Abstract
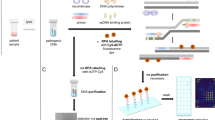
Fluorescent molecule-based direct labeling of amplified DNA is a sensitive method employed across diverse DNA detection and diagnostics systems. However, using pre-labeled primers only allows for the attachment of a single fluorophore to each DNA strand and any modifications of the system are less flexible, requiring new sets of primers. As an alternative, direct labeling of amplified products with modified nucleotides is available, but still poorly characterized. To address these limitations, we sought a direct and adaptable approach to label amplicons produced through Loop-mediated isothermal amplification (LAMP), using labeled nucleotides (dUTPs) rather than primers. The focus of this study was the development and examination of a direct labeling technique of specific genes, including those associated with drug resistance in Mycobacterium tuberculosis. We used 5-(3-Aminoallyl)-2′-deoxyuridine-5′triphosphate, tagged with 5/6-TAMRA (TAMRA-dUTP) for labeling LAMP amplicons during the amplification process and characterized amplification and incorporation efficiency. The optimal TAMRA-dUTP concentration was first determined based on amplification efficiency (0.5% to total dNTPs). Higher concentrations of modified nucleotides reduced or completely inhibited the amplification yield. Target size also showed to be determinant to the success of amplification, as longer sequences showed lower amplification rates, thus less TAMRA incorporated amplicons. Finally, we were able to successfully amplify all four M. tuberculosis target genes using LAMP and TAMRA-modified dUTPs.
Similar content being viewed by others
Introduction
As molecular diagnostics continue to evolve, isothermal amplification methods have emerged in the field of nucleic acid detection with the key advantage of conducting amplification at a constant temperature1. These isothermal techniques include, for example, recombinase polymerase amplification (RPA), helicase dependent amplification (HDA), as well as loop-mediated isothermal amplification (LAMP)2.
Since its development in 20003, loop-mediated isothermal amplification (LAMP), has been widely implemented as an alternative to conventional Polymerase Chain Reaction (PCR), particularly for point-of-care testing, as it obviates the need for a thermocycler to facilitate denaturation and temperature cycling. LAMP depends on 4–6 primers and a strong strand displacement polymerase to amplify the target DNA sequence, which allows for amplification at high specificity, sensitivity, and rapidity. LAMP has demonstrated the ability to attain comparable or even lower detection thresholds than PCR for specific targets4.
Traditional detection methods of LAMP products include visual detection methods, such as turbidimetry using ion indicators, agarose gel electrophoresis and observation of color change in the reaction mixture, with fluorescent intercalating dyes like SYBR Green I5. However, these methods are unable to differentiate between amplification of the desired sequence and spurious amplification occurring for example from primer-dimers6.
Various detection methods are employed alongside loop-mediated isothermal amplification (LAMP) to identify amplified products. Notably, these methods include coupling LAMP outputs with detection techniques like lateral flow assays (LFA)7,8 and microarrays9. This application is particularly suited for targeted detection, emphasizing the differentiation of mutations or single-nucleotide differences within LAMP products. Such approaches typically involve pre-labeled primers10,11,12 or probes12,13.
An alternative, less common method involves labeling the target during amplification using pre-labeled nucleotides, available with modifications like fluorophores and biotin. Some studies have examined the use of specific modified nucleotides, such as biotin-dUTP7,9, Cy5-dUTP9, FITC-dUTP14, and digoxigenin DIG-dUTP15 for direct LAMP labeling. Implementing nucleotide-based labeling can substantially enhance labeling efficiency by increasing the incorporation rates of labeling molecules, thereby increasing assay sensitivity9,16, in comparison to pre-labeled primers or probes which result in one label per amplicon. A further added advantage of this approach is its flexibility, as it eliminates the need to pre-label primer sets or probes when altering targets, labeling, or detection protocols17.
The incorporation of labeled nucleotides with distinct properties during LAMP has been noted in earlier research to yield differing impacts on both amplification efficiency and the resulting products9. This underscores the importance of investigating the influence of diverse modified-dUTP incorporations during a LAMP reaction.
In this context, our study seeks to examine the direct and flexible labeling of LAMP amplicons using 5-(3-Aminoallyl)-2′-deoxyuridine-5′triphosphate, labeled with 5/6-TAMRA (TAMRA-dUTP) during the amplification process. We compare the labeling and amplification efficiency across different Mycobacterium tuberculosis genes, including genes associated with drug resistance, by employing real-time LAMP (rtLAMP) and spectrofluorometric analysis.
