Abstract
Uric acid (UA) is associated with non-alcoholic fatty liver disease (NAFLD). However, it is unclear whether UA plays a predictive role in NAFLD prognosis. This study aimed to explore the relationship between UA levels and mortality in NAFLD patients without severe renal disease. Data were obtained from the Third National Health and Nutrition Examination Survey (NHANES). Time-dependent Cox regression was used to estimate the hazard ratio (HR) and 95% confidence interval (CI) for mortality. Overall, 2493 individuals with NAFLD and estimated glomerular filtration rate (eGFR) > 60 mL/min/1.73 m2 were included in this study. The median follow-up period was 26.58 years. Patients were divided into high and low-UA groups according to UA levels. Time-independent Cox regression showed that UA level was not an independent risk factor for mortality in NAFLD patients without decreased eGFR (P > 0.05). After matching for age and sex using the propensity score matching method, UA remained not independently associated with death in NAFLD patients (P > 0.05). Similar results were found for cardiovascular-related and cancer-related deaths. Although UA is closely related to NAFLD, UA levels are not independently associated with the long-term survival of patients with NAFLD without decreased eGFR.
Similar content being viewed by others
Introduction
NAFLD is characterised by excessive hepatic fat accumulation, associated with insulin resistance (IR), and defined by the presence of steatosis in > 5% of hepatocytes according to histological analysis or by a proton density fat fraction (PDFF, providing a rough estimation of the volume fraction of fatty material in the liver) > 5.6% assessed by proton magnetic resonance spectroscopy or quantitative fat/water selective magnetic resonance imaging (MRI)1. It is the most common liver disease worldwide, with a global prevalence of 25%2. Metabolic factors, such as obesity, type 2 diabetes, hyperlipidaemia, and hypertension, Sugar-sweetened beverages intake and a history of obesity in their early adulthood are closely associated with NAFLD and may accelerate its progression3,4,5.
Uric acid (UA) is the final product of purine metabolism. Hyperuricaemia is usually caused by a reduction in the renal excretion and/or overproduction of UA. Therefore, hyperuricaemia is often observed in patients with renal or metabolic diseases6. Previous studies have shown a significant association between hyperuricaemia and NAFLD7,8,9. For example, Li et al. demonstrated a higher prevalence of NAFLD in patients with hyperuricaemia than in those without7. Petta et al. showed that hyperuricaemia is independently associated with the severity of liver damage in NAFLD10. However, most previous studies have been cross-sectional, and the relationship between UA levels and NAFLD outcomes has not been fully explored. This study aimed to evaluate the role of UA levels in predicting the outcome of NAFLD in a large population-based survey cohort.
Method
Ethics
The study data were obtained from the Third National Health and Nutrition Examination Survey, a major nationwide survey conducted in the United States between 1988 and 1994. All study subjects were followed up until December 2015 to determine their survival status or cause of death. The study dataset was uploaded online for researchers worldwide. All datasets used in this study were obtained from the NHANES website (https://www.cdc.gov/nchs/nhanes/index.htm). This study was approved by the Institutional Review Board of the First Affiliated Hospital of Fujian Medical University (IEC-FOM-013-2.0).
The inclusion criteria for this study were adults with ultrasonography-confirmed fatty liver disease. The assess of hepatic steatosis was obtained by re-reviewing the archived gall bladder ultrasound video images derived from NHANES III between 1988 and 1994.
Patient exclusion criteria were as follows: 1. lost to follow-up; 2. missing data; 3. positive for HBsAg or anti-HCV antibody; 4. excessive alcohol consumption; and 5. decreased glomerular filtration rate (GFR), i.e., with estimated GFR (eGFR) < 60 mL/min/1.73 m2. eGFR was calculated according to the 2009 CKD-EPI equation as follows: GFR = 141 min(Scr/κ, 1)α × max(Scr/κ, 1)–1.209 × 0.993Age × 1.018 (if female) × 1.159 (if black), where Scr is serum creatinine, κ is 0.7 for females and 0.9 for males, α = –0.329 for females and –0.411 for males11.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation and compared using the Student’s t-test. Categorical variables are displayed as percentages and compared using the chi-square test. X-tile (Version3.6.1, http://tissuearray.org/) was used to determine the best UA cut-off value to discriminate between survival and death. A Kaplan–Meier survival curve was plotted to illustrate mortality between cohorts using R software (https://www.r-project.org/). Time-dependent Cox models were used to assess hazard ratios (HRs) and 95% confidence intervals (CI). Propensity score matching (PSM) was applied to match age and sex, to balance the baseline characteristics between the two study groups. All tests were two-tailed, and results with a P value less than 0.05 were considered statistically significant. The statistical analysis was conducted using SPSS 22.0 (SPSS Inc., Chicago, IL, USA).
