Abstract
Synthetic ester oils are widely used in many applications due to their ideal cleaning properties, lubricating performance and assured polarity. The majority of esters oils are more biodegradable. than any other base stock. For instance, oil soluble polyalkyleneglycols (PAGs) or polyalphaolephins (PAOs), are only biodegradable in the lower viscosity grades. The goal of this study is to create some synthetic base oils by two major protocols; the first is esterifying valeric acid with various glycols (ethylene glycol, propylene glycol, butylene glycol and poly (ethylene glycol 400). The second involves esterification of propanoic acid, heptanoic acid, or octanoic acid with ethylene glycol. The reaction yield varies between 85 and 94%. The chemical composition of the prepared esters was examined using various spectroscopic methods (Fourier-transform infrared (FT-IR) and proton nuclear magnetic resonance (1H-NMR) spectroscopy. The thermal properties investigation by thermo gravimetric analysis (TGA) showed pronounced thermal stability of the prepared esters. The biodegradability was verified versus two bacterial isolates (B1, B2). The results showed that percentage of degradation of the lube oil was in the range of 34% to 84% after 3 days of incubation. Moreover, the rheological study revealed that the prepared esters exhibited Newtonian rheological behaviours. Viscosity examination displayed that the esters based on ethylene glycol, such as (A), had the highest VI: 179 values when compared to those based on higher glycols. Viscosity and viscosity index results showed slight increase as the number of carbon atoms in the acid chain increases. At last, most of the synthesized esters possessed pour points ≤ − 32 °C: ≤ − 40 except in case of using higher acids like heptanoic acid and octanoic acid in preparation the pour point increases to − 9 °C and − 15 °C.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Introduction
Various microorganisms have the ability of utilizing hydrocarbons as their sole carbon source for metabolic activity, such microorganisms are plentiful in nature. The hydrocarbon used by each microbic strain is dependent on the chemical composition of the petroleum mixture, as well as other environmental considerations1. One of the common sources of the hydrocarbons is Lubricating oil which represents a widespread pollutant in both water and soil. Lubricating oil typically contains 80% hydrocarbon base oil and the rest is different additives. On the other hand, the green chemistry approach emphasizes the use of environmentally friendly materials- as possible- in different industries and applications2. This trend of replacing petroleum-based oils with more environmentally friendly insulating oils can also be seen in oil field transformer problems. Lubricant is emitted into the environment in the form of oil vapor and viscous micro droplets, creating a severe environmental risk. The power and impact of oil derivative interactions are directly related to the content, volume, and frequency of emissions in a particular area, as well as the attributes with open cutting system device [ref]. As known, mineral oils have a very low biodegradability [ref]. Also, Petroleum-derived oil poses significant risks to sawing operators in the natural environment. On the other hand, oil dangerously accumulates in different parts of plant, animal tissues, and even in groundwater causes various risks. More specifically, crude oil derived lubricants are a major danger to aquatic habitats even at very low concentrations3. However, Synthetic or vegetable oils are considered more suitable substitutes4. When designing the lubricant content, both environmental and application parameters must be taken into concern. As a result, it must be distinguished not just by a specific rate of biodegradability, but also by suitable physicochemical aspects3, such as the viscosity index range, dynamic and kinematic viscosities at different temperatures, pour point, evaporability, flash points, and the base or acid number. The use of appropriate (biodegradable) insulating oils would significantly improve the environmental aspects of all power transformer operations. This premise serves as the foundation for the primary intent of this paper, which is to investigate the possibilities of substituting suitable synthetic or natural products for petroleum-based oils4.
Transesterification and esterification reactions are commonly performed in hydrocarbon solvents such as toluene. The most common homogeneous acid catalysts for this purpose are sulfuric acid, methane sulfonic acid, and p-toluene sulfonic acid5,6. The usage of these catalysts poses a few obstacles, such as corrosion beside their environmental hazards. As a result, new methodologies are being developed involving more efficient catalysts that do not require solvent7,8. It is generally known that heterogeneous catalysts are used in liquid phase organic reactions, which can provide numerous advantages. After simple filtration, the catalyst is easily recovered, and the reaction product solution is clean. Therefore, some considerations have been taken into account in esterification processes to the employment of heterogeneous catalysts2. On the other hand, bioremediation is a type of biodegradation in which microorganisms are used to free the environment from organic and inorganic xenobiotics via their enzymatic activity9. Indeed, bacteria and fungi degrading hydrocarbons are abundant in marine, freshwater, and soil habitats10.
