Abstract
Cerebral white matter hyperintensities (WMH) have been associated with subclinical atherosclerosis including coronary artery calcification (CAC). However, previous studies on this association are limited by only cross-sectional analysis. We aimed to explore the relationship between WMH and CAC in elderly individuals both cross-sectionally and longitudinally. The study population consisted of elderly stroke- and dementia-free participants from the community-based Austrian Stroke Prevention Family Study (ASPFS). WMH volume and CAC levels (via Agatston score) were analyzed at baseline and after a 6-year follow-up period. Of 324 study participants (median age: 68 years), 115 underwent follow-up. Baseline WMH volume (median: 4.1 cm3) positively correlated with baseline CAC levels in multivariable analysis correcting for common vascular risk factors (p = 0.010). While baseline CAC levels were not predictive for WMH progression (p = 0.447), baseline WMH volume was associated CAC progression (median Agatston score progression: 27) in multivariable analysis (ß = 66.3 ± 22.3 [per cm3], p = 0.004). Ten of 11 participants (91%) with severe WMH (Fazekas Scale: 3) at baseline showed significant CAC progression > 100 during follow-up. In this community-based cohort of elderly individuals, WMH were associated with CAC and predictive of its progression over a 6-year follow-up. Screening for coronary artery disease might be considered in people with more severe WMH.
Similar content being viewed by others
Introduction
Cerebral white matter hyperintensities (WMH) of presumed vascular origin are considered as a hallmark neuroimaging feature of cerebral small vessel disease and a frequent incidental finding on brain MRI1,2. WMH are well-known to increase the risk for stroke, dementia and permanent disability, and are associated with long-term mortality3. Chronic hypoperfusion and ischemia in white matter regions, attributed to alterations in cerebral arterioles and capillaries, have been identified as key components of small vessel disease-related WMH4,5,6,7
Moreover, WMH have also been associated with subclinical atherosclerotic large vessel disease8,9. In particular coronary artery calcification (CAC), a well-known marker of coronary artery disease, has been related to WMH severity8,9,10,11. Apart from age, genetic predisposition, and classical vascular risk factors, previous studies suggested a more direct pathophysiological link between atherosclerotic large vessel disease and cerebral WMH9,12. Vessel wall stiffening of brain-supplying arteries was suspected to induce a more pulsatile blood flow that causes cerebral tissue damage and promotes WMH9,12,13. Consequently, coronary artery atherosclerosis has been postulated as a potential early indicator of WMH progression9. However, all studies published thus far are limited by their cross-sectional design8,9,10,11.
In this study, our objective was to investigate the association between WMH and coronary atherosclerotic disease, as indicated by CAC, over a long-term follow-up period in a stroke- and dementia-free elderly population.
Results
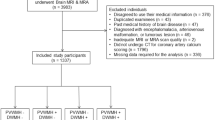
A total of 419 participants was included in the ASPFS in the studied period. Of those, 95 participants had to be excluded due to the unavailability of cardiac CT or brain MRI at baseline (22.7%). This resulted in a final study cohort of 324 participants (median age: 68 years; female: 59.8%) (Fig. 1).
Baseline
Of all included study participants, 75 (23.1%) had moderate/severe WMH on brain MRI at baseline. Median WMH volume was 4.1 cm3 (median PVWMH: 2.9 cm3; median DWMH: 0.9 cm3). Median baseline CAC level measured by the Agatston score was 7 (interquartile range, IQR: 112) and 37 participants fulfilled the criteria for severe calcification of the coronary arteries (Agatston score > 400, 8.8%).
In univariable analysis, WMH volume correlated with age (rs: 0.421, p < 0.001), arterial hypertension (rs: 0.279, p < 0.001), GFR (rs: − 0.189, p < 0.001), lacunes (rs: 0.362, p < 0.001), old cortical infarcts (rs: 0.204, p < 0.001) on baseline MRI, and baseline Agatston score (rs: 0.253, p < 0.001) (Table 1).
In multivariable linear regression analysis, age (ß: 0.19 ± 0.08 [per year], p = 0.011), arterial hypertension (ß: 3.89 ± 1.56, p = 0.015) and baseline Agatston score (ß: 0.60 ± 2.01 [per 100 points], p = 0.010) remained positively associated with baseline WMH volume after adjusting for important co-variables (Table 2). When employing the same model with PVWMH or DWMH volume as the target variable, instead of the total WMH volume, only PVWMH volume exhibited a significant association with the baseline Agatston score (ß: 0.40 ± 0.10, p = 0.008; DMWH, ß: 0.30 ± 0.10, p = 0.079).
