Abstract
The impact of small vessel disease (SVD) on stroke outcome was investigated either separately for its single features in isolation or for SVD sum score measuring a qualitative (binary) assessment of SVD-lesions. We aimed to investigate which SVD feature independently impacts the most on stroke outcome and to compare the continuous versus binary SVD assessment that reflects pronouncement and presence correspondingly. Patients with a first-ever anterior circulation ischemic stroke were retrospectively investigated. We performed an ordered logistic regression analysis to predict stroke outcome (mRS 3 months, 0–6) using age, stroke severity, and pre-stroke disability as baseline input variables and adding SVD-features (lacunes, microbleeds, enlarged perivascular spaces, white matter hyperintensities) assessed either continuously (model 1) or binary (model 2). The data of 873 patients (age 67.9 ± 15.4, NIHSS 24 h 4.1 ± 4.8) was analyzed. In model 1 with continuous SVD-features, the number of microbleeds was the only independent predictor of stroke outcome in addition to clinical parameters (OR 1.21; 95% CI 1.07–1.37). In model 2 with the binary SVD assessment, only the presence of lacunes independently improved the prediction of stroke outcome (OR 1.48, 1.1–1.99). In a post hoc analysis, both the continuous number of microbleeds and the presence of lacunes were independent significant predictors. Thus, the number of microbleeds evaluated continuously and the presence of lacunes are associated with stroke outcome independent from age, stroke severity, pre-stroke disability and other SVD-features. Whereas the presence of lacunes is adequately represented in SVD sum score, the microbleeds assessment might require another cutoff and/or gradual scoring, when prediction of stroke outcome is needed.
Similar content being viewed by others
Introduction
Small vessel disease (SVD) is considered to be a common cause of stroke and vascular dementia1. It manifests with various lesions, visible in imaging as small recent subcortical infarctions, lacunes, white matter hyperintensities (WMH), microbleeds, enlarged perivascular spaces (EPVS), and brain atrophy1. Among patients with similar degrees of SVD on brain imaging, clinical symptoms are often highly inconsistent in nature and clinical severity2. The overall burden of SVD lesions was shown to be associated with functional disability and cognitive performance3, and a higher risk of stroke and death4,5 even in young adults6.
There are two common forms of sporadic SVD: (1) hypertensive-related (arteriosclerotic/ cardiovascular risk factor-mediated) and (2) cerebral amyloid angiopathy7. To reflect the overall burden of the former form, the SVD score was introduced, which captures the presence of SVD features and their pronouncement in a binary way based on the given cut-off for major types of SVD lesions: lacunes, microbleeds, WMH, and EPVS8,9. The SVD score was demonstrated to be associated with global cognitive and functional impairment in stroke patients10 and poorer stroke outcome11. There is evidence for an impact on stroke outcome for each major type of SVD feature but EPVS12. Thus, the presence and severity of WMH13, multiple lacunes14 and microbleeds15 were shown to worsen stroke outcome. However, these studies assessed the impact of single SVD features without accounting for other ones. Taking into account a typical progression of pathophysiological developments of SVD1,16, one can expect that the pronouncement of different SVD features might correlate with each other and thus their independent impact on stroke outcome is still to be defined. In addition, whereas mainly simple binary measures for SVD are used, a continuous assessment of SVD features could potentially improve the prediction of stroke outcome, as it better reflects the severity of SVD pathology. Many existing studies on the impact of SVD also did not consider the pre-stroke level of disability in their prediction models.
Considering the gaps of knowledge, we aimed (i) to investigate what SVD feature impacts mostly stroke outcome independently from other SVD features and beyond clinical baseline characteristics; (ii) to compare continuous versus binary SVD assessment that reflect correspondingly the pronouncement and the presence of SVD specific features.