Ethical approval
This study was supported by Fund for nurse innovation research (No. 2022FY-HZ-20) and Natural Science Fundation of Fujian Province (No. 2023J01580). All methods performed in this study were carried out in accordance with the Declaration of Helsinki and approved by the the National Center for Health Statistics Ethics Review Board. Informed consent was obtained from all participants involved in the study.
Results
Baseline participant characteristics
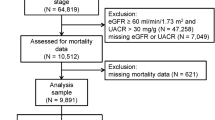
A total of 5949 adults with hepatic steatosis were screened. After excluding patients lost to follow-up (N = 634), with missing data (N = 745), positive for HBsAg or anti-HCV antibody (N = 151), excessive alcohol consumption (N = 1439), and decreased eGFR (N = 487), 2493 individuals with NAFLD remained eligible for final analysis (Fig. 1). Of these, 1410 (56.56%) were male, and the mean age was 44.21 ± 15.03 years. Diabetes and hypertension were diagnosed in 150 (17.69%) and hypertension in 1270 (50.94%) patients, respectively. The mean body mass index (BMI) level was 28.93 ± 6.83 kg/m2. The mean UA level was 311.94 ± 85.56 μmol/L, and 44.89% of cases had levels greater than 320 μmol/L.
Survival versus death groups
The median follow-up period was 26.58 (22.46, 28.42) years. During this period, 849 (34.06%) deaths were recorded. The baseline characteristics of the survival and death groups are presented in Table 1. Compared to those in the survival group, patients in the death group were significantly older with higher BMI. The metabolic profiles, including fasting plasma glucose, glycated haemoglobin, total cholesterol, and triglycerides, were higher in the death versus the survival group. There were more cases of hypertension in the death group. Patients who died were more likely to have more elevated renal function parameters (creatinine and urea nitrogen). The mean UA level was 325.63 ± 86.60 in the death group, which was significantly higher than that of the survival group (304.86 ± 84.17, P < 0.001). More individuals had UA levels greater than 320 μmol/L in the death group versus the survival group (52.53% vs. 40.94%, P < 0.001).
High versus low-UA groups
We used the X-tile software to determine the best UA cut-off value for assessing outcome. The results showed that at 320 μmol/L, serum UA level predicted the long-term survival of patients with NAFLD. A comparison of the high (> 320 μmol/L) and low (≤ 320 μmol/L) UA groups is presented in Table 2. The high-UA group was older and more likely to have increased BMI. Liver and renal injuries, evident by aspartate transaminase, alanine aminotransferase, creatinine, and eGFR, were more severe in the high versus low-UA group. Metabolic indices, such as total cholesterol and triglyceride levels, were significantly elevated in the high-UA group, whereas the low-UA group had higher high-density lipoprotein cholesterol levels. The death rate was 39.86% in the high-UA group, compared to 29.33% in the low-UA group. The survival probability between the two UA groups is illustrated as a Kaplan–Meier curve in Fig. 2A, indicating the association between baseline high UA levels and risk of death (P < 0.001). In the mild- and moderate group, patients with UA > 320 μmol/L had a higher risk of all-cause death (Supplementary Fig. 1A,B), while no difference was found in severe fatty liver group (Supplementary Fig. 1C).
Time-dependent cox regression analysis
As the risk of mortality may change over time, and participants with high UA levels were significantly older at baseline than those with low UA levels, we used time-dependent Cox regression to minimise the bias of time-dependent exposure. The results in Supplementary Table 1 showed that age (HR 1.016, 95%CI 1.004–1.027, P = 0.006) and glycated haemoglobin (HR 1.078, 95%CI 1.029–1.130, P = 0.002) were significant predictive factors for mortality, while UA (as a continuous variable, HR 1.000, 95%CI 1.000–1.000, P = 0.495 and as a dichotomous variable, HR 0.997, 95%CI 0.998–1.006, P = 0.508) was still not an independent predictor. Sex subgroup analysis showed that UA level was not a significant risk factor in either the male (HR 1.000, 95%CI 1.000–1.000, P = 0.659) or female subgroups (HR 1.000, 95%CI 1.000–1.000, P = 0.647) (Supplementary Table 2). In multivariate regression, UA level was not independently associated with outcomes in all three groups (Supplementary Table 3), which was consistent with the outcome without classifying patients according to the severity of fatty liver.