This research focuses on synthesis of diol diesters using Amberlyst®15 heterogenous catalyst by interaction of different dihydric alcohols (ethylene glycol, propylene glycol, butylene glycol, and poly ethylene glycol 400) with different monoacids (propanoic, heptanoic, octanoic, and valeric acids) and evaluating them to be used as highly performed synthetic lubricants and measuring the rate of biodegradability of these esters using two types of microorganisms.
Experimental
Materials
Acids (propanoic, heptanoic, octanoic, and valeric acids) were acquired from Aldrich Chemical Co. Ltd. (UK). Ethylene glycol (EG), propylene glycol (PG), and butylene glycol (BG) were acquired from VEB LABORCHEMIE APOLDA. Polyethylene glycol 400 (PEG 400) was maintained from Morgan Chemical Ind. Co. Other chemical reagents and organic solvents were of analytical quality and acquired from Carlo Erba Reagenti. Amberlyst 15 used came from Alfa Aesar Co. with CAS no. 6192-52-5.
Procedure
Esterification reaction
The preparation of three diesters was achieved by a facile methodology. Simply, 2 mol of valeric acid with one mole of different glycols (ethylene glycol, propylene glycol, butylene glycol, and polyethylene glycol 400) were placed inside a closed 1L. three-necked flask fitted with a Dean-Stark trap (filled with xylene). Also, the other three diol glycols were prepared by reacting two moles of different acids (propanoic, heptanoic, or octanoic acid) with one mole of ethylene glycol in the same manner in presence of Amberlyst®15 (0.5 wt%) as a catalyst the reaction was held in xylene. Slow flow of deoxygenated nitrogen 40 ml/min. was applied to avoid oxidation. The reactants with the same weight of xylene as a solvent are gradually heated at a controlled thermostat temperature up to 120 °C ± 0.5 °C and stirred at 500 rpm11. The reaction was held for 8 h to reach a product yield of 83–92%. To find out the rate of the reaction, the amount of freed water in Dean-Stark trap was measured.
Purification of prepared esters
A 10% by weight aqueous solution of sodium carbonate was used to wash the organic layer in the separating funnel, followed by multiple washing with distilled water. To remove xylene, under vacuum, a rotary evaporator was utilized. Then the products were dryed by using sodium sulfate anhydrous overnight12. The codes and composition of prepared esters are given in Table 1.
Characterization of prepared compounds:
Table 2 displays the analysis used to identify the chemical composition and examine the physicochemical properties of the prepared diesters.
Biodegradation measurements
Isolation of hydrocarbon utilizing bacteria
Oil degrading bacteria have been isolated via mineral salts medium (MSM). The MSM (g/L) includes the following ingredients: 0.5 MgSO4·7H2O; 0.5 KH2PO4; 0.1 KCl; 4.0 NH4NO3; 1.0 K2HPO4; 2.0 NaCl; 0.01 CaCl2; and 0.01 FeSO4·7H2O. The pH of the media was adjusted to 7.0 ± 0.2 by using 1% oil as the sole carbon source. The 250 mL conical flasks were inoculated with 100 mL of MSM broth The inoculated sample flasks were incubated for 7 days at 30 ± 2 °C in a shaking incubator at 150 rpm13. The bacterium colonies that grew on the plates were chosen for further investigation.
Selection of the most promising bacterial isolates
The most promising bacterial isolates for oil degradation (B1 and B2) grew faster on MSM mixed with oil as the main carbon source. They were chosen for further research.
Molecular identification of the bacterial isolate
The degrading bacterial isolates (B1 and B2) were identified in Sigma Scientific Services Co., Egypt, using 16s rDNA. After PCR product purification, the positive clone's DNA sequence was compared by the use of BLAST on the NCBI website (http://www.ncbi.nlm.nih.gov) and deposited in GenBank. Many relevant 16S rDNA gene sequences with currently published names were picked from the Gen-Bank as references.
Screening for the ability of bacterial isolates to degrade lubricating oil
By the use of mineral salt medium (MSM), the bacterial isolates were tested because of their capability of using lubricating oil as a main carbon source. Individual bacterial Cells were introduced into 250 ml Erlenmeyer conical flasks in amounts of 5 ml. Each containing 100 ml of sterile MSM containing 1% v/v of five different lubricants. The experiment was done in triplicate, with uninoculated flasks serving as controls. The flasks were all incubated at 30 °C for 72 h at 150 rpm and pH 7.5. The leftover crude oil samples have been taken out from various cultural microcosms and analyzed gravimetrically and chromatographically.14.