Tuberculosis (TB) is a bacterial infection caused by Mycobacterium tuberculosis. While it is a curable infection, it constitutes a health threat due to the presence of multidrug-resistant strains, which renders it unsusceptible to certain anti-TB agents, making treatment of drug resistant M. tuberculosis challenging18. Furthermore, the diagnosis of multidrug-resistant tuberculosis is currently conducted through drug sensitivity testing (DST) methods, which are both time-consuming and poorly available in resource-limited settings19. Therefore, rapid, and accurate detection of drug resistant M. tuberculosis holds vital importance as it addresses a growing global health concern. It is deemed crucial for devising appropriate treatment approaches and plays a pivotal role in curbing its transmission19,20.
Drug resistance in M. tuberculosis occurs due to genetic mutations, such as single nucleotide polymorphisms (SNPs), insertions, or deletions in specific genes21,22. The gene sequences used in this study were 16S rRNA, a highly conserved bacterial sequence that has been widely used for bacterial identification and classification23,24,25; IS6110, an insertion sequence found in the M. tuberculosis complex species26; katG and pncA, both genes with mutations associated with M. tuberculosis resistance against isoniazid (INH) and pyrazinamide (PZA)27 drugs, respectively.
Results
Finding the appropriate TAMRA-dUTP amount
Various amounts of TAMRA-dUTP were used for LAMP labeling of the 16S rRNA gene, a highly conserved bacterial sequence in M. tuberculosis genomic DNA strain. This was done to determine the appropriate TAMRA-dUTP concentration needed for efficient labeling during LAMP amplification. A percentage of 0.5–4% (10–80 µM) of labeled nucleotide and 2 mM unlabeled ones were used.
Through SYBR Green based real-time Loop-Mediated Isothermal Amplification (rtLAMP) (Fig. 1a), it was observed that the reactions containing TAMRA-dUTP exhibited a delayed onset of genomic DNA amplification compared to reactions without it. Additionally, this delay was found to be directly proportional to the concentration of labeled nucleotides, with increased concentrations of TAMRA-dUTP leading to further postponements in the initiation of the LAMP reaction. We observed that higher TAMRA concentrations completely inhibited the amplification reaction.
Determination of appropriate TAMRA-dUTP amount. LAMP reactions were performed for 20 min (65°C), using the 16S rRNA gene in M. tuberculosis DNA strain as target. (a: left) SYBR Green based rtLAMP curves for amplification of 1.00E + 05 copies/µl DNA target per reaction, with various ratios of TAMRA-dUTP (0.5–4%). (NTC: no template control, unlabeled), 0% TAMRA-dUTP: positive control, unlabeled). (a: right) Onset-values and corresponding Onset-value shifts performing amplification with unlabeled and x% TAMRA-dUTP-labeled nucleotides. (b) Gel electrophoresis and (c) graphical visualization of fluorescence signals with purified amplicons after LAMP reaction with different TAMRA-dUTP ratios compared to the unlabeled reaction (NTC no template control).
This was shown by the rtLAMP Onset-value shift from 8.23 min for the positive control (no TAMRA-dUTP) and 13.42 min using 0.5% TAMRA-dUTP to 19.45 min using 1% TAMRA-dUTP (Fig. 1a).
Sigmoidal curves could be observed with SYBR green fluorescence intensities, which showed a reduction in amplification efficiency that corresponded directly to the labeled nucleotide concentration. Highest fluorescence intensity was associated with the positive control (no TAMRA-dUTP), with subsequent declines observed in direct response to the increased concentrations of labeled nucleotides used in each LAMP reaction.
Examination of purified amplicons with various concentrations of labeled nucleotides by gel electrophoresis and fluorescence intensity (relative fluorescence units, rfu) revealed a consistent pattern of inhibition in the LAMP reaction using 2% and 4% TAMRA-dUTP (Fig. 1b,c) Conversely, the use of 0.5% TAMRA-dUTP resulted in no observable inhibition of the reaction and a higher fluorescence signal intensity of TAMRA labeled amplicons, while a concentration of 1% TAMRA-dUTP led to the production of only small amounts of target DNA, which was indicated with both the intensity of the bands on the gel and the lower fluorescence signal intensity of the TAMRA incorporated (Fig. 1b,c).
A lower concentration of TAMRA-dUTP (0.375%) was used to test if amplification efficiency could be increased. It was observed that while the reaction started ~ 3.5 min earlier, the fluorescence signal was lower than reactions with 0.5% TAMRA-dUTP, indicating less incorporation per amplified product (data not shown). Thus, based on these results, a concentration of 0.5% (10 µM) TAMRA-dUTP was used for labeling in further experiments.