Methods
Patients
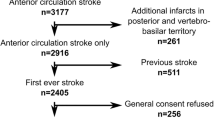
In this retrospective single-center cohort study, we included patients from the Bernese stroke register, admitted to the Bern stroke center for treatment of acute ischemic stroke between January 2015 and October 2020. Inclusion criteria were: (i) age ≥ 18 years old; (ii) the first-ever ischemic anterior circulation stroke to avoid heterogeneity introduced by distinct functional recovery trajectories in posterior circulation stroke; (iii) available MRI examination, which has been acquired routinely either at admission or 24 h post-stroke. According to the clinical guidelines, all patients admitted to our stroke center should be examined by MRI at admission or 24 h post-stroke. However, CT scans were acquired instead if the patient’s general condition was instable (e.g. low level of consciousness, instability of blood pressure), contraindications for MRI existed (e.g. due to the pacemaker), and, more rarely, when MR-scanner capacity was limited. Patients who only received CT were excluded from the study, as CT does not allow the evaluation of all SVD features. Exclusion criteria were: (i) new stroke within the 3 months post-stroke, complications, death or other events within 3 months post-stroke that hamper the interpretation of functional outcome 3 months post-stroke; (ii) absent general consent for the data usage and/or missing clinical stroke scores; (iii) additional stroke in the posterior circulation. All patients underwent standardized treatment according to the Bernese Stroke guidelines, as well as standardized rehabilitation after discharge from the stroke unit. The study was approved by the regional ethics committee (Kantonale Ethikkommission Bern). Written informed consent was obtained from all participants. All methods were carried out in accordance with relevant guidelines and regulations.
During the standardized treatment, all patients were assessed by a certified neurologist. The clinical examination included NIH Stroke Scale (NIHSS) at admission and 24 h after stroke onset; the pre-stroke disability and functional stroke outcome 3 months post-stroke were assessed using the modified Rankin Scale (mRS). The cardiovascular risk factors were documented during hospitalization.
MRI-Imaging and assessment of small vessel disease neuroimaging markers
We analyzed clinical MRI data, which have been acquired routinely either at admission or 24 h post-stroke. MRI examinations were performed on either a 1.5 T or 3 T scanner (Magnetom Avanto, Aera, Skyra, Verio, Vida, Siemens Medical Solutions, Erlangen, Germany). The following MRI sequences from the emergency stroke protocol were used for the analysis: axial diffusion-weighted imaging (DWI) with apparent diffusion coefficient (ADC) (4–5 mm slice thickness), fluid-attenuated inversion recovery (FLAIR, 4–5 mm slice thickness), susceptibility-weighted imaging (SWI, 1.5 mm slice thickness), and axial contrast-enhanced T1-weighted image (4–5 mm slice thickness). Only MRI data that included all necessary sequences—DWI, FLAIR, SWI, T1—were considered for the analysis. Patients with pronounced artifacts in the MRI images were excluded from the analysis.
The four major imaging features of SVD composing SVD score were rated: WMH pronouncement, the total number of lacunes, the total number of microbleeds, and the number of EPVS. We did not evaluate the SVD features of cerebral amyloid angiopathy such as cortical superficial siderosis as our focus was hypertensive/age-related SVD, due to the low probability of cerebral amyloid angiopathy in ischemic stroke populations and potential confounders such as previous head trauma etc. Lacunes were defined as cerebrospinal fluid isointense ovoid or round cavity ranging from 3 to 20 mm in diameter with surrounding gliosis seen as hyperintensity on the FLAIR image9,12. Microbleeds were defined as a small homogenous hypointense signal on the SWI sequence (any localizations)9,12.. Mineralization or calcification of the globus pallidus, suspected cavernoma with popcorn pattern, or petechial hemorrhage within the infarct were not considered microbleeds17. WMHs were divided into periventricular and deep white matter according to Fazekas 0–318, and we used the highest score as an overall measure of the severity of WMH. Perivascular spaces were assessed as fluid-filled spaces that were visible as either linear or round/ovoid CSF–like signals with an axial diameter < 3 mm that follow the orientation of penetrating arterioles in basal ganglia and centrum semiovale12. The severity of EPVS was assessed at the level of basal ganglia as follows: 0: no EPVS, 1: < 10 EPVS, 2: 11–20, 3: 21–40, and 4: > 4019. The procedure was based only on the hemisphere with more visible pathology to account for a possible asymmetry of perivascular spaces or a masking effect of the acute ischemic infarct. Usually, perivascular spaces are evaluated on the T2-sequence, which was not a standard sequence in the applied acute stroke MRI protocol, therefore we used the B0-image and ADC-map of the DWI in combination with FLAIR and T1 to count the number of perivascular spaces. We aimed to counter possible noise introduced by this limitation with the less fine-grain ranking EPVS scale (0–4) used in the SVD score9,12.