Follow-up
115 participants underwent a median long-term follow-up of 5.8 years (range: 5.2–6.4 years) including repeated brain MRI and CT of the coronary arteries. Vascular events occurred in 14 individuals during the follow-up period (12.1%; myocardial infarction: n = 7, stroke: n = 2, TIA: n = 5).
Median progression of the Agatston score during follow-up was 27 (IQR: 177) and median WMH volume progression from baseline to follow-up was 0.4 cm3 (IQR: 0.7; WMH progression of ≥ 1 score points on the Fazekas Scale: n = 24, 20.9%). CAC progression was associated with age (rs = 0.281, p < 0.001), arterial hypertension at baseline (rs = 0.354, p < 0.001), diabetes mellitus (rs = 0.187, p = 0.045), decreased GFR (rs = − 0.235, p = 0.012) as well as baseline WMH volume (rs = 0.383, p < 0.001) and baseline Agatston score (rs = 0.898, p < 0.001) (Table 3). Arterial hyertension as well as increased blood glucose and cholesterol levels during the follow-up period were not associated with CAC progression (p > 0.05 each).
In multivariable linear regression analysis, WMH volume at baseline remained significantly associated with CAC progression (ß = 66.3 ± 22.3 [per cm3], p = 0.004) as the sole variable, in addition to baseline Agatston score (ß = 50.2. ± 6.2 [per 100 points], p < 0.001) (Table 4). Again, the model was run with DWMH and PVWMH instead of total WMH volume: Both WMH subtypes tended to be predictive for CAC progression (DWMH, ß:12.0 ± 7.0, p = 0.097; PVWMH, ß:27.4 ± 16.2, p = 0.096). Of note, 10 of 11 participants with severe baseline WMH according to a Fazekas scale score of 3 had CAC progression of > 100 over the follow-up period (90.9% vs. 28.3%, p < 0.001).
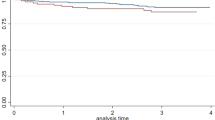
Vice versa, there was no association between CAC at baseline and WMH progression during the follow-period (rs = − 0.075, p = 0.447, univariable analysis) and even high baseline CAC scores > 400 (n = 29) did not predict a significant WMH progression during the follow-up period (WMH progression: 1.19 ± 1.66 vs. 1.07 ± 2.62, p = 0.884) (Fig. 2). Similarly, no associations were found between baseline CAC scores and WMH progression in both WMH subtypes (PVWMH progression: rs = − 0.092, p = 0.672; DWMH progression: rs = − 0.022, p = 0.801).
Discussion
In this cohort of stroke- and dementia-free elderly people, WMH volume on brain MRI was associated with calcification of the coronary arteries at baseline and predictive for its progression over long-term follow-up. Individuals with severe WMH, as indicated by a Fazekas score of 3, are at high risk for a substantial progression of CAC.
Our results are of interest as previous studies led to speculations about the mechanisms behind the association of cerebral WMH—a hallmark feature of cerebral small vessel disease—and (subclinical) large vessel atherosclerosis such as CAC8,9,10,11. A combination of shared classical vascular risk factors (predominantly arterial hypertension) and a genetic predisposition was suspected behind this phenomenon8,9,10,11. In this context, Johansen et al. identified differences in the strengths of the relationship between CAC and different WMH subtypes9. The more pronounced association observed between CAC and PVWMH, as opposed to DWMH, might be attributed to the typical vascular architecture of PVWMH-related short penetrating microvessels. These vessels may be more directly affected by arterial hypertension compared to the longer microvessels that supply the deep white matter14,15. Atherosclerotic changes in the penetrating branches of the large intracranial arteries might lead to hypoperfusion and ischemia, representing a key mechanism underlying WMH development16. In addition, similar genetic variations were identified in patients with calcification of coronary arteries and a high burden of PVWMH17.
In our analysis, PVWMH also showed a stronger correlation with baseline CAC levels (in multivariable analysis), but the difference between both WMH subtypes was rather small in absolute numbers. Future studies might analyze ultrastructural white matter changes using diffusion tensor imaging (DTI)18 and their association with macrovascular disease to improve the pathophysiological understanding of the presented association.
The unique longitudinal design of this study further allowed us to investigate potential associations between CAC as marker of (subclinical) atherosclerotic large vessel disease and WMH over a long-term follow-up period of 6 years. Apart from genetics and shared vascular risk factors, previous studies indicated a more direct link explaining the association between CAC and WMH. As atherosclerotic large vessel disease results in the stiffening of arteries, it is hypothesized that the subsequent increase in blood-flow pulsatility within the brain-supplying vessels directly damages the small cerebral vasculature, thereby promoting WMH9,12,13. For this reason, CAC was assumed to predict cerebral WMH progression, but previous studies only had cross-sectional data available8,9,10,11.
