Abstract
Acetylcarnitine is an essential metabolite for maintaining metabolic flexibility and glucose homeostasis. The in vivo behavior of muscle acetylcarnitine content during exercise has not been shown with magnetic resonance spectroscopy. Therefore, this study aimed to explore the behavior of skeletal muscle acetylcarnitine during rest, plantar flexion exercise, and recovery in the human gastrocnemius muscle under aerobic conditions. Ten lean volunteers and nine overweight volunteers participated in the study. A 7 T whole-body MR system with a double-tuned surface coil was used to acquire spectra from the gastrocnemius medialis. An MR-compatible ergometer was used for the plantar flexion exercise. Semi-LASER-localized 1H MR spectra and slab-localized 31P MR spectra were acquired simultaneously in one interleaved exercise/recovery session. The time-resolved interleaved 1H/31P MRS acquisition yielded excellent data quality. A between-group difference in acetylcarnitine metabolism over time was detected. Significantly slower τPCr recovery, τPCr on-kinetics, and lower Qmax in the overweight group, compared to the lean group was found. Linear relations between τPCr on-kinetics, τPCr recovery, VO2max and acetylcarnitine content were identified. In conclusion, we are the first to show in vivo changes of skeletal muscle acetylcarnitine during acute exercise and immediate exercise recovery with a submaximal aerobic workload using interleaved 1H/31P MRS at 7 T.
Similar content being viewed by others
Introduction
Acetylcarnitine can be detected in skeletal muscle tissue at 2.13 ppm by proton magnetic resonance spectroscopy (1H MRS)1,2 and has recently been suggested to be a very important metabolite in the control of muscle oxidative metabolism, glucose homeostasis, and insulin sensitivity3,4,5,6. The availability of free carnitine is recognized as a crucial factor in acetylcarnitine formation and the maintenance of metabolic flexibility3,4. The excessive buildup of acetyl-CoA serves as a strong allosteric inhibitor of pyruvate dehydrogenase, which serves as a key enzyme controlling the entry of pyruvate into the Krebs cycle7. The interconversion of acetyl-CoA and acetylcarnitine is catalyzed via the mitochondrial matrix enzyme carnitine acetyltransferase (CrAT)8. Although invasive measurements of acetylcarnitine following intensive exercise are well established9,10,11,12,13,14, there are only few reports on non-invasive dynamic measurements2,3.
Differences in baseline (non-exercise) acetylcarnitine concentrations have been shown between triathletes, active healthy individuals with normal glucose tolerance, patients with impaired glucose tolerance, and patients with type 2 diabetes mellitus (T2DM)15,16. It has been shown that a heavy workout (high-intensity exercise) leads to an immediate post-exercise increase of acetylcarnitine concentrations17. However, the in vivo dynamics of muscle acetylcarnitine content during exercise and early recovery in populations with different levels of cardiometabolic fitness has not yet been examined.
31P MRS is a well‐established method with which to investigate skeletal muscle energy metabolism in vivo18. Physiologically relevant parameters can be derived from time courses of phosphocreatine (PCr) and inorganic phosphate (Pi), that is, pH, the time constant of PCr resynthesis (τPCr recovery), the time constant of PCr decay at the onset of exercise (τPCr on-kinetics), and maximum oxidative capacity, Qmax19,20,21, which are linked to energy efficiency during contraction (exercise period). In particular, the τPCr recovery dynamics and Qmax are good measures of intracellular oxidative ATP production22. PCr-on-kinetics is assumed to be a marker of skeletal muscle mitochondrial inertia21. A prolonged duration signifies an extended dependence on PCr for ATP production, leading to a heightened skeletal mitochondrial inertia. The rate at which PCr is replenished serves as an indicator of oxidative capacity. A quicker recovery of PCr post-exercise signifies swifter replenishment and, consequently, superior mitochondrial function in restoring cellular energy. Moreover, from static fully relaxed muscle 31P MRS, we can calculate concentrations of phosphomonoesters (PMEs), phosphodiesters (PDEs), and nicotinamide adenine dinucleotide phosphate (NADP).