A binary evaluation of each SVD feature was created according to the suggested rating scheme for the total SVD burden8,9: the SVD features were scored 1 with the cutoffs > 0 for lacunes, > 0 for microbleeds, and ≥ 3 for EPVS rating. The binary evaluation of WMH was scored 1 with periventricular WMH = 3 or deep WMH ≥ 28,9. The total SVD burden score8,9 was computed by adding the binary scores of the four features, creating a scale with integer values ranging from 0 to 4.
Statistical analysis
We tested the data distribution of quantitative SVD measures with Shapiro–Wilk tests and found a significant deviation from the normal distribution for all scores. Therefore, we assessed Kendall’s nonparametric τ intercorrelation between the SVD parameters and age. For the regression analysis, we log-transformed (with ln(x) or ln(x + 1)) the quantitative measures. All non-binary regressors were z-standardized to ensure comparability in the interpretation of coefficients. The target variable stroke outcome was measured using the mRS 3 months post-stroke. As this variable is ordinal (range 0–6), we calculated ordered logistic regression with the baseline regressors age, pre-stroke functional disability (pre-stroke mRS), stroke severity 24 h after symptoms onset (NIHSS 24 h), and added SVD features. We did not introduce the application of the acute stroke treatment (= thrombolysis yes/no) and its success (TICI-grade) in the baseline model, as they are reflected in the stroke severity 24 h after symptoms onset (NIHSS 24 h) i.e. after application of the acute stroke treatment. The SVD features were assessed (i) continuously = quantitatively, or (ii) binary = qualitatively. (i) In model 1, we assessed the continuous or quantitative SVD measures: the transformed number of lacunes and microbleeds, and the transformed ratings for WMH (Fazekas) and EPVS respectively; (ii) in model 2, we included the four SVD features as binary variables. To investigate the independent impact of SVD features on stroke outcome, we used backward stepwise elimination of regressors guided by p-values (p < 0.05) to find out which SVD feature is associated with stroke outcome independent from other SVD lesions and clinical parameters. Additionally, we performed a principal component analysis to exclude the multicollinearity constraining the interpretation of regression analysis.
In an additional logistic regression analysis, we evaluated the predictive value of SVD scales in relation to binary long-term prognosis (favorable versus poor) to evaluate their use in bedside prognosis. We modeled the binarised mRS 3 months post-stroke, split into favorable (0–2) and poor (3–6) outcome. The area under curve (AUC) in this logistic regression and its confidence intervals allowed us to directly compare the value of full, non-hierarchical models for quantitative versus binary SVD scales versus the total SVD burden score. For direct comparability of quantitative versus binary SVD scales, we ensured that both models included the same amount of regressors. We chose models with all regressors, i.e. age, pre-stroke mRS, NIHSS 24 h, and the four SVD measures, thereby creating the most optimistic estimate for a predictive gain by SVD measures. For this analysis, we excluded 50 patients with a medium or higher degree of disability already before stroke (i.e. pre-stroke mRS > 2). The predictive value was assessed by a receiver operating characteristic on the outcome of the logistic regression. The AUC and its confidence intervals were bootstrapped with 10.000 resamplings. The likelihood ratio test was applied to compare the baseline models with those with added SVD parameters. The statistical analysis was performed in R-statistics using the MASS package20.
