Abstract
Analyses of registries and medical imaging suggest that laparoscopic surgery may be penalized with a high incidence of trocar-site hernias (TSH). In addition to trocar diameter, the location of the surgical wound (SW) may affect TSH incidence. The intra-abdominal pressure (IAP) exerted on the abdominal wall (AW) might also influence the appearance of TSH. In the present study, we used finite element (FE) simulations to predict the influence of trocar location and SW characteristics (stiffness) on the mechanical behavior of the AW subject to an IAP. Two models of laparoscopy patterns on the AW, with trocars in the 5–12 mm range, were generated. FE simulations for IAP values within the 4 kPa–20 kPa range were carried out using the Code Aster open-source software. Different stiffness levels of the SW tissue were considered. We found that midline-located surgical wounds barely deformed, even though they moved outwards along with the regular LA tissue. Laterally located SWs hardly changed their location but they experienced significant variations in their volume and shape. The amount of deformation of lateral SWs was found to strongly depend on their stiffness. Trocar incisions placed in a LA with non-diastatic dimensions do not compromise its mechanical integrity. The more lateral the trocars are placed, the greater is their deformation, regardless of their size. Thus, to prevent TSH it might be advisable to close lateral trocars with a suture, or even use a prosthetic reinforcement depending on the patient's risk factors (e.g., obesity).
Similar content being viewed by others
Introduction
The prevalence of trocar-site hernias (TSH) is not clear1. Analysis of registries suggests a high incidence of TSH2 and that, depending on their location, reparation of these hernias can be complex3. Moreover, the recently updated guidelines for closure of abdominal wall incisions4 provide no support to the notion that the fascial closure at the trocar site can benefit TSH prevention. These guidelines also mention that there is no evidence supporting the best trocar location and they recommend suturing the fascial defect for trocar sites of 10 mm or larger, and for trocars located at the umbilical site4.
Finite element (FE) simulations may strengthen our understanding of how surgical wounds (SWs) alter the mechanical behavior of the abdominal wall (AW)5,6. Our key assumption is that the response of the AW to intra-abdominal pressure (IAP) sometime after surgery will depend on the mechanical properties of the tissue regrown at the surgical sites, that is, the tissue conforming the SWs, and the laparoscopy pattern used in the surgery. The resilience of the AW to an applied IAP value, Pa, is mostly due to the stiffness of its tissues, which are of muscular or tendinous nature. Both types of tissue are characterized by the presence of fibers in their architecture7. The new SW tissue might be weak and, from a mechanical point of view, softer than expected8. On the other hand, the regrown tissue in the SW might become scarred9,10 and exceedingly stiff11.
In silico analysis could help to virtually simulate the influence of trocar location or trocar scar characteristics on the mechanical behavior of the AW. However, to the best of our knowledge, there is no study on how the SW characteristics, or its location alter the mechanical response of the AW. The aim of this work is to use FE simulations to predict AW deformation as a function of the stiffness and location of the SWs (mimicking trocar-site placement), and the level of IAP applied.
Models and methods
Geometry model
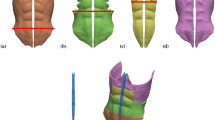
The present geometry model, illustrated in Fig. 1, is similar to the one used in our previous work5. It consists of external oblique (EO), internal oblique (IO), rectus abdominis (RA) and transverse abdominis (TR) muscles, as well as the linea alba (LA), which was considered with standard dimensions of 2 cm (i.e., without rectus diastasis)12. Notwithstanding, a novelty in the present geometry model is that the aponeurosis of the TR, EO and IO muscles is considered as a distinct tissue, having mechanical properties different from those of the regular muscle (see Fig. 1).
Geometry model of the abdominal wall. The elements involved in the model are: (a) right and left external oblique muscles (EO), (b) right and left internal oblique muscles (IO), (c) right and left rectus abdominis muscles (RA), (d) linea alba (LA) and (e) right and left transverse abdominis muscles (TR). (f) View of the whole model. Note that as the involved muscles are superimposed only those muscle regions below black lines in parts (a–c) are displayed. In parts (a,b,e) the different regions (regular, intermediate, and aponeurotic muscle tissue) are denoted with increasingly saturated color tones.
We investigated two laparoscopy patterns, denoted as models A and B (see Fig. 2a,b). We considered surgical wounds after surgery, i.e., assuming all trocar-sites closed. SWs were modeled as elliptic cylinders orthogonal to the outer AW surface (see Fig. 2c) with the ellipse major axis representing the trocar diameter, and the minor axis set to 2 mm in all cases. We will henceforth refer to the length and width of a laparoscopy SW instead of to the ellipse axes.