Our results do not support this hypothesis as we did not identify an association between CAC levels at baseline and WMH progression (neither total WMH nor WMH subtypes) during the follow-up period. Moreover, even participants with severe calcification of the coronary arteries at baseline (CAC score > 400) did not show a more pronounced WMH progression.
These results align with recently published data that failed to establish an association between intracranial pulsatility, measured in the middle cerebral artery, and both WMH volume at baseline and WMH progression during follow-up19.
Most notably, we found that baseline WMH volume predicted CAC progression during the follow-up period. Specifically, ten out of eleven participants with severe baseline WMH, as indicated by a Fazekas score of 3, showed substantial CAC progression of > 100. PVWMH and DWMH had a similar predictive value for CAC progression. Apart from a shared genetic predisposition, classical vascular risk factors (i.e., arterial hypertension) might be important factors behind this relation. In this context, all participants with severe baseline WMH (Fazekas 3) and CAC progression during follow-up also had underlying arterial hypertension. Our results therefore point towards a subgroup of WMH patients, in which intense and continuous vascular risk factor control might be crucial to avoid further damage of the brain, but also to avoid macrovascular changes in other vascular beds such as the coronary arteries. Moreover, treating physicians should be aware of signs and symptoms of cardiac disease in patients with high WMH load and initiate cardiological exploration or even coronary artery disease screening in case of any clinical suspicion.
Limitations
Our study is limited by the fact that only a subgroup of study participants (27%) underwent follow-up brain MRI and coronary artery CT (n = 115). However, this should not have influenced our results to a relevant extent as there were no differences in demographics, vascular risk factors and CAC or WMH volume at baseline between patients with and those without follow-up (p > 0.1, data not shown). Based on the small-sized follow-up cohort, we cannot exclude that we have overlooked a (small) predictive value of baseline CAC levels on WMH progression. We also only observed few outcome events in this study not allowing to report on the predictive value of WMH, CAC and associated vascular events, which should be addressed in larger prospective studies.
Conclusions
This study reinforces the correlation between cerebral WMH and large artery atherosclerosis. Moreover, WMH serve as predictors for the progression of coronary artery disease during a long-term follow-up period. Clinicians should be aware of this observed association and may consider to screen individuals with severe WMH for coronary artery disease. Intense control of vascular risk factors is essential for all such patients.
Materials and methods
All methods were performed in accordance with the relevant guidelines and regulations.
The study was approved by the ethics committee of the Medical University of Graz (Approval number: 17-088 ex 05/06). Written informed consent was obtained by all included study participants.
Selection of participants and data collection
All included study participants derive from the Austrian Stroke Prevention Family Study (ASPFS), an extension of the Austrian Stroke Prevention Study (ASPS). ASPFS is a prospective population-based study that was designed to assess the effects of vascular risk factors on brain structure, function and vessel damage in different vascular beds20. Between 2006 and 2013, study participants of the original study—ASPS—and their first-degree relatives were invited to enter the ASPFS study. Inclusion criteria included the absence of a history of cerebrovascular disease (stroke or transient ischemic attack) or dementia, as well as a normal neurological examination.
This is a single-center study. All participants were recruited at the University Hospital of Graz, Austria.
Baseline and follow-up assessments comprised blood pressure measurements, laboratory tests of vascular risk factors (such as blood glucose and serum lipid levels), a clinical evaluation of comorbidities as well as coronary artery computed tomography (CT) and brain magnetic resonance imaging (MRI). Vascular risk factors at baseline were defined according to latest guideline recommendations based on documented parameters (hypertension: systolic blood pressure > 140 mmHg, diastolic blood pressure > 90 mmHg; diabetes: fasting plasma glucose > 126 mg/dl; dyslipidemia: LDL cholesterol ≥ 116 mg/l) or if patients were already treated with respective medication (such as antihypertensives or statins)21,22,23. The guideline-based initiation of dedicated treatments was always verified by the study team. During the follow-up period, the same thresholds for increased blood pressure, hyperglycemia and dyslipidemia were used to identify patients with poor vascular risk factor control21,22,23.
Brain imaging
All brain MRI investigations were performed on a 3.0 T scanner (TimTrio; Siemens Healthcare, Erlangen, Germany) including standard T1- and T2-weighted pulse sequences (slice thickness = 3 mm), Fluid Attenuated Inversion Recovery (FLAIR; TR = 10,000 ms, TE = 69 ms, inversion time = 2500 ms) and high resolution T1 weighted rapid acquisition gradient-echo (MPRAGE; TR = 1900 ms, TE = 2.19 ms, inversion time = 900 ms).