Results
We included 873 patients in the present study. Table 1 summarizes the patients’ clinical characteristics. The pronouncement of all SVD features correlated weakly with each other (Kendall-tau, 0.283 > τ > 0.091, all p ≤ 0.001) and with age (Kendall-tau, 0.245 > τ > 0.109, all p < 0.001) except for WMH that strongly correlated with age (Kendall-tau, τ = 0.423, p < 0.001). An additional principal component analysis (the Jolliffe criterion, eigenvalues > 0.7) suggested that SVD features in the dataset were indeed four-dimensional enabling their further regression analysis.
Independent impact of SVD features on stroke outcome
We analyzed the impact of SVD features on stroke outcome (mRS, 0–6) independent from other SVD features and baseline clinical characteristics (age, pre-stroke disability and NIHSS 24 h). The analysis of model 1 with the continuous assessment of SVD features demonstrated that the number of microbleeds at the time of stroke improved prediction of stroke outcome (p = 0.002), whereas other SVD parameters—EPVS, WMH, and the number of lacunes—did not (backward elimination, ordered logistic regression, total McFadden’s pseudo-R2 = 0.122, AIC = 2454.7, Table 2; Supplementary Table S1). In model 2 with the binary evaluation of SVD features, prediction of stroke outcome was improved by the presence of lacunes (p = 0.01, pseudo R2 = 0.121, AIC = 2457.3, Table 2; Supplementary Table S2). The models were qualitatively comparable in the deviance explained. Importantly, age, stroke severity 24 h, and pre-stroke disability demonstrated a strong impact in both models (Table 2). Post-hoc, we additionally tested a model that included both the binary SVD score for lacunes and the number of microbleeds assessed continuously together with the baseline clinical parameters age, pre-stroke disability, and stroke severity 24 h. Both the presence of lacunes (p = 0.03) and number of microbleeds (p = 0.007) significantly contributed to the model together with the baseline variables (Table 2, pseudo R2 = 0.123, AIC = 2452.1). The direction of effects was always in the expected direction, meaning that the presence/severity of SVD features were associated with worse stroke outcome.
SVD measures in the prediction of favorable versus poor stroke outcome
We analyzed the impact of continuous and binary SVD measures in the prediction of binary stroke outcome, i.e. favorable (mRS 3 months 0–2) versus poor (mRS 3 months > 2) outcome. We excluded patients with pre-stroke disability (pre-stroke mRS > 2) from this analysis as they could bias the results. The comparison of AUCs between models (Fig. 1) with all continuous SVD measures (AUC = 0.876, 95% CI 0.849–0.906, pseudo R2 = 0.347), all binary SVD measures (AUC = 0.878, 95% CI 0.851–0.908, pseudo R2 = 0.348), the single total SVD burden score (AUC = 0.873, 95% CI 0.834–0.894, pseudo R2 = 0.311), and a baseline model without any SVD measures (AUC = 0.863, 95% CI 0.833–0.894, pseudo R2 = 0.325) found strongly overlapping confidence intervals between all three models that included SVD variables. Likelihood ratio tests indicated that the baseline model was outperformed by all those models—the model with continuous SVD predictors (X2(4) = 18.6, p < 0.001), the model with binary predictors (X2(4) = 20.0, p < 0.001), and the model with the total SVD burden score (X2(1) = 12.0, p < 0.001), though the overall model improvement was numerically small.
Receiver operating characteristics. Receiver operating characteristic curves that underlie the computation of the area under curve (AUC) for the logistic regression models of favorable (3-month mRS 0–2) versus poor (3-month mRS 3–6) stroke outcome. Blue: model including continuous SVD measures; yellow: model including binary rating of SVD measures; purple: model including the total SVD burden score; red: baseline model with baseline clinical parameters without any SVD measure.
Discussion
In contrast to the previous studies, the present study investigated the association of individual SVD features with functional stroke outcome adjusting for the presence and severity of other SVD markers, besides basic clinical parameters. We compared the continuous versus binary assessment of SVD features reflecting correspondingly the pronouncement and the simple presence of specific SVD features, while also taking relevant clinical parameters into account. As significant predictors of stroke outcome remained the continuous assessment of the number of microbleeds and, in the binary model, the presence of lacunes. A post hoc analysis showed that both features independently contributed to the prediction of stroke outcome when assessed with mRS 0–6. A statistically significant improvement in the simple binary prediction of stroke outcome (favorable versus poor) was observed by adding information on SVD features either only binary, only continuous, or as a total burden score, though the improvement in overall model fit was small (Fig. 1).