Analysis of lubricating oil biodegradation
Each flask's remaining oil was extracted using the US-EPA Gravimetric Method (1999) after the predetermined incubation period. In a nutshell, an oil-containing sample was gathered in a 500-mL separating funnel. By adding 6 N HCl, the pH was reduced to 2. Carbon tetrachloride was used twice to extract the oil in the samples. The solution's remaining solvent was then evolved in a rotary evaporator. Finally, the concentrated oil flask was placed in a desiccator for drying. Gravimetric analysis was used to determine the amount of residual oil recovered15. The percentage of oil content reduction was calculated as (A − B)/A × 100, where A is the initial crude oil weight and B is the remaining crude oil weight.
Determination of normal and iso-paraffins
The residual hydrocarbons were quantified chromatographically with Agilent 6890 plus capillary gas chromatography (GC)16. The peak area of each resolved constituent (whether n- or iso-paraffins) was measured separately. While the UCMs were made up of non-n-paraffins, primarily cycloparaffins and aromatics with long side chains, they were only detected as a total hump.
Results and discussion
There is an urgent need to develop pollution-free and environmentally friendly lubricants as an alternative to mineral-based lubricants17. Rape, soybean, and palm oils are among examples of basic oils. They possess good tribological properties. Despite their obvious benefits, they have limited corrosion resistance and are in danger of hydrolytic and oxidative instability18. Esters of glycols are an effective replacement for mineral and vegetable oils. This research focuses on the synthesis of diesters, the rate of biodegradability of the prepared esters, and their suitability for use as synthetic lubricants.
Preparation of diesters
Diesters were produced by individually reacting valeric acid with various glycols (ethylene glycol, propylene glycol, butylene glycol, or polyethylene glycol 400). Then, ethylene glycol reacts with lower (propanoic acid) and higher (heptanoic or octanoic acid) acids separately. The overall esterification reaction is shown in Fig. 1.
Structure confirmation of the prepared esters
FTIR
FTIR spectroscopy was used to ensure the completion of esterification reactions. The spectra of all esters are nearly the same with slight differences as seen in Figs. 2a–d. Generally, the FTIR spectra display the following: the disappearance of strong peaks –OH at 3500 cm−1 ± 10 cm−1 and acid group band C=O at 1730 cm−1 at the end of esterification and their replacement by ester group bands C=O at 1735 cm−1 and 1265 cm−1 ± 10 cm−1, respectively. This demonstrates that the esterification procedure was carried out successfully11.
1H-NMR spectroscopy
1H-NMR spectroscopy was used to investigate the composition of the products A-F. Figures 3a–f demonstrate diesters' chemical shift as follows: 1H-CH3, triplet at 0.96 ppm (a), 1H-CH2, multiplet at 1.33 ppm (b), 1H-C–C(O)OR esters, multiplet at 1.68 ppm (c), 1H-C(O)-OC, triplet at 2.25 ppm (d), 1H-O-C(O)R, triplet at 4.36 ppm (e), as well as the loss of RCOOH carboxylic acid, which approve the complete acids esterification.
Thermal stability of prepared esters
The diesters’ (A–G) thermal properties were also investigated. For example in Fig. 4a–f the complete degradation occurs between 150 and 300 °C, demonstrating a reasonable thermal stability of prepared esters. So, their use is consistent with the temperature restrictions depicted in the thermograms.
Physicochemical properties of prepared esters
Rheological behavior of prepared esters
The synthesized compounds have Newtonian rheological properties, as illustrated in Fig. 5a,b. This means that Newtonian fluids follow Newton's law of viscosity. Viscosity is unaffected by shear rate. In lubrication, Newtonian fluids like motor oil, are utilized for lubrication purposes in engines and machinery to reduce friction, dissipate heat and protect against wear and tear. Newtonian lubricant is preferable for improving tribological performance. Newtonian fluid can significantly increase the load-carrying capacity of bearing19.
Biological degradability of prepared esters
Culture media (MSM) detected seventeen bacterium isolates (B1-B17) from a sample of oil-contaminated water. On MSM media, the bacterium isolates B1 and B2 are considered predominant.15 determined two biodegrader bacterium species as having the best rate of growth on MSM medium.