Labeling various M. tuberculosis genes
In the second part of the experiments, the method developed with the 16S rRNA gene was applied, using 10 µM (0.5%) TAMRA-dUTP, to test TAMRA incorporation in different M. tuberculosis genes, including genes associated with drug resistance.
For this, genomic DNA of three different strains of M. tuberculosis were used for the LAMP labeling of 16S rRNA and IS6110 insertion sequences, as well as the labeling of katG and pncA genes. The LAMP primers were designed to amplify these genes in both wild-type and resistant M. tuberculosis strains.
The fluorescence signal intensities of amplified gene sequences labeled with TAMRA revealed a range in mean intensities. These ranged from approximately 74,000 relative fluorescence units (rfu) for the IS6110 gene sequence amplicons to 99,000 rfu and 115,000 rfu for the 16S rRNA and katG amplicons, respectively, and reached up to 128,000 rfu for the pncA sequence amplification product (Fig. 2a). Comparing the fluorescence signal intensities with the length of the fragments produced during LAMP showed that the shorter fragments produced higher signal intensities.
Detection of four different M. tuberculosis gene sequences: IS6110, 16S rRNA, katG, and pncA. LAMP reactions were performed for 30 min (65 °C), using 1.00E+05 copies/µl DNA target per reaction, and 0.5% TAMRA-dUTP. (a) Graphical visualization of fluorescence signals and (b) gel electrophoresis with purified amplicons after LAMP reaction with TAMRA-dUTP.
The LAMP products for pncA are the shortest with 162 nucleotides (nt) and a percentage of 17.9% dT, followed by the katG fragments with 209 nt (15.3% dT) and 16S rRNA fragments with 234 nt (19.7% dT). The IS6110 amplicons have the longest fragment length with 273 nt (17.6% dT) (Fig. 2b).
Total TAMRA-dUTP incorporation in each LAMP reaction was quantified using a calibration curve constructed from a serial dilution of free TAMRA-dUTP. Based on the response measurements, the concentrations of TAMRA-dUTP were determined as follows: 1.03 µM in pncA amplicons, 0.93 µM in katG, 0.80 µM in 16S rRNA, and 0.60 µM in the IS6110 amplified product (Fig. 3a).
Detection of TAMRA-dUTP in LAMP amplicons. LAMP reactions were performed for 30 min (65 °C), using 1.00E+05 copies/µl DNA target per reaction, and 0.5% TAMRA-dUTP. Incorporation of fluorescent-labeled nucleotides was analyzed by fluorescence spectroscopy. (a) Calibration curve for calculation of incorporated TAMRA-dUTP in amplified target DNA samples. (b) SYBR Green based graphical representation illustrating the quantity of amplified DNA targets, comparing reactions containing TAMRA-dUTP (w/) to reactions without the inclusion of TAMRA-dUTP (w/o). (c) Graphical visualization of TAMRA intensity signals within target amplicons at a concentration of 100 ng/µl, along with the proportion of thymidine (dT) associated with each amplified DNA fragment.
To further characterize the impact of TAMRA-dUTP incorporation on LAMP reactions, we examined the relative quantity of amplicons generated in reactions incorporating labeled nucleotides versus those without any additives. This comparison was facilitated by measuring the signal intensity resulting from SYBR Green intercalation in amplified products. The findings revealed a reduction of 9.8% in pncA amplification in reactions containing TAMRA-dUTP. Similarly, amplification of katG and 16S rRNA exhibited reductions of 16.2% and 15.4% respectively. Moreover, the analysis determined a decrease of 17.2% in amplification for the IS6110 target gene (Fig. 3b).
Fluorescence intensities resulting from TAMRA incorporation were measured for LAMP-labeled gene targets standardized to 100 ng/µl. The measured intensities were correlated to the percentage of thymidine (dT) within each amplified DNA sequence. Notably, it was observed that the signal intensities of TAMRA were relatively consistent in the labeled DNA targets. Specifically, the mean intensities measured were approximately 44,000 relative fluorescence units (rfu) for both the amplicons of IS6110 (273 bp, 17.6% dT) and pncA (162 bp, 17.9% dT) gene sequences. Slightly increased signals of 46.000 rfu and 57,000 rfu were measured for the katG (209 bp, 15.3% dT) and 16S rRNA (234 bp, 19.7% dT) amplicons, respectively (Fig. 3c).