The number of microbleeds improved the prediction of stroke outcome, whereas the simple presence or absence of one microbleed (binary model) was not informative for the prediction model. One might explain it by the high prevalence (up to 38.3%) of microbleeds in the elderly21 pointing that the presence of one single microbleed could not represent a cerebrovascular pathology and therefore does not impact stroke outcome. In contrast, the number of microbleeds appears to represent a better measure of cerebrovascular pathology. The number of microbleeds showed the highest variance across assessed SVD features, and this variance was best captured by continuous measurement. This suggests that microbleeds require another cut-off in the SVD score or should be captured gradually to reflect best the SVD severity and its potential relevance on stroke outcome. For the lacunes, its presence assessed in the binary model might indicate more severe SVD1, whereas the number of lacunes in the continuous model was outperformed by the number of microbleeds. Another possible explanation for the low predictive value of the number of lacunes on stroke outcome might be their heterogeneity: the higher number of lacunes in the absence of microbleeds was not associated with worse stroke outcome, whereas fewer lacunes in the presence of microbleeds did22.
The impact of microbleeds on stroke is mainly investigated in the context of intracerebral hemorrhage. Microbleeds were associated with high mortality and morbidity7, an increased risk of intracerebral hemorrhage, ischemic stroke, death4,23, a higher risk of vascular dementia24, and stroke outcome15. As the continuous measure of microbleeds outperformed other SVD features, this parameter might be a marker for a far-progressed SVD stage.
The functional impact of lacunes was only investigated in a few studies7. Lacunes assessed in CT scans were found to be associated with poor stroke outcome25. In contrast, in a meta-analysis of patients who underwent mechanical thrombectomy for acute ischemic stroke, lacunes were not associated with poor outcome at 90 days26. Multiple lacunes were associated with worse outcome than single ones14. But again, most studies examined the impact of lacunes without taking into account other SVD features that made further implications on its independent impact difficult. In the present study, the presence of lacunes was associated with worse stroke outcome independent of age, stroke severity, pre-stroke disability and other SVD-features.
We did not observe an independent impact of WMH on stroke outcome. Historically, WMH were the first described feature of SVD18,27, as it could be visualized with conventional MRI sequences and CT. Therefore, WMH is the most investigated SVD feature. It was related to the risk of stroke and mortality4, and stroke outcome13. On the other hand, WMH is one of the most common incidental findings on MRI and strongly correlates with age7. The progression of WMH is also strongly associated with an increase of the number of lacunes28, and a higher burden of microbleeds29. Although WMH alone are associated with stroke outcome, the typically co-occurring lacunes and microbleeds outperformed the predictive value of WMH in the present regression analysis and thus could better explain clinical variance in stroke outcome. On the other hand, the volumetric analysis of WMH might further improve its predictive value and should be investigated in future research.
Although perivascular spaces are one of the recognized features of SVD, the data on its clinical relevance is still controversial12,30. The heterogeneous anatomy and physiology of perivascular spaces may explain the controversies31. The number of visible perivascular spaces on MRI increases with age, vascular risk factors (particularly hypertension), and other features of SVD31 that may be confounding factors complicating the assessment of the independent impact of EPVS on stroke outcome. In addition, a meta-analysis of 94 eligible studies in SVD reported limited data on EPVS, suggesting the need for further investigations4. In the present study, EPVS had no independent effect on stroke outcome.