Molecular identification of the selected oil degrading bacteria
Under the light microscope, the bacterium isolate (B1) is a Gram-negative, rod-shaped organism. 16S rRNA identifies the bacterial isolate (B2) as Enterobacter hormaechei subsp. Xiangfangensis strain 10–17 having a 99.17% similarity. MEGA programme is being used. A sequence of Enterobacter hormaechei subsp. identified in this investigation matched with other Enterobacter species. Figure 6a depicts the phylogenetic tree built from Enterobacter hormaechei and closely related bacterial strains using the 16S rRNA gene's Neighbor-joining method.
(a) A phylogenetic tree of Enterobacter hormaechei subsp. Xiangfangensis strain 10–17 16S evolutionary relationships to other strains, taken from the NCBI database and the phylogenetic tree done by the Mega X program. (b) A phylogenetic tree of Pseudomonas aeruginosa ATCC 10145 evolutionary relationships to other strains, taken from the NCBI database and the phylogenetic tree done by the Mega X program.
Under a light microscope, the bacterium isolate (B2) appears as a rod-shaped, Gram-negative organism. Pseudomonas aeruginosa ATCC 10145 is recognised as the bacterial isolate (B2) by 16S rRNA with a similarity of 99.53%. using the MEGA programme. Pseudomonas aeruginosa sequences from this study's isolation were matched with Pseudomonas species via BLAST analysis. Figure 6b shows how the phylogenetic tree of Pseudomonas aeruginosa and closely related bacterial strains was reconstructed using the Neighbor-joining method of the 16S rRNA gene.
Biodegradation of different lubricating oil samples using the degrading bacterial strains
In the current study, the capacity of two different bacterial isolates (B1–B2) to biodegrade of the lubricating oil sample was evaluated. The pure bacterial isolates were inoculated into MSM media with different lubricating oil separately used as sole carbon source for 3 days. The oil samples were accurately weighted as a gravimetric analysis. The percentage of the oil that had biodegraded was estimated, and gravimetric analysis (GC) was used to find the change in chemical composition.
Gravimetric analysis of the different degraded lubricating oil
According to the results of gravimetric analysis in Table.3, different bacterial isolates (B1-B2) degraded lubricating oil in a range from 34 to 84% after incubation for three days. In comparison to other types of lubricating oil, the microcosms containing the individual bacterial isolates (B1 and B2) with A and D gave a higher percentage of degradation. Verma et al.20 stated that, after 5 days, Pseudomonas sp. SV 17's oily sludge degradation capacity represents around 60% of the saturates and aromatics components. El-Sheshtawy et al.16 demonstrated that after 7 days, the bacterial strains had broken down between 30 and 50% of the crude oil.
Gas chromatographic analysis
The first step in the mechanism of alkane degradation in lubricating oil by bacteria in aerobic conditions is the oxidation of alkanes by the class of oxygenase enzymes (enzymes that catalyze the incorporation of oxygen into the substrate) namely alkane hydroxylase enzymes that catalyze the addition of hydroxyl groups by attacking the O atom through oxidation during the alkane hydroxylation reaction. Alkanes are oxidized to alcohol and subsequently become fatty acids21. The next pathway of fatty acid metabolism can be through cellular lipid pathways, β-oxidation, and α-oxidation. Through the β-oxidation pathway fatty acids will be converted into acetyl CO-A and enter into the TCA cycle, converted into CO2 and energy. If through the fatty acid α oxidation pathway it will be converted directly into CO2 and fat derivatives22.
In this study, the biodegradation of various lubricating oils was examined in the current study utilising the GC for aliphatic compounds after three days of the incubation period. Following biodegradation by two different bacterial strains, the residual n-paraffin and iso-paraffin percentages contained in various lubricating oils were calculated and contrasted with the control sample of Tables 4, 5.
The results demonstrated that iso-paraffins degraded faster than n-paraffins, which consider more resistant for the biodegradation process in the most of different microcosms separately. In the literature study The hydrocarbons vary in their sensitivity to microbial attack, and they were previously classed in the following order of decreasing sensitivity: n-alkanes ˃ branched alkanes ˃ aromatics with low molecular weight cycloalkanes6. On the other hand, additional high molecular weight substances consumed can be blamed for an increase in any compound's percentage, which causes a proportional buildup of that hydrocarbon component on the low molecular weight chemicals. The data above indicate that (A and D) types of lubricating oil were more consistently degraded by two separate bacterial strains than other types of lubricating oil.