Propensity score matching
To further balance the baseline characteristics, we used PSM to match the age and sex between the two UA groups. The characteristics of the high and low-UA groups after PSM are presented in Table 2. The Kaplan–Meier curve is presented in Fig. 2 B. After matching for age and sex, survival probability increased in the low versus high UA group on the Kaplan–Meier curve (P < 0.05). On the time-dependent Cox regression, there was no independent association between higher UA (as a continuous variable HR 1.000, 95%CI 1.000–1.000, P = 0.554 and as a dichotomous variable, HR 1.005, 95%CI 0.994–1.015, P = 0.413) and mortality (Table 3).
Cause-specific death
The Kaplan–Meier analysis showed there was different in high- versus low-UA groups (P < 0.001) for cardiovascular disease (CVD)-related death, whereas identified no difference between the high and low-UA groups for cancer-related deaths in terms of survival probability (P > 0.05) (Figs. 2C and 2D). Time-dependent Cox regression showed that UA was not an independent risk factor for mortality from CVD or cancer (all P values > 0.05, Supplementary Tables 4 and 5).
The overall summary of the association between UA levels and mortality is shown in Table 4.
Sensitivity analysis
We included overall population (cases with and without decreased eGFR) in this sensitivity analysis. The results of time-dependent COX regression, as presented in Supplementary Table 6, showed that UA level was not significantly related to all-cause mortality of NAFLD participants irrespective of eGFR levels (P > 0.05).
To exclude the impact of uric acid lowering drugs, we investigated the correlation between uric acid and the prognosis of NAFLD in individuals with or without uric acid treatment. The Kaplan–Meier curve is presented in Supplementary Fig. 1D,E. After excluding patients with uric acid treatment, survival probability increased in the low UA group (P < 0.05). This difference was not found in patients using uric acid treatment (P = 0.2). But the result of time-dependent cox regression, which had adjusted confounding factors, showed that UA was not independently risk factor for the prognosis of NAFLD in NAFLD without using uric acid treatment (P > 0.05) (Supplementary Table 7). And using uric acid treatment was not an independent risk factor for all-cause death in all population (Supplementary Table 8).
Discussion
The main finding of this study was that although UA level was associated with more severe metabolic dysfunction in patients with NAFLD, it was not an independent risk factor for mortality without severe renal dysfunction.
Although NAFLD patients with high UA levels had a relatively higher mortality rate, UA level with NAFLD was not an independent factor for survival. Metabolic disorders may play a mediating role in the relationship between UA level and NAFLD outcome. Numerous studies have indicated that increased UA levels are associated with metabolic syndrome12, CVD13, hypertension14, type 2 diabetes15, and hypertriglyceridemia16. The underlying mechanism among high UA levels, NAFLD, and metabolic syndrome may be insulin resistance caused by hyperuricaemia. Increased UA levels lead to endothelial dysfunction, oxidative stress, insulin resistance, and inflammation17. Insulin resistance is a key factor in the development of NAFLD18 and other metabolic diseases19,20,21. In addition, insulin resistance can promote lipolysis in adipocytes, which increases hepatic triglyceride accumulation and leads to hepatic steatosis18. In this study, the high-UA group had significantly more severe metabolic dysfunction than the low-UA group, which is consistent with previous studies22. After adjustment for the above metabolic profiles, the effect of UA level was no longer significant. The complex interaction between UA and metabolic syndrome may contribute to the association between UA levels and NAFLD prognosis.
At least 70% of the daily UA production is excreted by the kidney23. To reduce the effect of renal dysfunction, we excluded persons whose eGFR was below 60 mL/min/1.73 m2. By excluding patients whose eGFR was < 60 mL/min/1.73 m2, the influence of renal dysfunction on the results was minimised. But the association between UA and long-term outcomes of NAFLD was not statistically significant, and this result was not dependent on the baseline renal function level, as the sensitivity analysis have included patients with decreased eGFR. To reduce and evaluate the effect of uric acid-lowering drugs to renal function, which may result in an underestimation of mortality, we have performed multivariate regression with different GFR levels and the results kept unchanged.
Sex differences in the relationship between UA levels and death are controversial. A previous study reported that serum UA level was a predictor of all-cause, CVD, and cancer mortality in men24. However, another study reported that both low and high UA levels were predictive of increased mortality in both men and women25. In this study, UA was not identified as an independent risk factor for all-cause, CVD, or cancer mortality. A previous study showed that among patients with eGFR < 60 mL/min/1.73 m2, men had worse prognosis than women26. To reduce the effect of renal dysfunction, we have excluded persons whose eGFR was below 60 mL/min/1.73 m2. The different inclusion criteria of this study may explain the different results regarding sex disparities. Previous surveys showed that severity of NAFLD were associated with mortality27. In this study, UA level was not independently associated with outcomes in all three groups based on the grade of fatty liver, which was in line with Angulo et al. study28.