The independent impact of single SVD features on stroke outcome was not investigated yet: The majority of studies investigated either the impact of one single SVD feature or the impact of total SVD burden measured via SVD sum score9. Several studies demonstrated the association between a higher SVD sum score with poorer stroke outcome32, whereas other studies failed to confirm this association for both ischemic10,11 and hemorrhagic stroke33, when compared to the usual clinical predictors, such as age and initial stroke severity (baseline NIHSS). Only a few studies assessed the impact of SVD features continuously, and the results were contradicting: one study failed to confirm this association34, whereas another one did10. In the present study, the prediction of stroke outcome (favorable versus poor) was statistically improved by adding all SVD features assessed either continuously or binarily. However, the effect of this improvement was negligible (Fig. 1). In other words, the severity of SVD lesions does not contribute substantially to poor outcome in a bedside setting but should be considered in big data studies or large trials. The small effect of adding SVD parameters might be explained by the outcome measure (favorable versus poor), which is mainly influenced by age and stroke severity. Besides differences in the sample size, the contradictory findings on SVD and stroke outcome might be explained by the applied statistical models, applied adjustment for the basic clinical parameters and pre-stroke disability. Furthermore, the cutoffs for binarization of individual SVD features introduced in the total SVD score have been chosen arbitrarily in a descriptive manner and have not been validated. Thus, the predictive value of the total SVD score might be improved by the application of improved and validated cutoffs for the individual SVD features. The current study has several strengths: first, microbleeds were assessed with SWI scans that are more sensitive than T2* MRI sequence. Second, we analyzed a relatively large cohort with standardized MRI sequences in the stroke protocol. Third, the study subjects represented the homogenous stroke population concerning stroke anatomy (anterior circulation), absence of the previous stroke, and absence of critical events within the observation interval that might worsen stroke outcome and in this way hamper data interpretation. The applied inclusion and exclusion criteria thus led to the homogeneity of the studied population and reduced the potential “composition bias” and consequently the Type II error35. Finally, we controlled for the pre-stroke functional disability that might be reduced due to accelerated aging, multimorbidity etc. However, the study also bears some limitations. As a standard procedure, perivascular spaces are evaluated on the T2 scans, which was not a standard MRI sequence in our acute stroke protocol. Therefore, we used the B0-image and ADC-map of the DWI scans in combination with FLAIR and T1 scans to rate the presence of EPVS. On the other hand, we assess the introduced noise as insignificant taking into account the rating procedure and strongly negative results. The anatomical distribution of microbleeds (basal ganglia versus lobar or cortical) and lacunes36 was not taken into consideration, as it is not required in the assessment of the SVD score; microbleeds localization was also not the aim of the present study. As we focused on hypertensive SVD (type 1) score, superficial siderosis was not taken into consideration and the criteria for cerebral amyloid angiopathy were not checked explicitly. This constrains the etiological interpretation of microbleeds. On the other hand, the cerebral amyloid angiopathy is much rarer than hypertensive SVD and its impact in the present result is unlikely to be decisive. MRI availability might introduce bias by exclusion of patients with more severe stroke or multimorbidity (= higher frailty) associated with contraindications for MRI examination or constraining its feasibility. However, these patients usually have a poor stroke outcome independent of SVD, whereas there is need to improve prediction of stroke outcome in patients with an uncertain prognosis. In the present study, the overall predictive values of models assessing mRS in the ordinal way were not high. This highlights the challenges of prediction of functional stroke outcome assessed in the ordinal form compared to the dichotomous one. On the other hand, the models included only basic clinical parameters. The analysis of lesion location or even simple lesion size37, vascular risk factors, and parameters of brain reserve and cognitive reserve38 might potentially improve model’s performance.
To conclude, whereas the reasonable theoretical framework for the assessment of SVD feature to predict stroke outcome is missing, the present study demonstrated that the method of assessment (continuous versus binary) is important and might differ across SVD features. From the major SVD features, only the number of microbleeds and the presence of lacunes were associated with stroke outcome independent of age, initial stroke severity, pre-stroke disability and other SVD features. Whereas the presence of lacunes is adequately assessed in the SVD sum score, microbleeds seem to require another cut-off or should be captured gradually, when better prediction of stroke outcome is needed. Simple binary prediction of stroke outcome (favorable versus poor) was improved by adding either continuous or binary SVD measures to clinical parameters. While this prediction improvement was not substantial for bedside settings, it should be considered in big data studies or large trials. Though the results should be confirmed in future studies, the present findings demonstrated the different value of the presence and severity of distinct SVD features that provide deeper insight into its pathophysiological and clinical impact on stroke outcome.