Determination of reaction sensitivity
A dilution series of M. tuberculosis genomic DNA in a range from 10 to 1.0E+05 DNA copies/µl was used to evaluate the sensitivity of the LAMP labeling reaction. As before, the LAMP was performed by using 0.5% (10 µM) TAMRA-dUTP. The target gene labeled was the 16S rRNA.
More than 100,000 rfu were detected for 1.0E+05 DNA copies/µl of the 16S rRNA target. There was a concentration dependent decrease in the fluorescence signal intensity with different amounts of the target genes corresponding to the decreasing number of DNA copies used for the LAMP reactions. At 10 DNA copies/µl the fluorescence intensity was 25,000 rfu for the 16S rRNA amplified product (Fig. 4).
LOD determination for the 16S rRNA gene. LAMP reactions were performed for 30 min (65 °C), using a serial dilution of 10 to 1.00E+05 copies/µl target genomic DNA per reaction, and 0.5% TAMRA-dUTP. Fluorescence intensities were measured of purified amplicons after LAMP reactions with TAMRA-dUTP. (NTC no template control; Buffer: 1 × Isothermal buffer).
Even with taking the fluorescence of the no template controls (NTC) into consideration, the limit of detection (LOD) for the 16S rRNA gene was as low as 10 DNA copies/µl per reaction.
Discussion
While the substitution of dTTP with modified dUTP during amplification is still not widely adopted, a handful of studies have employed this method to label products of polymerase chain reaction (PCR)28,29, rolling circle amplification (RCA)16, recombinase polymerase amplification (RPA)17,30, and loop-mediated isothermal amplification (LAMP)7,9,14,15 techniques.
Previous studies have demonstrated the modification of amplified products through the utilization of labeled nucleotides. These investigations highlight that the efficiency of amplification, and the incorporation effectiveness are dependent on factors such as the structural features, size, and concentration of the used labeled nucleotides9,16.
In this study we showed the successful incorporation of 5-(3-Aminoallyl)-2′-deoxyuridine-5′triphosphate, labeled with 5/6-TAMRA (TAMRA-dUTP) in four different M. tuberculosis gene sequences directly during the LAMP reaction. To our knowledge, this is the first time TAMRA-dUTP has been used in direct labeling during the LAMP process.
Performing different detection methods such as rtLAMP, fluorescence spectroscopy and agarose gel electrophoresis, we were able to characterize TAMRA-dUTP incorporation into amplicons during LAMP reaction. Furthermore, the effect of the labeled nucleotides on the LAMP process and the ratio of incorporation were analyzed.
Warmt et al. demonstrated a positive correlation between the fluorescence intensity of the amplicons in LAMP and the increasing concentration of labeled nucleotides. In that study, the quantities of Cy5-dUTP employed per LAMP reaction ranged from 4 (0.28%) to 200 µM (12.5%). Notably, employing 1.5% Cy5-dUTP introduced a 3.5-min delay in the LAMP reaction. The study suggested an optimal ratio of 1–2% of fluorescence-labeled dUTP, striking a balance between fluorescence intensity and the speed of amplification.
Similarly, in this study, the best ratio of labeled nucleotides used in a LAMP reaction was determined to be 10 µM (0.5%) TAMRA-dUTP to 2 mM non-labeled nucleotides. A delay in the onset of the amplification reaction was observed to be an effect of using higher concentrations of labeled nucleotides. An Onset-value shift of more than 11.00 min from the non-labeled LAMP reaction to 1% amount of TAMRA-dUTP per reaction in samples was observed. While using 2% or higher concentrations was seen to inhibit LAMP reaction completely. Based on that, TAMRA-dUTP/dTTP substitution rate was assumed to be 1 per 200 dTTP when using 0.5% labeled dUTP during the LAMP reaction.
Comparable inhibitory effects were observed in rolling circle amplification (RCA) reactions with incorporation of (sulfo-Cy3-dUTP, sulfo-Cy5-dUTP, sulfo-Cy5.5-dUTP, BDP-FL-dUTP, and NH2-dUTP) labeled nucleotides. It was reported that reaction yields decreased with an increasing percentage of labeled nucleotides. Consequently, an optimal percentage of each labeled nucleotide had to be determined to avert the observed reduction in reaction efficiency16.
M. tuberculosis genome has a high GC content (65.6%)31, this is also the case in all genes targeted in this study. Amplifying GC-rich templates are reported to be more challenging compared to non-GC-rich targets due to their high melting temperatures (Tm)32, making the GC-rich DNA target, as well as the primers more prone to hairpin or secondary structure formation, thus interfering in the amplification process33,34.