This study included a larger general population and a longer follow-up period; therefore, the results were relatively robust compared with those of other studies. This study had some limitations. First, the use of UA-lowering drugs was not available in the original database; thus, we were unable to analyse the effects of medication on UA levels. Second, UA levels were tested only at the beginning of the survey. The changes in UA levels and their effects on survival remain unclear.
In conclusion, UA level was not associated with long-term survival in patients with NAFLD without decreased eGFR.
Data availability
The datasets are available online at https://www.cdc.gov/nchs/nhanes/index.htm.
Abbreviations
- NAFLD:
-
Non-alcoholic fatty liver disease
- UA:
-
Uric acid
- NHANES III:
-
Third National Health and Nutrition Examination Survey
- eGFR:
-
Estimated glomerular filtration rate
- BMI:
-
Body mass index
- WHR:
-
Waist-to-hip ratio
- FPG:
-
Fasting plasma glucose
- HBA1C:
-
Glycated haemoglobin
- TCHO:
-
Total cholesterol
- TG:
-
Triglycerides
- HDL:
-
High-density lipoprotein cholesterol
- CR:
-
Creatinine
- BUN:
-
Blood urea nitrogen
- AST:
-
Aspartate transaminase
- ALT:
-
Alanine aminotransferase
References
Blond, E. et al. EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. Diabetologia 59, 1121–1140. https://doi.org/10.1007/s00125-016-3902-y (2016).
Cotter, T. G. & Rinella, M. Nonalcoholic fatty liver disease 2020: The state of the disease. Gastroenterology 158, 1851–1864. https://doi.org/10.1053/j.gastro.2020.01.052 (2020).
Bao, T. et al. Association between serum uric acid and nonalcoholic fatty liver disease in nonobese postmenopausal women: A cross-sectional study. Sci. Rep. 10, 10072. https://doi.org/10.1038/s41598-020-66931-9 (2020).
Naomi, N. D. et al. Sugar-sweetened beverages, low/no-calorie beverages, fruit juice and non-alcoholic fatty liver disease defined by fatty liver index: The SWEET project. Nutr. Diabetes 13, 6. https://doi.org/10.1038/s41387-023-00237-3 (2023).
Wang, L., Yi, J., Guo, J. & Ren, X. Weigh change across adulthood is related to the presence of NAFLD: Results from NHANES III. J. Transl. Med. 21, 142. https://doi.org/10.1186/s12967-023-04007-8 (2023).
Konta, T. et al. Association between serum uric acid levels and mortality: A nationwide community-based cohort study. Sci. Rep. 10, 6066. https://doi.org/10.1038/s41598-020-63134-0 (2020).
Li, Y., Xu, C., Yu, C., Xu, L. & Miao, M. Association of serum uric acid level with non-alcoholic fatty liver disease: A cross-sectional study. J. Hepatol. 50, 1029–1034. https://doi.org/10.1016/j.jhep.2008.11.021 (2009).
Ryu, S., Chang, Y., Kim, S. G., Cho, J. & Guallar, E. Serum uric acid levels predict incident nonalcoholic fatty liver disease in healthy Korean men. Metabolism 60, 860–866. https://doi.org/10.1016/j.metabol.2010.08.005 (2011).
Xu, C., Yu, C., Xu, L., Miao, M. & Li, Y. High serum uric acid increases the risk for nonalcoholic Fatty liver disease: A prospective observational study. PLoS ONE 5, e11578. https://doi.org/10.1371/journal.pone.0011578 (2010).
Petta, S., Cammà, C., Cabibi, D., Di Marco, V. & Craxì, A. Hyperuricemia is associated with histological liver damage in patients with non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 34, 757–766. https://doi.org/10.1111/j.1365-2036.2011.04788.x (2011).
Levey, A. S. et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 150, 604–612. https://doi.org/10.7326/0003-4819-150-9-200905050-00006 (2009).
Ciarla, S. et al. Serum uric acid levels and metabolic syndrome. Arch. Physiol. Biochem. 120, 119–122. https://doi.org/10.3109/13813455.2014.924145 (2014).
Ndrepepa, G. Uric acid and cardiovascular disease. Clin. Chim. Acta 484, 150–163. https://doi.org/10.1016/j.cca.2018.05.046 (2018).