Distribution of the incisions on the AW for the two laparoscopy patterns studied in this work. (a) Laparoscopy model A, with three 5-mm long and three 12-mm long incisions. (b) Laparoscopy model B, with one 10-mm long, one 12-mm long and three 5-mm long surgical wounds (see Table 1 for details on the length, surface area and volume of each laparoscopic SW). (c) Sketch of the elliptic profile for the three geometry models of laparoscopy surgical wounds assumed in the present study.
Length, surface area and volume of each SW geometry are summarized in Table 1. Model A (Fig. 2a) comprises six surgical wounds. Two 12-mm long incisions are located on the LA (A1, A4), two 5-mm long incisions are located on the left lateral region (A2, A3), and two more SWs, respectively 5- and 12-mm long, are on the right lateral region (A5, A6). Model B (Fig. 2b) consists of five surgical wounds. Two incisions, a 10-mm long supraumbilically placed (B1) and a 5-mm long infraumbilically placed (B4), are embedded in the LA. Two 5-mm long incisions are located on the left lateral region (B2, B3) and a 12-mm long SW is placed on the right lateral region (B5).
Material properties
Following our previous work5 we assumed a linear elastic behavior of the AW tissues,
where σ is the stress tensor, ε is the strain tensor, accounting for the relative deformation of tissues, and E is the elastic modulus. The specific E values for each tissue, together with the bibliographic source, are listed in Table 2. A value of ν = 0.49 was assumed for the Poisson's ratio of muscular tissues. The aponeurosis of the TR, EO and IO muscles forms the so-called rectus sheath13,14. The tissue in the rectus sheath is more fibrous (higher E) than regular muscular tissue. Figure 3 illustrates the definition of the three regions (regular muscle, rectus sheath, and a thin intermediate transition region). In the transition region, we assumed an E value halfway the values for rectus sheath and regular muscle.
Illustration of the transition between the regular muscle tissue (yellow), intermediate tissue (red) and aponeurotic tissue (green). (a) Frontal view of the left and right IO muscles that surround most of the RA muscles (blue). (b) Axial slices taken at the height denoted by the horizontal black line in part (a); the upper plot shows only the IO and RA tissues whereas the lower plot shows also the EO and TR muscle tissues for the sake of completeness.
A wide variability in the mechanical properties of wounds can be found in the literature. For example, Ibrahim et al.15 reported E values of a few kPa for uninjured human skin, whereas Samartsev et al.16 obtained elastic moduli of a few GPa in their experiments with sutured samples. In the current study, we performed simulations within a wide range of E values for the SWs, namely 0.1 MPa ≤ Ew ≤ 10,000 MPa. The Poisson ratio for SW tissue was set to νw = 0.4016.
Numerical simulation
The current simulations were performed using the Code Aster open-source FE software17. As illustrated in Fig. 4, fixed (zero deformation) boundary conditions were prescribed at the edge of the abdominal wall, where the muscles would attach to the bones. The IAP was uniformly applied to the AW inner surface. For each geometry, we performed simulations for five uniformly distributed values pf Pa between 4 and 20 kPa, a normal IAP range during typical activities of daily living18. In each simulation, the resulting deformations of the geometry were computed and analyzed.
Views of the AW geometry with a dark blue color denoting areas where the fixed boundary condition is applied. (a) Front view. (b) Side view. (c) Rear view. Note that the surfaces of the different geometry elements (muscles) have been made translucent to enhance the visibility of fixed-boundary areas.
The geometry model was generated by refinement of the raw model available in the BodyParts3D database for anatomy, which in turn was generated from CT images of an adult man19. In this refinement process, internally consistent, high quality triangular surface meshes were generated for each of the AW components in our model. Subsequently, the corresponding volume meshes, consisting of linear tetrahedra, were constructed. Table 3 shows the dimensions of the three computational meshes used in the present study, i.e., the mesh for the base case (no-trocar) and the respective meshes for the trocar patterns A and B. Special care was taken to ensure the homogeneity of the computational meshes. That is, the values of the (mesh wide) mean triangle surface area and mean tetrahedron volume, listed in Table 3, are characteristic of most of the mesh elements. A exception to this rule is found in the particularly refined meshes that were generated for the trocar elements (see Table 3). These trocar meshes replaced the corresponding portions of the base mesh at the trocar sites and the mesh elements in the surrounding regions were correspondingly adapted to ensure continuity and smoothness of the resulting computational mesh. As an example, Fig. 5a shows a section of the surface mesh of the RA muscles and LA whereas Fig. 5b provides a detailed view of the mesh on the internal surface separating, and common to, the left RA and LA volumes.