Four different gene sequences in M. tuberculosis were successfully labeled using TAMRA-dUTP in LAMP. Amplification efficiency and incorporation rate were compared between these four DNA targets. It was observed that the total TAMRA fluorescence intensity depends highly on the length of the amplified fragment. The shortest amplicon (pncA, 162 bp) had more than double the fluorescence signal intensity compared to the longest amplified fragment (IS6110, 273 bp). To further analyze the total incorporated TAMRA-dUTP in each amplification reaction of the target genes, a calibration curve was constructed from the measurement of the signal intensity of a serial dilution of the free labeled nucleotide. We observed that LAMP reactions of the shortest fragment had 40% more TAMRA-dUTP when compared to the longest fragment. Further results determined that the variation in TAMRA signals stemmed from differing amplification rates associated with the length of the target DNA product.
Similar results have been reported in the literature, evaluating the fragment length in recombinase polymerase amplification (RPA) reactions to fluorescent signal intensity on incorporated Cy5-labeled nucleotides30. It was observed that the shortest RPA fragment (141 bp) gave a 20-fold higher fluorescence signal compared to the largest fragment (809 bp).
Furthermore, this study revealed that the incorporation rate of TAMRA-dUTP was relatively consistent regardless of the length of the amplified target gene. However, the length of the amplicon significantly influences amplification efficiency, correlating with the quantity of generated products. Amplification of the DNA target was seen to increase as the fragment length decreased. Consequently, this gives rise to varying quantities of labeled amplicons generated with distinct DNA targets. This was evident through the measurement of TAMRA signal intensities in target DNA products with equal concentrations. Notably, it was seen that there was a slight increase in the signal intensity of the 16S rRNA gene sequence amplicon (Fig. 3c), which could be related to the higher proportion of thymidine (dT) within the DNA sequence, compared to the other DNA targets.
Length of the amplified product can indeed impact both amplification efficiency and the quantity of amplicons produced35,36. The effectiveness of LAMP is influenced by the size of the target DNA due to the involvement of strand displacement DNA synthesis as a rate-limiting step in this method. It has been determined that optimal outcomes were achieved with DNA target size smaller than 200 base pairs, while DNA of more than 500 bp amplified, but very poorly3.
Additionally, upon conducting a comparative analysis of amplification levels in LAMP reactions that incorporated labeled vs. unlabeled nucleotides, it became evident that the incorporation of TAMRA-dUTP led to an overall reduction in amplification efficiency. This reduction in LAMP products ranged from 9 to 17% when compared with unlabeled reactions. These results reinforce the observed impact of TAMRA-dUTP incorporation in diminishing the efficiency of amplification within LAMP reactions.
The assessment of sensitivity and efficacy of the LAMP reaction while labeling lower DNA copy numbers involved using serial dilutions of M. tuberculosis DNA. The results indicated that the labeled amplification reaction remained functional even at a minimum of 10 DNA copies per reaction. This finding underscores that the utilized concentration of 10 µM TAMRA-dUTP was satisfactory for effective labeling of M. tuberculosis even at low copy numbers. Importantly, this concentration of labeled nucleotide did not impede the reaction's progression despite the lower amount of target DNA in the reaction.
This study showed the successful integration of the TAMRA fluorophore into four distinct target genes of M. tuberculosis. This incorporation was achieved through a direct labeling technique applied during the LAMP process. Moreover, the efficiency of both their amplification and TAMRA integration exhibited variability based on factors such as the length of the amplified product and the concentration of the labeled nucleotide implemented. Furthermore, it was established that TAMRA-dUTP exhibits a consistent incorporation rate throughout LAMP reactions. However, variations in the signal output align with the quantity of generated amplicons, a relationship directly influenced by the length of the DNA fragment.
In conclusion, employing labeled nucleotides for direct amplicon labeling during LAMP reactions provides adaptability and harmonizes with various detection methods like fluorescence spectrometry and microarray technology. This enables simultaneous analysis of multiple LAMP products, eliminating the need for an extensive array of labeled primers and probes, as well as the need for additional post-amplification labeling steps; thus, saving time and resources, and making it suitable for point-of-care settings where rapid and accurate results are essential. Nonetheless, when applying TAMRA-labeled nucleotides in LAMP reactions, certain factors warrant consideration. These factors encompass the effectiveness of the chosen primer set, the length of the target gene arranged for amplification, the elongation time, and the proportion of labeled nucleotides integrated per reaction.