Although the detection of acetylcarnitine using 1H single-voxel MRS and the exercise-challenged time course of PCr and Pi concentrations using dynamic 31P MRS are well established and there is a good consensus about their standardization23,24, the concurrent interleaved measurement25 of these metabolites has not yet been performed. Reproducing the exact same exercise load under identical metabolic conditions in two sequentially repeated measurements is notoriously difficult. Hence, an assessment of acetylcarnitine, PCr, and Pi time courses during a single exercise-recovery experiment increases the volunteer’s compliance with the experimental procedures and could improve potential correlation between the metabolites. Recent improvements in interleaved MRS methodology25,26,27,28,29 and an advanced double-tuned multi-channel surface RF coil30 are the best pre-requisites for such an experiment.
Based on our previous experiments we hypothesize qualitatively similar trends between acetylcarnitine and PCr during the rest/exercise/recovery period, and therefore we expect correlations between dynamics of those two processes. Moreover, based on findings published previously2,15,31,32, we hypothesize lower acetylcarnitine concentrations at rest in overweight and sedentary volunteers.
Thus, we aimed to explore the time-dependent behavior of acetylcarnitine in more detail during rest, plantar flexion exercise, and recovery in the human gastrocnemius muscle (GM) and PCr and Pi evolution under aerobic conditions during a single experiment using non-invasive interleaved 1H/31P MRS at 7 T. To demonstrate the physiologic value of this measurement, we have included two metabolically different groups of healthy volunteers (lean and active and overweight and sedentary). Last but not least, we aimed to perform a bivariate analysis of the interrelations between acetylcarnitine and 31P-derived parameters, as post-exercise phosphocreatine recovery rates had previously been shown to be positively associated with CrAT activity and coincided with dramatic shifts in muscle acetylcarnitine dynamics33.
Methods
Participant characteristics
Ten healthy, lean, and active volunteers (Age: 30 ± 6 years, BMI: 21.3 ± 2.4 kg/m2, sex: 7 f/3 m) and nine overweight and sedentary volunteers (Age: 33 ± 6 years, BMI: 32.7 ± 3.0 kg/m2, sex: 5 f/4 m) participated in the study. Forty-five to ninety minutes of continuous exercise, regardless of endurance or resistance training, was considered as one training unit. Written, informed consent was provided in accordance with the local ethics committee requirements. The study was approved by the ethics committee of the Medical University of Vienna (NR:1081/2020) and all experiments were performed in accordance to the guidelines of the Declaration of Helsinki.
To determine maximal oxygen uptake (VO2max), all participants underwent a standardized protocol with continuous increments, until exhaustion, on a cycle ergometer (Lode Excalibur, Groningen, The Netherlands). Measurement of VO2max and other parameters was performed via "breath-by-breath" spiroergometry (Master CPX, VIASYS Healthcare).
The maximal voluntary contraction force (MVC) of the right leg was measured with a leg-press dynamometer (FPES CU, Bratislava, Slovakia) and served later to adjust the exercise load for the in-magnet challenge.
Magnetic resonance (MR) measurements
All MR measurements were performed on a 7 T whole-body MR system (Terra Dot Plus, Siemens Healthineers, Erlangen, Germany). A double-tuned surface coil transceiver array with two 1H channels (d = 17 cm, l = 12.5 cm) and three 31P channels (d = 15 cm, l = 10 cm shaped to the anatomy of the human calf30 was used to acquire spectra from the gastrocnemius medialis/lateralis of the right leg. An MR-compatible ergometer (Trispect, Ergospect, Innsbruck, Austria) was used for the plantar flexion exercise. The setup of coil and ergometer inside of the scanner is depicted in Fig. 1A and B.
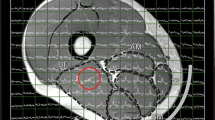
Whole setup of volunteer lying on the 7 T MR system table with right calf muscle placed on the double-tuned 1H/31P coil and on the ergometer pedal fixed with belts (A, B), Axial localizer image of a calf muscle with depicted slice selection of the DRESS localization sequence from 31P MRS (white slab), VOI for acetylcarnitine acquisition with 1H MRS (yellow voxel), and shimming volume (green rectangle) (C), interleaved rest/exercise/recovery session (2 min rest, 15 min exercise at 30–40% of MVC, 23 min recovery) and acquisition of DRESS-slab-localized 31P MR spectra from white slab and semi-LASER-localized 1H MR spectra from yellow voxel followed by plantar flexion exercise—twice pressed pedal within a TR of 6 s (D).