(a) Detail of the computational mesh on a sector of the RA muscles (pink) and LA (brown) frontal surfaces; the edges of the surface-mesh triangles are denoted with blue lines. (b) Side view of a cross-section of the left RA mesh showing the lateral surface (yellow, with blue triangle edges) that is shared with the LA mesh.
Characterization of the deformation patterns of incisions
For each laparoscopy pattern (A and B), we first performed a reference case simulation in which Ew values for every SW were set to the average E of the muscles surrounding it (that is, SWs were considered to be healed). Then we performed a set of simulations in which one SW at a time was softened to Ew = 0.1 MPa. Since the predicted displacements of each computational node are available in the FE simulation output, we computed the corresponding changes in surface area and volume of the deformed SWs by means of numerical integration along the mesh surface elements (triangles) and volume elements (tetrahedra), respectively.
There are two main effects in the deformation of a SW: (i) an undulation of its outer surface, and (ii) a reduction in its length (depth) in the direction normal to the AW surface.
Even though the concept of surface undulation arises intuitively from the visualization of the SW deformed geometry—obtained from the FE simulation output—its quantification is by no means straightforward. In what follows we explain in some detail the methodology that we developed to quantify this concept. Specifically, our main goal is to associate a map of the predicted SW surface changes to a quantity that estimates the SW degree of surface undulation. In the non-deformed geometry, each SW is an elliptic cylinder with two end lids, respectively located on the AW inner and outer surfaces. For every computational node ("i") along the rim of a lid, we determined the nearest node ("j") in the rim of the opposite lid, and we measured the Euclidean distance (di,j) and the minimum geodesic distance (Di,j) between these two nodes. A geodesic distance between two points on a surface is the length of a path between the two points along the surface. The full set of distances between rim node pairs was used to compute averaged Euclidean (d) and minimum geodesic (D) distances for every SW. In the original geometry (Fig. 6a), the SW surface is hardly undulated, and we initially have D0 ≈ d0. After a FE simulation was completed, the final Euclidean (df) and geodesic (Df) distances were computed for the deformed SW (see Fig. 6b). The SW undulation was characterized using the averaged tortuosity,
as well as its variation,
Results from the simulations with Pa = 20 kPa for soft (Ew = 0.1 MPa) A4 (a,c) and B2 (b,d) laparoscopy surgical wounds. (a,b) Deformation of the entire AW external surface is plotted for the simulations with a soft A4 (a) and B2 (b) surgical wound. (c,d) Comparison of the original and deformed geometries of A4 (c) and B2 (d) surgical wounds.
To characterize the compression of the SW due to a decrease in its depth, we defined the averaged geodesic deformation as:
Results
Table 4 shows predicted Gd and \(\Delta \overline{\tau }\) values for the simulations with Pa = 20 kPa. Surgical wounds within the LA (A1, A4, B1 and B4) hardly experience any deformation (Gd ≈ 1 and \(\Delta \overline{\tau }\) ≈ 0) but they travel a significant distance outward, together with the rest of the LA tissue. Figure 6 shows the predicted deformations for the simulations with Pa = 20 kPa and either a soft A4 or B2 SW. The deformation behavior of a SW in the LA is illustrated in Fig. 6c for a soft A4 (Ew = 0.1 MPa). Laterally located surgical wounds (A2, A3, A5, A6, B2, B3 and B5) hardly change their location instead but their shape is significantly modified (see Table 4). This second behavior is illustrated in Fig. 6d for a soft B2. The depth of laterally placed surgical wounds is reduced, with Gd values in the 0.96–0.97 range. Also, lateral surgical wounds located in the upper lumbar region (A2, A6 and B2) experience significant surface undulations, as characterized by higher \(\Delta \overline{\tau }\) values in Table 4.