The method described in this study presents a new and efficient strategy to label isothermal amplification products. It is especially promising when used in combination with sequence-specific detection systems. Development of fast, accurate and portable detection platforms of SNPs in genes associated with resistance of M. tuberculosis to antibiotics will be key to a more rapid diagnosis and treatment.
Methods
LAMP amplification
LAMP was carried out for each strain in a total 25 µl reaction mixture containing 1.6 µM each FIP and BIP, 0.2 µM each F3 and B3, 0.6 µM each FL and BL, 200 µM each dNTP (roboklon, EURx, Cat.No. E0503-01), 0.4 M betaine, 1 × Isothermal buffer (New England Biolabs, B0537), 6 mM MgSO4 (New England Biolabs, B1003) and 0.32 units/µl Bst3.0 polymerase (New England Biolabs, M0374L). Depending on the experiment the mixture was incubated at 65 °C for an amplification time of 20–30 min, then heated at 85 °C for 3 min to terminate the reaction.
Aminoallyl-dUTP-5/6-TAMRA (Jena Bioscience, NU-803-TAM) were used for labeling of LAMP products with final concentrations ranging from 10 µM to 80 µM (0.5–4%) per reaction. The amount of nuclease free water was adjusted to fit 25 µl reaction volume. Each LAMP reaction contained 1µl of genomic DNA with varied concentrations according to the experiment. For the analysis of LAMP reaction in real-time (rtLAMP) (Analytik Jena, qTOWER3G touch thermocycler, Farbmodul 1), 1µl of 50 × SYBR™ Safe DNA Gel Stain (Thermo Fisher Scientific; S33102) was added per reaction.
As part of the experiments, non-templated controls (NTC) were carried out. These controls contained all components of a LAMP reaction except for the inclusion of the genomic DNA template.
Post-LAMP purification
For the detection and analysis of the LAMP efficiency and TAMRA-dUTP incorporation, via gel electrophoresis, and fluorescence spectroscopy, the products were purified with the GeneMATRIX PCR / DNA Clean-Up Purification Kit (roboklon, Cat.No.E3520) according to the manufacturer’s instructions with a finishing elution step in 25 µl Elution buffer.
Gel electrophoresis
For gel electrophoretic analysis, 1 µl DNA Gel Loading Dye (6 ×) (Thermo Fisher Scientific; SM0334) was added to 5 µl of the purified samples. For band visualization, a 2% agarose gel was prepared in 1 × TAE buffer (50 × TAE buffer; Jena Bioscience; BU-119–50) containing 1 µl SYBR™ Safe DNA Gel Stain (Thermo Fisher Scientific; S33102) per 50 ml gel to visualize the band. A total volume of 6 µl of the purified and prepared samples were used for electrophoresis which ran at 95 V for 35 min. Detection was done via E-Box gel imaging system (Vilber). GeneRuler DNA Ladder Mixture, Ready to Use (Thermo Fisher Scientific; SM0334) was used for calculation of the fragment length.
Fluorescence spectroscopy
The fluorescence labeling with TAMRA-dUTP was analyzed with the MARS data analysis software (BMG LABTECH) and FLUOstar Omega microplate reader (BMG LABTECH). Using the fluorescence spectrometer, fluorescence was measured with 15 µl of purified LAMP products and using the following parameters: 544 nm excitation and 590 nm emission filter; gain = 1500. All measurements were conducted as triplicates.
A standard calibration line for the incorporation of TAMRA was created by measuring free TAMRA-dUTP at a dilution range of 10 µM to 4.88 nM.
The evaluation of SYBR Green intercalation was also conducted to perform a relative quantification of amplicons produced within a LAMP reaction. To carry out this analysis, the MARS data analysis software (BMG LABTECH) and the FLUOstar Omega microplate reader (BMG LABTECH) were used. The fluorescence spectrometer was employed to analyze 15 µl of purified LAMP products, using the following settings: an excitation wavelength of 485 nm and an emission wavelength of 520 nm with a gain value of 1000. All measurements were conducted as triplicates.
A baseline signal of 1 × Isothermal buffer (New England Biolabs, B0537) was included in all fluorescence measurements to help detect any intrinsic fluorescence or background noise.
Concentration measurements
Concentration measurements were conducted using NanoDrop 2000 UV–Vis spectrophotometer (Thermo Fisher Scientific) with 2 µl purified LAMP products.