Measurements were performed in the morning, after an overnight fast in a single exercise-recovery session. Volunteers were in the supine position with the right calf muscle placed on the double-tuned 1H/31P coil, on the ergometer, inside the 7 T MR system.
For volume-of-interest (VOI) positioning, localizer MR images were acquired. The VOI for 1H MRS acetylcarnitine detection (13 × 20 × 40 mm3) and the slab for 31P MRS PCr and Pi detection (thickness of 18 mm) were carefully placed predominantly within the gastrocnemius medialis and lateralis muscles (Fig. 1C). The linewidth of the water signal after shimming was in the range of 35–45 Hz, in magnitude spectra.
First, fully relaxed, static long-TR 31P MR spectra (TR = 15 s, NA = 8) were obtained.
Afterwards, semi-LASER34,35 localized 1H MR spectra (TE = 300 ms) (acetylcarnitine) and slab-localized (DRESS) (31P MR spectra (PCr, Pi)36 were acquired in an interleaved fashion, in a single exercise/recovery session (2 min rest, 15 min exercise at 30–40% of MVC, 23 min recovery) with a TR of 6 s, similar to what has already been published27 (Fig. 1D). Volunteers were instructed to press the pedal twice within every TR, with the noise of the spoiler gradients serving as an audio cue, to ensure data acquisition in a relaxed state of the muscle. For absolute quantification of acetylcarnitine, the fully relaxed water signal was measured separately (TR = 6000 ms, TE = 30 ms).
Post processing
All 1H/31P spectroscopy data were extracted and processed from raw data using in-house-developed Python scripts (http://www.python.org) for phasing and channel combination. Signals were phased to the highest peak magnitude of PCr, water, or lipids in the frequency domain after 7 Hz Lorentzian apodization and 2 × zero-filling. The channel combination was then performed by weighted averaging of the raw data (that is, without apodization and zero-filling).
All spectra were analyzed with the fitting routine AMARES, using jMRUI v6.0 alpha, and acetylcarnitine concentrations were calculated as described in16 in mmol/L tissue volume units (further mmol/L only). In all 19 volunteers, we evaluated a time series of 400 spectra. For the further post hoc tests, we averaged 20 blocks at each of the following time intervals: rest (0–1st minute); middle of exercise (7th–8th minute); end of exercise (15th–16th minute); recovery (18th–19th minute); 10 min of recovery (27th–28th minute); 15 min of recovery (32nd and 33rd minute); and end of recovery (38th–39th minute). Moreover, we calculated the difference between acetylcarnitine concentrations from the end of the recovery period to the middle of exercise (Δ[Ac] recovery-exercise). IMCL CH2 at 1.3 ppm was corrected for T1 and T2 relaxation effects and expressed as a percentage of water content.
The static, fully relaxed 31P MR spectra provided concentrations of PCr, Pi, NADP, PDEs-glycerolphoshocholine (GPC) and glycerolphosphoethanolamine (GPE), and PMEs-phosphocholine (PC) and phopshoethanolamine (PE) in mmol/L units. The interleaved/dynamic 31P MRS protocol yielded the rate of PCr depletion during the exercise (τPCr on-kinetics), the rate of PCr resynthesis during recovery (τPCr recovery), the maximal rate of oxidative phosphorylation, i.e., mitochondrial capacity (Qmax), the initial recovery rate (VPCr), and the time course of intracellular pH changes in skeletal muscle37,38. The chemical shift between PCr and Pi was used to calculate intramyocellular pH. ADP levels were calculated assuming creatine kinase (CK) to be at equilibrium (with KCK = 1.66 × 109 M–1) and upon the assumptions that PCr represents 85% of the total creatine concentration at rest and [ATP] was equal to 8.2 mmol/L39. To calculate the time constant of τPCr on-kinetics and τPCr recovery, [PCr] time courses were fitted with a mono-exponential function using curve fitting in MATLAB. VPCr and Qmax were calculated as described in Valkovic et al.24,38.