All MRI scans were reviewed by blinded neuroradiological experts (C.E., R.S.). WMH, lacunes and (chronic) cortical infarcts were assessed on T2 and FLAIR images. WMH severity was rated according to the Fazekas rating scale in deep and periventricular locations24. For quantitative assessment, WMH areas were first segmented manually and consecutively added to a total lesion volume using FMRIB Software Library (FMRIB, Oxford, UK; freely available at https://fsl.fmrib.ox.ac.uk)25.
Coronary artery calcification
Participants underwent cardiac CT with a 64-channel multidetector computed tomography (GE Imatron, San Francisco, USA) at baseline and follow-up. ECG triggering was used at 80% of the cardiac cycle to obtain images (slice thickness: 3 mm; image acquisition time: 100 ms). CAC was defined as a minimum of three contiguous pixels with a CT density ≥ 130 Hounsfield units. An experienced radiologist (GR) specialized in cardiac CT imaging and blinded to clinical data read all the CT images and calculated CAC scores according to the Agatston method26.
Patient and public involvement
Patients or the public were not involved in the design, conduct, reporting or dissemination plans of our research.
Statistics
Statistical analyses were performed using IBM SPSS Statistics, version 28. Aside from analyzing WMH volumes and CAC levels as continuous variables, WMH were dichotomized using the Fazekas visual grading scale scores 0–1 (i.e., no or mild WMH) versus 2–3 (i.e., moderate to severe WMH))27. If WMH in either deep (DWMH) or periventricular (PVWMH) location were graded as Fazekas 2 or higher, they were classified as moderate to severe WMH. In a second step, participants were divided according to CAC score severity (quartile 4 versus quartiles 1–3). Pearson’s chi-square or Fisher’s exact test was used to compare dichotomous variables. All quantitative variables were first tested for Gaussian distribution with the Kolmogorov–Smirnov test and, if Gaussian distribution was identified, a two-sample independent t-test was utilized to compare the variables. The Mann–Whitney-U-Test was used for non-parametric data.
As WMH volumes and CAC levels at baseline and follow-up were not normally distributed, Spearman’s rank correlation was performed for bivariable correlations including these parameters. A p-value less than 0.05 was considered statistically significant.
A multivariable linear regression model was fitted to identify factors that were independently associated with WMH volume and CAC levels at baseline.
Besides age and sex, the model included variables that were related to baseline WMH (target variable) and baseline CAC volume in univariable analysis (p < 0.05): arterial hypertension, diabetes and glomerular filtration rate (GFR). However, after testing for multicollinearity and interactions, GFR was removed from the multivariable analysis because of its strong correlation with age (variance inflation index > 10).
In a second step, the same model was again calculated with DWMH and PVWMH as the target variables (instead of total WMH volume) to test for the influence of different WMH subtypes.
For follow-up analyses, predictors for WMH or CAC progression were first analyzed via univariable statistics as described above. Based on these analyses, CAC progression was set as the target variable in a multivariable linear regression analysis. The model included age and sex, and further variables that were predictive for CAC progression in previous studies or in univariable analysis (arterial hypertension, diabetes, GFR, baseline WMH volume)8,9,10,11. Again, GFR had to be excluded based on a variance inflation index > 10 and the model was also recalculated twice including baseline volume of DWMH and PVWMH instead of (total) baseline WMH volume.
Data availability
Study data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Moran, C., Phan, T. G. & Srikanth, V. K. Cerebral small vessel disease: A review of clinical, radiological, and histopathological phenotypes. Int. J. Stroke 7, 36–46 (2012).
Debette, S. & Markus, H. S. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: Systematic review and meta-analysis. BMJ. 341, c3666 (2010).
Georgakis, M. K., Duering, M., Wardlaw, J. M. & Dichgans, M. WMH and long-term outcomes in ischemic stroke: A systematic review and meta-analysis. Neurology 92, e1298–e1308 (2019).
Wardlaw, J. M., Smith, C. & Dichgans, M. Mechanisms of sporadic cerebral small vessel disease: Insights from neuroimaging. Lancet Neurol. 12, 483–497 (2013).
Wardlaw, J. M. et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration: A united approach. Lancet Neurol. 12, 822–838 (2013).
Brickman, A. M. et al. Long-term blood pressure fluctuation and cerebrovascular disease in an elderly cohort. Arch. Neurol. 67, 564–569 (2010).