To further investigate the mechanical response of the SWs as a function of their toughness (Ew), we selected one SW from each pattern, namely A6 and B2, which featured the largest surface undulation in terms of Gd and \(\Delta \overline{\tau }\) (see Table 4). Figure 7a–d illustrates the relative change of surface area and volume of A6 and B2 as a function of Pa. Figure 7a,c shows that for a very tough SW (Ew = 2000 MPa) the surface area of both A6 and B2 is preserved. The surface area of these wounds decreases with increasing IAP, up to about 1% at Pa = 20 kPa, when their stiffness is comparable to that in the surrounding muscles (Ew = 1–2 MPa), and it slightly decreases (for Pa < 15 kPa) or increases (for Pa ≥ 15 kPa) when the wounds are soft (Ew = 0.1–0.2 MPa). Figure 7b,d shows that the volume of A6 and B2 increases steadily with increasing IAP and with decreasing Ew, with a maximum expansion of 5.3% for A6 with Ew = 0.1 MPa. Figure 7e,f shows that for a fixed IAP level of 20 kPa changes in Ew lead to modest changes in SW volume and surface area, with some increase in surface area only observed for the softest SWs (Ew = 0.1 MPa). In the 0.2 MPa ≤ Ew ≤ 10 MPa range SW surface area decreases instead for both A6 and B2, with a minimum of about Ew = 1 MPa.
Relative change in surface area (a,c,e) and volume (b,d,f) of the A6 and B2 laparoscopy surgical wounds. In parts (a–d), changes in SW geometry are plotted against the level of applied IAP for several values of Ew whereas in parts (e,f) the predicted changes in surface area and volume are plotted against Ew for the simulations with Pa = 20 kPa. Calculated values are marked with a symbol whereas the solid line segments are intended just as a visual aid.
Discussion
Even though laparoscopic surgery can be associated with benefits in different procedures when compared with open surgery20,21 it might be penalized with an unclear incidence of TSH1. TSH incidence turns out to be high when we analyze data from the real-world evidence (i.e., registries)2 or we examine medical imaging such as ultrasound and CT scans, which help to diagnose a considerable amount of TSH that would be otherwise clinically undetectable1.
Evidence supporting the benefits of trocar-site closure after completing the surgical procedure is very limited. This data uncertainty might be the reason why some authors believe it is not necessary to close the trocar-site wound to prevent a TSH22. Nevertheless, the most recent clinical guidelines recommend the closure of trocars with large diameters (10 mm or larger)4. In addition to trocar diameter4, the location of the trocar-site may affect TSH incidence22. Literature reviews suggest higher TSH incidence rates for trocars in the LA, compared to the off midline locations22. Moreover, the umbilical trocar site along with the use of larger trocars and obesity have been reported as risk factors for developing TSH23. The IAP exerted on the abdominal wall, and consequently on the trocar-site wounds, might also influence the appearance of TSH. Correlation between central obesity and increase in IAP has been described in several works24,25,26.
In the present study, we performed virtual simulations of the mechanical behavior of the AW for different properties of the SW tissues and different IAP levels. Our results indicate that laparoscopic SWs embedded in the LA barely deform as a result of the AW expansion. These wounds, having a diameter smaller than the standard LA width (2 cm), are surrounded by the stiffer LA tissue and thus have very little room for deformation, even when they are very soft (Ew = 0.1 MPa). On the contrary, laterally placed laparoscopic SWs significantly deform when the AW is expanded. The volume growth of laterally placed SWs increases with decreasing Ew, up to a maximum of 5% for Ew = 0.1 MPa. A soft SW opposes less resistance to the motion of the muscular tissue (TR and EO) around it and the SW tissue just follows the muscles and adjusts to fill the new space generated. The increase in SW volume is accompanied by either an increase or a decrease in its surface area, depending on the Ew value. When a pressure push is applied on the inner AW surface, the innermost muscular layer (TR and EO) is slightly compressed, that is, its depth is slightly reduced. Consequently, the depth of laterally placed SWs is similarly reduced. However, even though SW depth is reduced the increase in SW volume entails an increase in both its length and width. As the total SW surface area is proportional to its depth, length and width, an increase of the two latter quantities can partly compensate, or even outreach, the decrease in the former.
Study limitations
As a rule, virtual simulations ought to be validated by comparison with experimental results. To the best of our knowledge, no available experimental data exists on the present subject, a fact resulting from the difficulties that are inherent to experimentation with living persons. Nonetheless, the absence of verification constitutes a limitation of the present study.