Evaluation of the prepared esters
A lubricant's primary characteristic is that it is anticipated to lubricate. The lubricity of a molecule refers to how well it coats the metal surface, competes for it, and flows over itself. Because they get in contact with metal surfaces and minimise the amount of metal-to-metal contact during sliding motion, esters are typically regarded as effective border lubricants. The chain length, degree of branching, and position of links inside the molecule are structural elements that affect lubricity. Due to oxygen's electronegativity, there is a permanent dipole in the ester chemical reaction. This inherent polarity has a number of effects and contributes significant lubricating properties. Permanent dipoles have greater internal cohesion than pure hydrocarbons and are attracted to one another by electrical power, which reduces their evaporation rate and volatility.
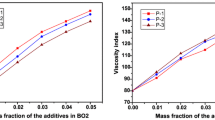
If the viscosity is too high, it could result in low oil flow, causing oil starvation and dry startups. When the viscosity is too low, greater mechanical friction may occur, which can lead to heavier loads. You also would see more wear due to film loss.
A higher viscosity index number means the lubricant changes viscosity at a lower rate based on the temperature. A higher VI is more desirable because it enables the lubricant to provide a more stable lubricating film over a wider temperature range.
The synthetic esters' viscosity was tested three times at 40 °C and 100 °C. The average values were displayed in Table 6. By raising the number of carbon atoms in the glycols utilised, viscosity index values drop, as seen in Fig. 7a. Consequently, utilising ethylene glycol in (A) results in superior viscosity index values VI: 179. The viscosity index values in Fig. 7b show increase with increasing internal chain length of the acid up to valeric acid (5 carbons) in (A) with VI: 179, then decrease with the use of heptanoic acid (7 carbons) with VI: 170 and octanoic acid (8 carbons) with VI: 109 because the attraction among molecules of monoalcohols increases as the acid's carbon number increases. As a result, oils might be classified as multirange oils. Except for those prepared by heptanoic and octanoic acid, all created esters had pour points equal to or less than − 42 as a result of a rise in molecular weight. As indicated in Table 6, density values decrease as the chain length of the aliphatic glycols increases. All of the produced compounds have densities ranging from 0.90 to 1.06, and flash point range 140: 200.
Conclusion
According to the findings of this investigation, the ester formed by the reaction of valeric acid with ethylene glycol as (A) exhibits better value of VI: 145 and by different glycols used, the VI decreases by raising the chain length of glycol used. Most of the prepared esters provide pour points from ≤ − 30 °C to ≤ − 42 °C except in case of using higher acids like heptanoic acid and octanoic acid in preparation the pour point increases to − 9 °C and − 15 °C. All of the esters that have been prepared are Newtonian fluids. It was found that the rate of biodegradability of the prepared esters by degrading bacterial isolates (B1, B2) in the range from 34 to 84% after 3 days of incubation period. The ester resulting from reaction of valeric acid with ethylene glycol give higher biodegradability with 84%. We recommend that the prepared esters be used as a biodegradable synthetic lubricating oil based on our findings.
Data availability
Data available on request from the corresponding author Reham I. El Shazly, E.mail: reham_chemidt21@yahoo.com.
References
Adeline, S. Y., Carol, H. C. T. & Aw, C. S. Hydrocarbon-degradation by isolate Pseudomonas iundensis UTAR FPE2. Malays. J. Microbiol. 15, 23. https://doi.org/10.21161/mjm.15609 (2009).
El-Shazly, R. I., Haseeb, M. E., Nassar, A. M. & Ahmed, N. S. Synthesis and characterization of synthetic lubricants based on dibasic acid. Can. J. Chem. Eng. 101, 6372–6384 (2023).
Nowak, P., Kucharska, K. & Kamí, M. Ecological and health effects of lubricant oils emitted into the environment. Int. J. Environ. Res. Public Health https://doi.org/10.3390/ijerph16163002 (2019).
Mentlík, V., Polanský, R., Prosr, P., Pihera, J. & Trnka, P. Synthetic ester-based oils and their application in power industry. Renew. Energy Power Qual. J. 1, 215–219 (2009).
Juárez, R., Martín, R., Álvaro, M. & García, H. (Perfluoro)sulfonic acids having an imidazolium tag as homogeneous and reusable ionophilic Brönsted acid catalysts for carboxylic acid esterification. Appl. Catal. A 369, 133–137 (2009).