Data availability
Upon contacting the corresponding author, the authors will share the anonymized data that support the findings of this study with qualified investigators whose proposal of data use has been approved by the independent local review committee.
Abbreviations
- mRS:
-
Modified Rankin scale
- NIHSS:
-
National Institutes of Health Stroke Scale
- SVD:
-
Small vessel disease
- WMH:
-
White matter hyperintensities
- EPVS:
-
Enlarged perivascular spaces
References
Wardlaw, J. M., Smith, C. & Dichgans, M. Small vessel disease: Mechanisms and clinical implications. Lancet Neurol. 18, 684–696 (2019).
ter Telgte, A. et al. Cerebral small vessel disease: From a focal to a global perspective. Nat. Rev. Neurol. 14, 387–398 (2018).
Jokinen, H. et al. Global burden of small vessel disease-related brain changes on MRI predicts cognitive and functional decline. Stroke 51, 170–178 (2020).
Debette, S. et al. Clinical Significance of magnetic resonance imaging markers of vascular brain injury: A systematic review and meta-analysis. JAMA Neurol. 76, 81–94 (2019).
Del Brutto, V. J. et al. Total cerebral small vessel disease score and all-cause mortality in older adults of Amerindian ancestry: The Atahualpa Project. Eur. Stroke J. 6, 412–419 (2021).
De Giuli, V. et al. Subclinical vascular brain lesions in young adults with acute ischemic stroke. Stroke 53, 1190–1198 (2022).
Pasi, M. & Cordonnier, C. Clinical relevance of cerebral small vessel diseases. Stroke 51, 47–53 (2020).
Klarenbeek, P. et al. Ambulatory blood pressure in patients with lacunar stroke: Association with total MRI burden of cerebral small vessel disease. Stroke 44, 2995–2999 (2013).
Staals, J. et al. Stroke subtype, vascular risk factors, and total MRI brain small-vessel disease burden. Neurology 83, 1228–1234 (2014).
Georgakis, M. K. et al. Cerebral small vessel disease burden and cognitive and functional outcomes after stroke: A multicenter prospective cohort study. Alzheimers Dement J. Alzheimers Assoc. https://doi.org/10.1002/alz.12744 (2022).
Coutureau, J. et al. Cerebral small vessel disease MRI features do not improve the prediction of stroke outcome. Neurology 96, e527–e537 (2021).
Wardlaw, J. M. et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 12, 822–838 (2013).
Georgakis, M. K. et al. WMH and long-term outcomes in ischemic stroke: A systematic review and meta-analysis. Neurology 92, e1298–e1308 (2019).
Arauz, A. et al. Prospective study of single and multiple lacunar infarcts using magnetic resonance imaging. Stroke 34, 2453–2458 (2003).
Sakuta, K. et al. Cerebral microbleeds load and long-term outcomes in minor ischemic stroke. J. Stroke Cerebrovasc. Dis. 30, 105973 (2021).
Kim, B. J. & Lee, S.-H. Prognostic impact of cerebral small vessel disease on stroke outcome. J. Stroke 17, 101–110 (2015).
Greenberg, S. M. et al. Cerebral microbleeds: A field guide to their detection and interpretation. Lancet Neurol. 8, 165–174 (2009).
Fazekas, F. et al. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am. J. Roentgenol. 149, 351–356 (1987).
Doubal, F. N. et al. Enlarged perivascular spaces on MRI are a feature of cerebral small vessel disease. Stroke 41, 450–454 (2010).
Venables, W. N. & Ripley, B. D. Modern Applied Statistics with S (Springer, Berlin, 2002).
Vernooij, M. W. et al. Prevalence and risk factors of cerebral microbleeds: The Rotterdam Scan Study. Neurology 70, 1208–1214 (2008).