Statistical analysis
Data were tested for normality, the differences between groups, and the differences in the values of the acetylcarnitine concentration during rest-exercise-recovery. Changes were tested for significance by a general linear model repeated measures with tests of within-subjects effects (over time period during rest-exercise-recovery) and tests of between-subjects effects over time (between two groups). Additionally, Bonferroni correction per time point as post hoc tests were performed. All analyses were done in SPSS (version 28.0; IBM SPSS, Chicago, Illinois, USA). The relationships between metabolic parameters determined using 1H and 31P MRS were analyzed by linear correlations using Pearson’s correlation coefficient (two-tailed probability values) to estimate the strength of the relationship. The correlation coefficient of an absolute value of 0.46, which corresponded to a 95% confidence agreement, was taken as significant. All values are provided as mean ± standard deviations and a p value < 0.05 was considered significant.
Ethics approval and consent to participate
Written, informed consent and MR safety forms signed by the participants was provided in accordance with the local ethics committee requirements (Ethikkommission der Medizinischen Universität Wien, EK Nr. 1081/2020).
Results
Participant characteristics
Participant characteristics are shown in Table 1. Overweight and sedentary volunteers had significantly higher BMI, lower VO2max, and lower MVC.
MR measurement
All volunteers accepted the timing and exercise load prescribed in the protocol. The time-resolved interleaved 1H/31P MRS acquisition yielded excellent data quality (Figs. 2 and 3). Acetylcarnitine linewidth was 16.42 ± 4.21 Hz. Numerical results derived from both 1H and 31P data are summarized in Table 2.
Representative average of 20 scans of 1H spectroscopy (acetylcarnitine acquisition) acquired in an interleaved 1H/31P MRS session during 2 min at rest, 15 min of plantar flexion exercise, and 23 min of recovery from GM of one volunteer from the lean and active (A) and one from the overweight and sedentary group (B). Representative time series of acetylcarnitine [mmol/L] evolution during exercise/recovery from two groups (C). On the timeline, the red colour marks exact minutes used to average the acetylcarnitine data.
A between-group changes in acetylcarnitine metabolism over time was detected, therefore differences between groups depend on the time point. At rest, we observed significantly lower acetylcarnitine concentrations in the overweight/sedentary group than in the lean/active group (overweight/sedentary: 1.52 ± 0.59 mmol/L, lean/active: 2.88 ± 1.05 mmol/L; p = 0.003). There were significant differences between the overall averaged acetylcarnitine concentrations between the two groups (p < 0.001) at almost every interval during exercise and recovery, except for the last two intervals (15 and 23 min after exercise) (Fig. 2C). Further, in the lean/active group, acetylcarnitine content started to decay upon cessation of exercise, while, in the overweight/sedentary group, acetylcarnitine content continued to rise for the next 15 min, after which it reached a plateau until the end of the measurement time (Fig. 2C). The lean/active group reached the highest concentrations right after exercise (5.7 ± 1.63 mmol/L), whereas the overweight/sedentary group reached highest concentrations 15 min after exercise (3.49 ± 2.25 mmol/L).
In the dynamic 31P MRS data, as expected, PCr concentrations decreased during exercise, reached a steady state, and recovered toward basal values after exercise in all volunteers (Fig. 3A). The plantar flexion exercise protocol of 15 min at 30–50% of the individuals’ predetermined MVC yielded a PCr depletion of 40.5% ± 11.3% in all subjects.
The ADP metabolism differed over time between the groups (p = 0.029) (Fig. 3B). Prior to exercise, ADP levels (μM) at rest were similar across the groups. At the onset of exercise, ADP levels increased rapidly in both groups (Fig. 3B). When PCr reached a steady state during exercise, we observed a trend toward lower end-exercise ADP in lean/active subjects but not in overweight/sedentary subjects, which did not reach significance (p = 0.06).
No significant correlations between acetylcarnitine and PCr, Pi, and ADP concentrations were found.