Drelinkiewicz, A., Kalemba-Jaje, Z., Lalik, E. & Kosydar, R. Organo-sulfonic acids doped polyaniline-based solid acid catalysts for the formation of bio-esters in transesterification and esterification reactions. Fuel 116, 760–771 (2014).
Ahmad, U. et al. A review on properties, challenges and commercial aspects of eco-friendly biolubricants productions. Chemosphere 309, 136622 (2022).
Khan, Z. et al. Current developments in esterification reaction: A review on process and parameters. J. Ind. Eng. Chem. 103, 80–101 (2021).
Philp, J. C. & Atlas, R. M. Bioremediation of contaminated soils and aquifers. Bioremediation https://doi.org/10.1128/9781555817596.ch5 (2005).
Atlas, R. M. & Bartha, R. Stimulated biodegradation of oil slicks using oleophilic fertilizers. Environ. Sci. Technol. 7, 538–541 (1973).
El-shazly, R. I., Kamal, R. S., Nassar, A. M., Ahmed, N. S. & Sayed, G. H. The behavior of some terpolymers as lubricating oil additives. Appl. Petrochem. Res. 10, 115–123 (2020).
Kamal, R. S., Nassar, A. M. & Ahmed, N. S. Study the efficiency of some esters based on 2- ethyl hexanoic acid as synthetic lubricants. Egypt. J. Chem. 64, 7191–7200 (2021).
Larik, I. A. et al. Stenotrophomonas maltophilia strain 5DMD: An efficient biosurfactant-producing bacterium for biodegradation of diesel oil and used engine oil. Int. J. Environ. Sci. Technol. 16, 259–268 (2019).
El-Sheshtawy, H., Khalil, N., Ahmed, W. & Amin, N. Enhancement the bioremediation of crude oil by nanoparticle and biosurfactants. Egypt. J. Chem. 60, 835–848 (2017).
El-Sheshtawy, H. S., Aman, D. & Nassar, H. N. A novel bioremediation technique for petroleum hydrocarbons by bacterial consortium immobilized on goethite-chitosan nanocomposite. Soil Sediment. Contam. 31, 176–199 (2022).
El-Sheshtawy, H. S., Khalil, N. M., Ahmed, W. & Abdallah, R. I. Monitoring of oil pollution at Gemsa Bay and bioremediation capacity of bacterial isolates with biosurfactants and nanoparticles. Mar. Pollut. Bull. 87, 191–200 (2014).
Lakkoju, B. & Vandana, V. Preparation and properties of bio-lubricants of neopentylglycol esters from various acids. Res. J. Chem. Environ. 26, 9–18 (2021).
Emelianov, V. V., Krasnykh, E. L., Portnova, S. V. & Levanova, S. V. Synthetic oils based on pentaerythritol esters. Vapor pressure and enthalpy of vaporization. Fuel 312, 122908 (2022).
Abbaspur, A., Norouzi, M., Akbarzadeh, P. & Vaziri, S. A. Analysis of nonlinear viscoelastic lubrication using Giesekus constitutive equation. Proc. Inst. Mech. Eng. Part J. 235, 1124–1138 (2020).
Verma, S., Bhargava, R. & Pruthi, V. Oily sludge degradation by bacteria from Ankleshwar, India. Int. Biodeterior. Biodegrad. 57, 207–213 (2006).
Blasig, R. et al. Degradation of long-chain n-alkanes by the yeast Candida maltosa. Appl. Microbiol. Biotechnol. 28, 589–597 (1988).
Al-Hawash, A. B. et al. Principles of microbial degradation of petroleum hydrocarbons in the environment. Egypt. J. Aquat. Res. 44, 71–76 (2018).
Acknowledgements
The authors are greatly thankful to the Egyptian Petroleum Research Institute (EPRI) for its extreme support.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
R.I.E.: writing original draft and visualization, experimental, formal analysis, investigation, data curation. H.S.E.: writing, experimental, formal analysis, investigation, data curation of biodegradation part. N.S.A.: conceptualization, methodology, supervision, project administration, review and editing. A.M.N.: conceptualization, methodology, supervision, project administration, review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shazly, R.I.E., El-Sheshtawy, H.S., Ahmed, N.S. et al. Synthesis and biodegradation testing of some synthetic oils based on ester. Sci Rep 14, 3416 (2024). https://doi.org/10.1038/s41598-024-53331-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-53331-6
- Springer Nature Limited