Sperber, C. et al. A typology of cerebral small vessel disease based on imaging markers. J. Neurol. https://doi.org/10.1007/s00415-023-11831-x (2023).
Wilson, D. et al. Cerebral microbleeds and stroke risk after ischaemic stroke or transient ischaemic attack: A pooled analysis of individual patient data from cohort studies. Lancet Neurol. 18, 653–665 (2019).
Ding, J. et al. Space and location of cerebral microbleeds, cognitive decline, and dementia in the community. Neurology 88, 2089–2097 (2017).
Arba, F. et al. Leukoaraiosis and lacunes are associated with poor clinical outcomes in ischemic stroke patients treated with intravenous thrombolysis. Int. J. Stroke 11, 62–67 (2016).
Xu, T. et al. Small vessel disease burden and outcomes of mechanical thrombectomy in ischemic stroke: A systematic review and meta-analysis. Front. Neurol. https://doi.org/10.3389/fneur.2021.602037 (2021).
Young, I. R. et al. Nuclear magnetic resonance (NMR) imaging in white matter disease of the brain using spin-echo sequences. J. Comput. Assist. Tomogr. 7, 290–294 (1983).
Gouw, A. A. et al. Heterogeneity of small vessel disease: A systematic review of MRI and histopathology correlations. J. Neurol. Neurosurg. Psychiatry 82, 126–135 (2011).
Yamada, S. et al. Periventricular and deep white matter leukoaraiosis have a closer association with cerebral microbleeds than age. Eur. J. Neurol. 19, 98–104 (2012).
Mahammedi, A. et al. Small vessel disease, a marker of brain health: What the radiologist needs to know. AJNR Am. J. Neuroradiol. 43, 650–660 (2022).
Wardlaw, J. M. et al. Perivascular spaces in the brain: anatomy, physiology and pathology. Nat. Rev. Neurol. 16, 137–153 (2020).
Appleton, J. P. et al. Imaging markers of small vessel disease and brain frailty, and outcomes in acute stroke. Neurology 94, e439–e452 (2020).
Hostettler, I. C. et al. Cerebral small vessel disease and functional outcome prediction after intracerebral hemorrhage. Neurology 96, e1954–e1965 (2021).
Bu, N. et al. Imaging markers of brain frailty and outcome in patients with acute ischemic stroke. Stroke 52, 1004–1011 (2021).
Xie, Y. Population heterogeneity and causal inference. Proc. Natl. Acad. Sci. 110, 6262–6268 (2013).
Pasi, M. et al. Distribution of lacunes in cerebral amyloid angiopathy and hypertensive small vessel disease. Neurology 88, 2162–2168 (2017).
Sperber, C. et al. Stroke lesion size—Still a useful biomarker for stroke severity and outcome in times of high-dimensional models. NeuroImage Clin. 40, 103511 (2023).
Umarova, R. M. Adapting the concepts of brain and cognitive reserve to post-stroke cognitive deficits: Implications for understanding neglect. Cortex 97, 327–338 (2017).
Acknowledgements
The authors thank all colleagues from the stroke unit of the University Hospital Bern, Switzerland and PhD Mattia Branca for his support with statistical analysis.
Funding
This work is funded by the Dementia Research Switzerland—Synapsis Foundation and Heidi Seiler Foundation.
Author information
Authors and Affiliations
Contributions
R.U. and A.H. researched literature and conceived the study; RU developed protocol, gained ethical approval; U.F., M.A., R.U., H.A., R.W., L.G. were involved in patients recruitment and data acquirement; A.H., F.W., B.R.K. and E.J. acquired SVD scores; C.S., R.U. performed statistical analysis; R.U., A.H., and C.S. wrote the first draft of the manuscript. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hakim, A., Gallucci, L., Sperber, C. et al. The analysis of association between single features of small vessel disease and stroke outcome shows the independent impact of the number of microbleeds and presence of lacunes. Sci Rep 14, 3402 (2024). https://doi.org/10.1038/s41598-024-53500-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-53500-7
- Springer Nature Limited