The present geometrical and mathematical models have several limitations. Only one linea alba geometry, 2 cm wide, has been considered and the present model does not account for the placement of sutures or prostheses at trocar sites. Simulations of surgical wounds located on the LA at the umbilical site level were dismissed. Moreover, a linear isotropic elastic behavior was assumed for all abdominal wall tissues to constrict the computational costs. Even though it is well established that the AW tissues are fibrous and thus anisotropic27, our simulations predict modest strain levels even at the highest IAP values considered. That is, proximity to the stress–strain (zero) origin would partly justify the use of the linear constitutive model.
Another limitation of the present study is that only one geometry model, obtained from a specific subject, is considered. This raises a crucial question on the applicability of the present results to the broader population. The substantial inter-subject variability in both morphology and the mechanical properties of the different AW tissues introduces a significant challenge. Therefore, a generalization of the present results would be meaningful at a qualitative level, and even so, it should be approached with caution.
Despite these limitations, we think that the present methodology establishes the basis for future advancements. Specifically, virtual simulations may serve as the basis for a more refined, patient-specific assessment on how trocar placement influences the mechanics of the abdominal wall. Future work should therefore aim to the automation of the geometry building process from individual CT scans, thus offering a more personalized and clinically relevant perspective.
Conclusion
In conclusion, trocar incisions placed in a LA with non-diastatic dimensions (up to 2 cm) do not seem to compromise the LA mechanical integrity. However, we previously found that trocar holes exceeding the dimensions of a non-diastatic LA can cause an alteration in its mechanical integrity, especially trocars placed in the lowermost area of the LA (hypogastric)5. This can be especially important when a surgeon must decide whether to close a trocar placed in the LA, mainly if we consider the “pivot point” or “fulcrum” clinical concept1 that affects the part of the trocar held by the abdominal wall muscles around which the trocar performs its movement. Depending on the characteristics of the intervention, the trocar will pivot around a distinct set point, potentially causing shearing injury to the abdominal wall, enlarging the defect in the abdominal wall musculature. Maybe robotic trocars, which move with more precision, would maintain the integrity of the LA without exceeding its dimensions because they are pivoting in the same set point all time, regardless of the characteristics of the intervention. Laterally placed trocar incisions (beyond the rectus abdominis muscles) experience greater deformation with IAP than those placed on LA. The more lateral the trocars are placed the greater is their deformation, regardless of their size. It could be speculated that the mechanical behavior of lateral trocars may be compromised if left only at the expense of scar resistance, with potential risk of finally developing TSH. It might be, therefore, advisable to close lateral trocars with a suture or even use a prosthetic reinforcement depending on the patient's risk factors (e.g., obesity) and regardless of the size of the trocar.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
de Beaux, A. C. & East, B. Thoughts on trocar site hernia prevention. A narrative review. J. Abdom. Wall Surg. 1, 11034 (2022).
Pereira, J. A., López-Cano, M., Hernández-Granados, P., Feliu, X. & del grupo EVEREG, en representación. Initial results of the national registry of incisional hernia [Resultados iniciales del registro español de hernia incisional]. Cirugía Española 94, 595–602 (2016).
Ciscar Belles, A. et al. Umbilical incisional hernias (M3): Are trocar-site hernias different? Comparative analysis of the EVEREG Registry. Cirugía Española 100, 336–344 (2022).
Deerenberg, E. B. et al. Updated guideline for closure of abdominal wall incisions from the European and American Hernia Societies. Br. J. Surg. 109, 1239–1250 (2022).
Tuset, L. et al. Virtual simulation of the biomechanics of the abdominal wall with different stoma locations. Sci. Rep. 12, 3545 (2022).
Karrech, A., Ahmad, H. & Hamdorf, J. M. Biomechanical stability of hernia-damaged abdominal walls. Sci. Rep. 13(1), 4936 (2023).
Abat González, F., Turmo Garuz, A., Campos Moraes, J. & Capurro Soler, B. Physiology and mechanobiology of tendon and muscle tissue. Rev. Esp. Artroscopia Cirugía Articular 29, 3–12 (2022).
Garg, S., Dahiya, N. & Gupta, S. Surgical scar revision: An overview. J. Cutan. Aesthet. Surg. 7(1), 3–13 (2014).
Mann, C. J. et al. Aberrant repair and fibrosis development in skeletal muscle. Skelet. Muscle 1, 21 (2011).
Laumonier, T. & Menetrey, J. Muscle injuries and strategies for improving their repair. J. Exp. Orthop. 3, 15 (2016).
Corr, D. T. & Hart, D. A. Biomechanics of scar tissue and uninjured skin. Adv. Wound Care 2, 37–43 (2013).