In vivo skeletal muscle mitochondrial capacity, as determined by the rate constant of PCr resynthesis after exercise (τPCr recovery), was significantly different across the groups (p < 0.001), with a significantly slower PCr recovery in the overweight/sedentary group compared to the lean/active group. Likewise, significant differences in τPCr on-kinetics (PCr at the onset of exercise) were observed between those two groups of volunteers (p = 0.002), with a slower τPCr on-kinetics, again, in the overweight/sedentary group. In addition, in this group, a significantly lower Qmax (p = 0.002) was observed (Fig. 4).
In addition to the results from the dynamic, interleaved protocol, we evaluated static 1H and 31P MR spectra and found significant differences in IMCL and PDE between the lean/active and the overweight/sedentary groups (both p = 0.04), with higher IMCL content and higher GPC concentrations in the overweight and sedentary group.
Correlations between the measured parameters
Linear relations between τPCr on-kinetics, τPCr recovery, VO2max, and in vivo skeletal muscle acetylcarnitine content are shown in Fig. 5A–G. Furthermore, GPC positively correlated with BMI (r = 0.46, p = 0.04) and negatively correlated with VO2max and Qmax (r = − 0.60, p = 0.006; r = − 0.58, p = 0.009), while IMCL and BMI were positively correlated (r = 0.56, p = 0.01) and IMCL positively correlated with both GPC and PDE (r = 0.53, p = 0.02; r = 0.50, p = 0.03). Another interesting correlation was between τPCr on-kinetics and τPCr recovery, which were both negatively correlated with VO2max and MVC (τPCr on-kinetics vs. VO2max: r = − 0.68, p = 0.001; τPCr recovery vs. VO2max: r = − 0.74, p < 0.001; τPCr on-kinetics vs. MVC: r = − 0.50, p = 0.02; τPCr recovery vs. MVC: r = − 0.61, p = 0.005). Moreover, VPCr positively correlated with both VO2max and MVC (r = 0.62, p = 0.004; r = 0.67, p = 0.001).
Discussion
Acetylcarnitine as a relatively low-concentration metabolite that fulfills a major role in maintaining pyruvate dehydrogenation activity. This is further connected to whole-body glucose homeostasis and metabolic flexibility3, and is, therefore, of high interest in skeletal muscle research. It is already known that a heavy exercise workout leads to increased acetylcarnitine concentrations immediately after the exercise challenge17; however, the in vivo behavior of muscle acetylcarnitine content during exercise with MRS has not been shown.
To the best of our knowledge, we are the first to show in vivo changes of skeletal muscle acetylcarnitine during submaximal acute exercise (aerobic exercise) and immediate recovery from exercise using a protocol that can be tolerated by a broad range of volunteer and patient populations. The straightforward detection and quantification of acetylcarnitine is challenging, due to the strong overlap of the 2.13-ppm line with lipid resonances, but the differences in T2 relaxation times of acetylcarnitine and lipids and superior spectral resolution at 7 T allow for the detection of the 2.13-ppm line at rest, using a long-TE 1H MRS1,2, even in overweight and sedentary volunteers (Fig. 2B). In some of overweight group the visibility of acetylcarnitine peak can be limited due to the spectral overlap with adjanced lipid resonances which in general are more pronounced. Another added value of this reasearch is that we have assessed skeletal muscle acetylcarnitine together with PCr during a single dynamic session using interleaved 1H/31P MRS. We were, therefore, able to quantify both time courses in one single experiment, which allowed for the measurement of different parameters under a unique exercise load and metabolic conditions (which would otherwise be difficult or impossible to reproduce in the exact same fashion)25. It has the further benefit of increasing volunteer compliance with the experimental procedures, due to a shortened protocol, compared to consecutive acquisitions. We examined metabolites from gastrocnemius medialis and lateralis muscles. One can argue that 31P DRESS slab as applied here includes small parts of soleus muscle as well, however plantar flexion with the straight leg is described as a suitable model for dynamic data collection from gastrocnemius muscles40. In other words, it is true that a small fraction of soleus tissue contributes to the total signal, but because soleus is mostly inactive, it only produces a (small) constant offset to the metabolite signals of PCr, Pi and ATP, with minor influence on recovery rates41.