Taniguchi, Y. et al. Serum uric acid and the risk for hypertension and Type 2 diabetes in Japanese men: The Osaka Health Survey. J. Hypertens. 19, 1209–1215. https://doi.org/10.1097/00004872-200107000-00005 (2001).
King, C. et al. Uric acid as a cause of the metabolic syndrome. Contrib. Nephrol. 192, 88–102. https://doi.org/10.1159/000484283 (2018).
Xu, J., Liu, C., Fu, L., Li, L. & Wang, T. The association of serum uric acid with metabolic syndrome and its components-From a single-clinical centre in China. Int. J. Clin. Pract. 75, e13845. https://doi.org/10.1111/ijcp.13845 (2021).
Zhou, Y., Wei, F. & Fan, Y. High serum uric acid and risk of nonalcoholic fatty liver disease: A systematic review and meta-analysis. Clin. Biochem. 49, 636–642. https://doi.org/10.1016/j.clinbiochem.2015.12.010 (2016).
Sun, D. Q. et al. Serum uric acid: A new therapeutic target for nonalcoholic fatty liver disease. Expert Opin. Ther. Targets 20, 375–387. https://doi.org/10.1517/14728222.2016.1096930 (2016).
Adnan, E., Rahman, I. A. & Faridin, H. P. Relationship between insulin resistance, metabolic syndrome components and serum uric acid. Diabetes Metab. Syndr. 13, 2158–2162. https://doi.org/10.1016/j.dsx.2019.04.001 (2019).
Bagry, H. S., Raghavendran, S. & Carli, F. Metabolic syndrome and insulin resistance: Perioperative considerations. Anesthesiology 108, 506–523. https://doi.org/10.1097/ALN.0b013e3181649314 (2008).
Gobato, A. O., Vasques, A. C., Zambon, M. P., Barros FilhoAde, A. & Hessel, G. Metabolic syndrome and insulin resistance in obese adolescents. Rev. Paulista Pediatr. 32, 55–62. https://doi.org/10.1590/s0103-05822014000100010 (2014).
Choi, H. K. & Ford, E. S. Prevalence of the metabolic syndrome in individuals with hyperuricemia. Am. J. Med. 120, 442–447. https://doi.org/10.1016/j.amjmed.2006.06.040 (2007).
Sharaf El Din, U. A. A., Salem, M. M. & Abdulazim, D. O. Uric acid in the pathogenesis of metabolic, renal, and cardiovascular diseases: A review. J. Adv. Res. 8, 537–548. https://doi.org/10.1016/j.jare.2016.11.004 (2017).
Chang, D. Y., Wang, J. W., Chen, M., Zhang, L. X. & Zhao, M. H. Association between serum uric acid level and mortality in China. Chin. Med. J. 134, 2073–2080. https://doi.org/10.1097/cm9.0000000000001631 (2021).
Cho, S. K., Chang, Y., Kim, I. & Ryu, S. U-shaped association between serum uric acid level and risk of mortality: A cohort study. Arthritis Rheumatol. 70, 1122–1132. https://doi.org/10.1002/art.40472 (2018).
Swartling, O. et al. CKD progression and mortality among men and women: A nationwide study in Sweden. Am. J. Kidney Dis. 78, 190-199.e191. https://doi.org/10.1053/j.ajkd.2020.11.026 (2021).
Simon, T. G., Roelstraete, B., Khalili, H., Hagström, H. & Ludvigsson, J. F. Mortality in biopsy-confirmed nonalcoholic fatty liver disease: Results from a nationwide cohort. Gut 70, 1375–1382. https://doi.org/10.1136/gutjnl-2020-322786 (2021).
Angulo, P. et al. Liver fibrosis, but no other histologic features, Is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 149, 389-397.e310. https://doi.org/10.1053/j.gastro.2015.04.043 (2015).
Acknowledgements
The authors thank the National Health and Nutrition Examination Survey program for providing the data for this study.
Funding
This study was supported by Fund for nurse innovation research (No. 2022FY-HZ-20) and Natural Science Fundation of Fujian Province (No. 2023J01580).
Author information
Authors and Affiliations
Contributions
X.Y. conducted statistical analysis and drafted the manuscript. S.L., J.H., Y.W., Y.L. and Y.C. edited and revised the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, X., Lin, Y., Huang, J. et al. Serum uric acid levels and prognosis of patients with non-alcoholic fatty liver disease. Sci Rep 14, 5923 (2024). https://doi.org/10.1038/s41598-024-55845-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-55845-5
- Springer Nature Limited