Dufouil, C. et al. Longitudinal study of blood pressure and white matter hyperintensities: The EVA MRI cohort. Neurology. 56, 921–926 (2001).
Choi, J. et al. Association of cerebral white matter hyperintensities with coronary artery calcium in a healthy population: A cross-sectional study. Sci. Rep. 12, 21562 (2022).
Johansen, M. C. et al. Association of coronary artery atherosclerosis with brain white matter hyperintensity. Stroke. 52, 2594–2600 (2021).
Bos, D. et al. Calcification in major vessel beds relates to vascular brain disease. Arterioscler. Thromb. Vasc. Biol. 31, 2331–2337 (2011).
Chen, Y. C. et al. Correlation between intracranial arterial calcification and imaging of cerebral small vessel disease. Front. Neurol. 10, 426 (2019).
Mitchell, G. F. et al. Arterial stiffness, pressure and flow pulsatility and brain structure and function: The age Gene/Environment Susceptibility-Reykjavik study. Brain. 134, 3398–3407 (2011).
Shi, Y., Thrippleton, M. J., Marshall, I. & Wardlaw, J. M. Intracranial pulsatility in patients with cerebral small vessel disease: A systematic review. Clin. Sci. 132, 157–171 (2018).
Blanco, P. J., Müller, L. O. & Spence, J. D. Blood pressure gradients in cerebral arteries: A clue to pathogenesis of cerebral small vessel disease. Stroke Vasc. Neurol. 2, 108–117 (2017).
Wardlaw, J. M. et al. Standards for Reporting Vascular Changes on Neuroimaging (STRIVE v1). Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 12, 822–838 (2013).
Nam, K. W. et al. Cerebral white matter hyperintensity is associated with intracranial atherosclerosis in a healthy population. Atherosclerosis. 265, 179–183 (2017).
Armstrong, N. J. et al. Common genetic variation indicates separate causes for periventricular and deep white matter hyperintensities. Stroke. 51, 2111–2121 (2020).
Pasi, M., van Uden, I. W., Tuladhar, A. M., de Leeuw, F. E. & Pantoni, L. White matter microstructural damage on diffusion tensor imaging in cerebral small vessel disease: Clinical consequences. Stroke. 47(6), 1679–1684 (2016).
Kneihsl, M. et al. Intracranial pulsatility in relation to severity and progression of cerebral white matter hyperintensities. Stroke. 51, 3302–3309 (2020).
Schmidt, R. et al. Assessment of cerebrovascular risk profiles in healthy persons: Definition of research goals and the Austrian stroke prevention study (ASPS). Neuroepidemiology. 13, 308–313 (1994).
Williams, B. et al. ESC Scientific Document Group. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur. Heart J. 39, 3021–3104 (2018).
Chamberlain, J. J., Rhinehart, A. S., Shaefer, C. F. Jr. & Neuman, A. Diagnosis and management of diabetes: Synopsis of the 2016 American Diabetes Association standards of medical care in diabetes. Ann. Intern. Med. 164, 542–552 (2016).
Grundy, S. M. et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 73, e285–e350 (2019).
Fazekas, F. et al. White matter signal abnormalities in normal individuals: Correlation with carotid ultrasonography, cerebral blood flow measurement, and cerebrovascular risk factors. Stroke 19, 1285–1288 (1988).
Seiler, S. et al. Magnetization transfer ratio relates to cognitive impairment in normal elderly. Front. Aging Neurosci. 6, 263 (2014).
Agatston, A. S. et al. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 15, 827–832 (1990).
Schmidt, R., Enzinger, C., Ropele, S., Schmidt, H. & Fazekas, F. Austrian Stroke Prevention Study Progression of cerebral white matter lesions: 6-year results of the Austrian Stroke Prevention Study. Lancet. 361(9374), 2046–2048 (2003).
Author information
Authors and Affiliations
Contributions
M.K.: conceptualization, data curation, formal analysis, writing—original draft; T.G.: conceptualization, methodology, project administration, supervision; E.H.: formal analysis; P.R.: Writing—review and editing; G.R.: data curation, writing—review and editing; S.F.H.: writing—review and editing; M.H.: writing—review and editing; S.P.: methodology; C.E.: writing—review and editing; R.S.: conceptualization, methodology, project administration, writing—review and editing, supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kneihsl, M., Gattringer, T., Hofer, E. et al. Cerebral white matter hyperintensities indicate severity and progression of coronary artery calcification. Sci Rep 14, 4664 (2024). https://doi.org/10.1038/s41598-024-55305-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-55305-0
- Springer Nature Limited