A between-group difference in acetylcarnitine metabolism over time was detected. First, we observed significantly lower acetylcarnitine concentrations in the overweight/sedentary group than in the lean/active group. Lindeboom et al.1 found similar results in the vastus lateralis muscle with 3 T MRS, where obese, sedentary subjects had lower acetylcarnitine concentrations than lean subjects (lean sedentary subjects: 1.28 ± 0.22 mmol/kgww; obese sedentary subjects: 0.70 ± 0.22 mmol/kgww). In our research16 focused on the soleus and tibialis anterior muscles at 3 T, we found similarly lower acetylcarnitine content in impaired glucose tolerance (IGT) patients than in volunteers with normal glucose tolerance (NGT) (SOL: IGT: 1.9 ± 0.2 mmol/L tissue volume vs. NGT: 2.3 ± 0.6 mmol/L tissue volume, TA: 0.9 ± 0.4 mmol/L tissue volume vs. 1.2 ± 0.8 mmol/L tissue volume). The BMI of this IGT group was similar to the BMI of the overweight and sedentary group in the present study. It is difficult to compare the exact concentrations; however, calculated concentrations in mmol/L units are slightly higher than in mmol/kgww, as they were corrected for the specific weight of skeletal muscle tissue. Furthermore, here we investigated another type of muscle, i.e., the gastrocnemius medialis and lateralis, in which the proportion of type I and type II muscle fibers is typically 50:5042,43. This is more comparable to the vastus lateralis muscle than to the soleus muscle (predominantly type I fibers)43 and the m. tibialis anterior (predominantly type II fibers)44.
We report resting acetylcarnitine concentrations of 1.5 to 2.8 mmol/L, which is in good agreement with vast majority of results of biopsy studies9,10,11,12,13,14,45 when these are converted to mmol/L (ranging from ~ 0.5 to 4.8 mmol/kg dry mass). These studies reported acetylcarnitine concentrations assessed from biopsy samples after 10-, 30- and 60-min of high intensity exercise (70, 80 and 65% VO2max resp.), where acetylcarnitine content increased up to 15 mmol/kg dry mass12,13,14. We are not able to compare acetylcarnitine concentrations from such a high intensity exercise challenge as the goal of our exercise challenge is to maintain aerobic conditions to avoid acidifying of muscles, peak-splitting of the Pi resonance in 31P MR spectrum during exercise and bi-exponential behaviour of PCr recovery.
When we looked at the time course of acetylcarnitine (2 min rest, 15 min exercise, 23 min recovery) in both groups, we recognized significant differences between the overall means of acetylcarnitine concentrations between those two groups at almost every interval during the exercise and recovery phases, except for the last two points (15 min of recovery and end of recovery) (Fig. 2C), which may indicate distinct states of skeletal muscle metabolism. It is known that obesity and T2DM affect the regulation of muscle fat oxidation, particularly during exercise, and carnitine availability may limit fat oxidation46. The uptake and/or oxidation of fatty acids have also been shown to be impaired during post-absorptive conditions in overweight subjects and/or in subjects with T2DM. Also, human studies have shown that the muscle of subjects who are overweight and/or have T2DM is characterized by an inability to increase fatty acid uptake and/or fatty acid oxidation during exercise47,48,49,50.
Regarding the recovery period for acetylcarnitine content, in the lean/active group, the increased level of acetylcarnitine content after exercise started to decay, suggesting increased consumption, while, in the sedentary/overweight group, levels of acetylcarnitine continued to rise (Fig. 2C). Similar observations were found by Seiler et al.33 in which muscle levels of acetylcarnitine were measured in trained and untrained subjects (n = 4) by 1H-MRS before, 15 min after, and during recovery from 30 min of cycle ergometer exercise performed at 50% of maximal workload.