Templates and primers
The extracted and purified genomic DNA of M. tuberculosis was purchased from the Leibniz Institute DSMZ-German Collection of Microorganisms and Cell Cultures GmbH (DSM 43990). Extracted and purified M. tuberculosis mutant strains were purchased from the Institute of Microbiology and Laboratory Medicine—IML red GmbH (IML-00020, IML-02020). IML-00020 strain was used to amplify katG gene that contained S315T mutation, while IML-02020 strain was used to amplify pncA gene that contained H57D mutation.
Primers were designed with Geneious Prime (Dotmatics). Each reaction contained 6 LAMP-primers specific to the respective M. tuberculosis resistance gene (Table 1). Primers were designed based on the genome mutation sequences (provided by Fraunhofer Institute for Cell Therapy and Immunology, Branch Bioanalytics and Bioprocesses IZI-BB). All primers were synthesized and purchased from Biomers.net.
Data availability
The data that support the findings of this study are available from the corresponding author [B.A.] upon reasonable request.
References
Zhao, Y., Chen, F., Li, Q., Wang, L. & Fan, C. Isothermal amplification of nucleic acids. Chem. Rev. 115, 12491–12545 (2015).
Glökler, J., Lim, T. S., Ida, J. & Frohme, M. Isothermal amplifications–a comprehensive review on current methods. Crit. Rev. Biochem. Mol. Biol. 56, 543–586 (2021).
Notomi, T. et al. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 28, E63 (2000).
Khan, M. et al. Comparative evaluation of the LAMP assay and PCR-based assays for the rapid detection of Alternaria solani. Front. Microbiol. 9, 2089 (2018).
Panno, S. et al. Loop mediated isothermal amplification: Principles and applications in plant virology. Plants (Basel) 9, 461 (2020).
Varona, M. & Anderson, J. L. Advances in mutation detection using loop-mediated isothermal amplification. ACS Omega 6, 3463–3469 (2021).
Agarwal, S. et al. Lateral flow–based nucleic acid detection of SARS-CoV-2 using enzymatic incorporation of biotin-labeled dUTP for POCT use. Anal. Bioanal. Chem. 414, 3177–3186 (2022).
Du, Y. et al. Coupling sensitive nucleic acid amplification with commercial pregnancy test strips. Angew. Chem. Int. Edition 56, 992–996 (2017).
Warmt, C., Yaslanmaz, C. & Henkel, J. Investigation and validation of labelling loop mediated isothermal amplification (LAMP) products with different nucleotide modifications for various downstream analysis. Sci. Rep. 12, 7137 (2022).
Varona, M. & Anderson, J. L. Visual detection of single-nucleotide polymorphisms using molecular beacon loop-mediated isothermal amplification with centrifuge-free DNA extraction. Anal. Chem. 91, 6991–6995 (2019).
Dong, Y. et al. Multiplex, real-time, point-of-care RT-LAMP for SARS-CoV-2 detection using the HFman probe. ACS Sens. 7, 730–739 (2022).
Phillips, E. A., Moehling, T. J., Bhadra, S., Ellington, A. D. & Linnes, J. C. Strand displacement probes combined with isothermal nucleic acid amplification for instrument-free detection from complex samples. Anal. Chem. 90, 6580–6586 (2018).
Hashimoto, K., Nakamura, N. & Ito, K. Rapid, highly sensitive and highly specific gene detection by combining enzymatic amplification and DNA chip detection simultaneously. Sens. Biosensing Res. 8, 27–30 (2016).
Zhang, C. et al. Rapid one-pot detection of SARS-CoV-2 based on a lateral flow assay in clinical samples. Anal. Chem. 93, 3325–3330 (2021).
Hashimoto, M. et al. In situ loop-mediated isothermal amplification (LAMP) for identification of Plasmodium species in wide-range thin blood smears. Malar. J. 17, 235 (2018).
Goryunova, M. S., Arzhanik, V. K., Zavriev, S. K. & Ryazantsev, D. Y. Rolling circle amplification with fluorescently labeled dUTP-balancing the yield and degree of labeling. Anal. Bioanal. Chem. 413, 3737–3748 (2021).
Warmt, C., Broweleit, L.-M., Fenzel, C. K. & Henkel, J. An experimental comparison between primer and nucleotide labelling to produce RPA-amplicons used for multiplex detection of antibiotic resistance genes. Sci. Rep. 13, 15734 (2023).
