population-based study. Hepatology 39, 754–763. https://doi.org/10.1002/hep.20149 (2004).
Illouz, F. et al. Distribution of adipose tissue: Quantification and relationship with hepatic steatosis and vascular profiles of type 2 diabetic patients with metabolic syndrome. Diabetes Metab. 34, 68–74. https://doi.org/10.1016/j.diabet.2007.10.007 (2008).
Chen, V. L. et al. Body composition and genetic lipodystrophy risk score associate with nonalcoholic fatty liver disease and liver fibrosis. Hepatol. Commun. 3, 1073–1084. https://doi.org/10.1002/hep4.1391 (2019).
Hsing, J. C. et al. Associations between body fat, muscle mass, and nonalcoholic fatty liver disease: A population-based study. Hepatol. Commun. 3, 1061–1072. https://doi.org/10.1002/hep4.1392 (2019).
Miwa, T. et al. Impact of body fat accumulation on metabolic dysfunction-associated fatty liver disease and nonalcoholic fatty liver disease in Japanese male young adults. Hepatol. Res. 53, 691–700. https://doi.org/10.1111/hepr.13906 (2023).
Imamura, Y. et al. Changes in body composition and low blood urea nitrogen level related to an increase in the prevalence of fatty liver over 20 years: A cross-sectional study. Hepatol. Res. 51, 570–579. https://doi.org/10.1111/hepr.13631 (2021).
Burton, R. F. The fat mass index: Why its height exponent should be 3 and not 2. Am. J. Clin. Nutr. 98, 1367. https://doi.org/10.3945/ajcn.113.068205 (2013).
Weber, D. R., Moore, R. H., Leonard, M. B. & Zemel, B. S. Reply to RF Burton. Am. J. Clin. Nutr. 98, 1368–1369. https://doi.org/10.3945/ajcn.113.068379 (2013).
Flegal, K. M. Commentary: Is there a best index of weight for height?. Int. J. Epidemiol. 40, 44–46. https://doi.org/10.1093/ije/dyq266 (2011).
Tsochatzis, E. A. et al. Elastography for the diagnosis of severity of fibrosis in chronic liver disease: A meta-analysis of diagnostic accuracy. J. Hepatol. 54, 650–659. https://doi.org/10.1016/j.jhep.2010.07.033 (2011).
Sasso, M. et al. Controlled attenuation parameter (CAP): A novel VCTE guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: Preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med. Biol. 36, 1825–1835. https://doi.org/10.1016/j.ultrasmedbio.2010.07.005 (2010).
Unger, R. H. Lipid overload and overflow: Metabolic trauma and the metabolic syndrome. Trends Endocrinol. Metab. 14, 398–403. https://doi.org/10.1016/j.tem.2003.09.008 (2003).
Azzu, V., Vacca, M., Virtue, S., Allison, M. & Vidal-Puig, A. Adipose tissue-liver cross talk in the control of whole-body metabolism: Implications in nonalcoholic fatty liver disease. Gastroenterology 158, 1899–1912. https://doi.org/10.1053/j.gastro.2019.12.054 (2020).
Bijnen, M. et al. Adipose tissue macrophages induce hepatic neutrophil recruitment and macrophage accumulation in mice. Gut 67, 1317–1327. https://doi.org/10.1136/gutjnl-2016-313654 (2018).
Friedman, S. L., Neuschwander-Tetri, B. A., Rinella, M. & Sanyal, A. J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 24, 908–922. https://doi.org/10.1038/s41591-018-0104-9 (2018).
Liu, P., Ma, F., Lou, H. & Liu, Y. The utility of fat mass index vs. body mass index and percentage of body fat in the screening of metabolic syndrome. BMC Public Health 13, 629. https://doi.org/10.1186/1471-2458-13-629 (2013).
Ramirez-Velez, R. et al. Percentage of body fat and fat mass index as a screening tool for metabolic syndrome prediction in Colombian University students. Nutrients https://doi.org/10.3390/nu9091009 (2017).
Yki-Jarvinen, H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2, 901–910. https://doi.org/10.1016/S2213-8587(14)70032-4 (2014).
Ghaben, A. L. & Scherer, P. E. Adipogenesis and metabolic health. Nat. Rev. Mol. Cell Biol. 20, 242–258. https://doi.org/10.1038/s41580-018-0093-z (2019).
Sohn, W., Kwon, H. J., Chang, Y., Ryu, S. & Cho, Y. K. Liver fibrosis in Asians with metabolic dysfunction-associated fatty liver disease. Clin. Gastroenterol. Hepatol. 20, e1135–e1148. https://doi.org/10.1016/j.cgh.2021.06.042 (2022).
Castera, L., Friedrich-Rust, M. & Loomba, R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology 156, 1264–1281 e1264 (2019). https://doi.org/10.1053/j.gastro.2018.12.036
Karlas, T. et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J. Hepatol. 66, 1022–1030. https://doi.org/10.1016/j.jhep.2016.12.022 (2017).
Cassinotto, C. et al. Non-invasive assessment of liver fibrosis with impulse elastography: Comparison of Supersonic Shear Imaging with ARFI and FibroScan®. J. Hepatol. 61, 550–557. https://doi.org/10.1016/j.jhep.2014.04.044 (2014).
Buuren, S. V. & Groothuis-Oudshoorn, K. mice: Multivariate Imputation by Chained Equations in R. J. Stat. Softw. 45, 1–67. https://doi.org/10.18637/jss.v045.i03 (2011).
Funding
The work was supported by the National Natural Science Foundation of China [Grant Number 82000540]; Suzhou Clinical Center of Digestive Diseases [Grant Number Szlcyxzx202101]; the Youth Program of Suzhou Health Committee [Grant Number KJXW2019001]; and the Open Fund of Key Laboratory of Hepatosplenic Surgery, Ministry of Education, Harbin, China [grant number GPKF202304].
Author information
Authors and Affiliations
Contributions
L.L. (Lihe Liu), M.Y., and J.L. took charge of the study design. J.G. and X.L. took charge of data extraction. L.L. (Lihe Liu) and L.L. (Lu Liu) performed statistical analyses and interpretation. L.L. (Lihe Liu), M.Y., and J.L. were responsible for manuscript drafting. J.Z. and A.W. took charge of the modification. The final manuscript has been read and approved by all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, L., Lin, J., Yin, M. et al. Association of the fat mass index with hepatic steatosis and fibrosis: evidence from NHANES 2017–2018. Sci Rep 14, 6943 (2024). https://doi.org/10.1038/s41598-024-57388-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-57388-1
- Springer Nature Limited