Hernández-Granados, P. et al. European Hernia Society guidelines on management of rectus diastasis. Br. J. Surg. 108, 1189–1191 (2021).
Martins, P. et al. Mechanical characterization and constitutive modelling of the damage process in rectus sheath. J. Mech. Behav. Biomed. Mater. 8, 111–122 (2012).
Deeken, C. R. & Lake, S. P. Mechanical properties of the abdominal wall and biomaterials utilized for hernia repair. J. Mech. Behav. Biomed. Mater. 74, 411–427 (2017).
Ibrahim, M. M. et al. A novel immune competent murine hypertrophic scar contracture model: A tool to elucidate disease mechanism and develop new therapies. Wound Repair Regener. 22, 755–764 (2014).
Samartsev, V. A., Kuchumov, A. G. & Gavrilov, V. A. Sutures in abdominal surgery: biomechanical study and clinical application. Cent. Eur. J. Med. 9, 849–859 (2014).
Electricité de France. code_aster. Structures and Thermomechanics Analysis for Studies and Research (2023).
Cobb, W. S. et al. Normal intraabdominal pressure in healthy adults. J. Surg. Res. 129, 231–235 (2005).
Mitsuhashi, N. et al. BodyParts3D: 3D structure database for anatomical concepts. Nucleic Acids Res. 37, D782–D785 (2009).
Ozair, A. et al. Minimally invasive versus open hepatectomy for the resection of colorectal liver metastases: a systematic review and meta-analysis. Surg. Endosc. 36, 7915–7937 (2022).
Luo, W., Wu, M. & Chen, Y. Laparoscopic versus open surgery for elderly patients with colorectal cancer: a systematic review and meta-analysis of matched studies. ANZ J. Surg. 92, 2003–2017 (2022).
Gutierrez, M., Stuparich, M., Behbehani, S. & Nahas, S. Does closure of fascia, type, and location of trocar influence occurrence of port site hernias? A literature review. Surg. Endosc. 34, 5250–5258 (2020).
Ahlqvist, S. et al. Trocar site hernia after gastric sleeve. Surg. Endosc. 36, 4386–4391 (2022).
Smit, M. et al. How central obesity influences intra-abdominal pressure: A prospective, observational study in cardiothoracic surgical patients. Ann. Intensive Care 6, 1–10 (2016).
Lambert, D. M., Marceau, S. & Forse, R. A. Intra-abdominal pressure in the morbidly obese. Obes. Surg. 15, 1225–1232 (2005).
Wilson, A., Longhi, J., Goldman, C. & McNatt, S. Intra-abdominal pressure and the morbidly obese patients: The effect of body mass index. J. Trauma Injury Infect. Crit. Care 69, 78–83 (2010).
Tuset, L., Fortuny, G., Herrero, J., Puigjaner, D. & López, J. M. Implementation of a new constitutive model for abdominal muscles. Comput. Methods Progr. Biomed. 179, 104988 (2019).
Cardoso, M. Experimental Study of the Human Anterolateral Abdominal Wall: Biomechanical Properties of Fascia and Muscles 7–16, 52 (Faculdade de Engenharia, Universidade do Porto, 2012).
Cooney, G. M. et al. Uniaxial and biaxial tensile stress-stretch response of human linea alba. J. Mech. Behav. Biomed. Mater. 63, 134–140 (2016).
Ben Abdelounis, H., Nicolle, S., Otténio, M., Beillas, P. & Mitton, D. Effect of two loading rates on the elasticity of the human anterior rectus sheath. J. Mechan. Behav. Biomed. Mater. 20, 1–5 (2013).
Acknowledgements
This project has been partially founded by AGAUR research group 2021SGR-00111: "ASCLEPIUS: Smart Technology for Smart Healthcare".
Author information
Authors and Affiliations
Contributions
Conception and design: all authors; Methodology: L.T., G.F., J.M.L., J.H., D.P.; Numerical simulations: L.T.; Analysis and interpretation of data: all authors; Study supervision: J.H. and D.P.; Writing Original Draft: all authors; Writing Review and Editing: all authors. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tuset, L., López-Cano, M., Fortuny, G. et al. A virtual simulation approach to assess the effect of trocar-site placement and scar characteristics on the abdominal wall biomechanics. Sci Rep 14, 3583 (2024). https://doi.org/10.1038/s41598-024-54119-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-54119-4
- Springer Nature Limited