Seung, K. J., Keshavjee, S. & Rich, M. L. Multidrug-resistant tuberculosis and extensively drug-resistant tuberculosis. Cold Spring Harb. Perspect. Med. 5, 1 (2015).
Jang, J. G. & Chung, J. H. Diagnosis and treatment of multidrug-resistant tuberculosis. Yeungnam Univ. J. Med. 37, 277–285 (2020).
Gupta, A. & Anupurba, S. Detection of drug resistance in Mycobacterium tuberculosis: Methods, principles and applications. Indian J. Tuberc. 62, 13–22 (2015).
Singh, V. & Chibale, K. Strategies to combat multi-drug resistance in tuberculosis. Acc. Chem. Res. 54, 2361–2376 (2021).
Palomino, J. C. & Martin, A. Drug resistance mechanisms in Mycobacterium tuberculosis. Antibiotics 3, 317–340 (2014).
Sontakke, S., Cadenas, M. B., Maggi, R. G., Diniz, P. P. V. P. & Breitschwerdt, E. B. Use of broad range16S rDNA PCR in clinical microbiology. J. Microbiol. Methods 76, 217–225 (2009).
Böttger, E. C. Rapid determination of bacterial ribosomal RNA sequences by direct sequencing of enzymatically amplified DNA. FEMS Microbiol. Lett. 53, 171–176 (1989).
Choi, Y. et al. Conventional and real-time PCR targeting 16S ribosomal RNA for the detection of Mycobacterium tuberculosis complex. Int. J. Tuberculosis Lung Dis. 19, 1102–1108 (2015).
Roychowdhury, T., Mandal, S. & Bhattacharya, A. Analysis of IS6110 insertion sites provide a glimpse into genome evolution of Mycobacterium tuberculosis. Sci. Rep. 5, 12567 (2015).
Suthum, K., Samosornsuk, W. & Samosornsuk, S. Characterization of katG, inhA, rpoB and pncA in Mycobacterium tuberculosis isolates from MDR-TB risk patients in Thailand. J. Infect. Dev. Ctries 14, 268–276 (2020).
Hyun Kim, S. et al. DNA sequence encodes the position of DNA supercoils. Elife 7, e36557 (2018).
Šimoníková, D. et al. Chromosome painting facilitates anchoring reference genome sequence to chromosomes in situ and integrated karyotyping in banana (Musa Spp.). Front. Plant Sci. 10, 1503 (2019).
Warmt, C., Fenzel, C. K., Henkel, J. & Bier, F. F. Using Cy5-dUTP labelling of RPA-amplicons with downstream microarray analysis for the detection of antibiotic resistance genes. Sci. Rep. 11, 20137 (2021).
Cole, S., Brosch, R., Parkhill, J. et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393, 537–544 (1998).
Mamedov, T. G. et al. A fundamental study of the PCR amplification of GC-rich DNA templates. Comput. Biol. Chem. 32, 452–457 (2008).
Kumar, A. & Kaur, J. Primer based approach for PCR amplification of high GC content gene: Mycobacterium gene as a model. Mol. Biol. Int. 2014, 1–7 (2014).
Frey, U. H., Bachmann, H. S., Peters, J. & Siffert, W. PCR-amplification of GC-rich regions: ‘Slowdown PCR’. Nat. Protoc. 3, 1312–1317 (2008).
Van Holm, W. et al. A viability quantitative PCR dilemma: Are longer amplicons better?. Appl. Environ. Microbiol. 87, e02653-e2720 (2021).
Shagin, D. A., Lukyanov, K. A., Vagner, L. L. & Matz, M. V. Regulation of average length of complex PCR product. Nucleic Acids Res. 27, 1 (1999).
Acknowledgements
We acknowledge that this work is part of FluoResYst project funded by BMBF. We would also like to acknowledge all our partners in the project.
Funding
Open Access funding enabled and organized by Projekt DEAL. This project was funded by the Federal Ministry of Education and Research (BMBF) with a grant number 13N15814.
Author information
Authors and Affiliations
Contributions
B.A. planned and performed the experiments, analyzed the data, and wrote the manuscript. J.U. conducted parts of the experiments. E.M. and A.P. provided the M. tuberculosis target sequences and concentration measurements of LAMP products. R.M. and F.B. reviewed the data and the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Altattan, B., Ullrich, J., Mattig, E. et al. Direct TAMRA-dUTP labeling of M. tuberculosis genes using loop-mediated isothermal amplification (LAMP). Sci Rep 14, 5611 (2024). https://doi.org/10.1038/s41598-024-55289-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-55289-x
- Springer Nature Limited