Further, in our volunteers, the lean/active group reached the highest acetylcarnitine concentrations shortly after exercise, whereas, in the overweight/sedentary group, the acetylcarnitine content increased during a longer period, reaching its maximum 15 min after exercise. Previous studies have shown that acute exercise can lead to an increase in acetylcarnitine concentrations in skeletal muscle51,52. The surge is believed to be a reaction to the increased requirement for energy generation from fatty acids while exercising52,53. Variations in the timing of the highest acetylcarnitine levels during recovery and the degree of wash-out could indicate the intensity and length of prior exercise, along with physiological distinctions among the observed muscle groups. Time series of unaveraged acetylcarnitine evolution during exercise/recovery period from all volunteers is shown in supplementary figure.
In skeletal muscle, non-invasive 31P MRS measurements of the post-exercise recovery kinetics contain valuable information about muscle mitochondrial function and cellular pH homeostasis in vivo24,54. The method has been an established research tool with which to assess muscle energy metabolism and to distinguish between different metabolic and pathologic states. In our dynamic 31P MRS data, we observed decreased PCr concentrations during exercise which reached a steady state and recovered toward steady state values after exercise. Fifteen minutes of plantar flexion exercise yielded a PCr depletion of 40.5% ± 11.3% in all subjects. MVC was used as a measure to set the exercise workload, as this takes into account direct power production of the muscle involved in the given exercise. Yet, one could contend that we overlooked the variation in power output associated with the lactate threshold. However, the measured intracellular pH at the end of exercise did not show substantial acidosis in either of the groups (pHend of exercise in overweight/sedentary: 6.99 ± 0.04; lean/active: 6.97 ± 0.03). Individuals who are trained exhibit increased maximal aerobic power, as evidenced by their elevated VO2max scores. This signifies their enhanced ability to transport oxygen to muscles and facilitate its transfer to mitochondria, where it acts as an electron acceptor during ATP production. In a review by McMahon and Jenkins, the authors suggest that people boasting a higher VO2max are expected to have improved capacity in replenishing PCr after engaging in intense exercise55. The linear relations we found between τPCr (both at on-kinetics and recovery), VPCr, and VO2max, as well as with MVC, appear to corroborate these results consistently, with the notion that elevated VO2max results in a faster PCr resynthesis. As a result, it is not surprising that the rate of PCr resynthesis following moderate exercise represents the muscle’s oxidative capacity, which has been found to be faster in endurance-trained athletes, compared to sedentary controls55. Our results from dynamic 31P MRS are consistent with this statement, since τPCr (both at on-kinetics and recovery), was significantly slower in the overweight/sedentary group compared to the lean/active group. In addition, in the overweight/sedentary group, there was a significantly lower Qmax.
The further value of this interleaved 1H/31P MRS protocol is shown in the ability to calculate the time course of ADP concentrations39. Earlier findings indicated a decrease in basal ADP levels and reduced sensitivity to ADP in individuals facing metabolic challenges or those in older age groups56,57. Likewise, Mancilla et al. reported higher ADP levels during exercise in T2DM patients and obese patients compared to lean/trained individuals21. Our observations were very similar, since we found that ADP metabolism differed over time between the groups. While pre-exercise ADP levels were comparable across the groups, we observed a marginal difference in ADP levels across the groups in the time point when PCr reached a steady state during exercise. Although the difference at this time point was not significant, the difference in the whole-time course of ADP metabolism was found to be significant between the groups. Mancilla et al. proposed that increased ADP levels are necessary to activate the mitochondrial oxidative phosphorylation system in groups facing metabolic challenges. This could potentially offer an alternative mechanistic rationale for the disparities observed in PCr kinetics among groups at the start of exercise21. These findings could imply that metabolically compromised individuals require greater metabolic stress to trigger oxidative ATP production, leading to an extended reliance on substrate-level phosphorylation21. Moreover, in their work, their findings demonstrated a strong correlation between CrAT protein activity in muscle tissue and PCr kinetics at the start of exercise. Additionally, metabolically compromised individuals exhibited reduced skeletal muscle acetylcarnitine content at rest, which significantly correlated with slower PCr kinetics when exercise began21.
In this work, we found linear relations between τPCr (both at on-kinetics and recovery) and in vivo skeletal muscle acetylcarnitine content during exercise. Furthermore, Δ[Ac] rec-exe was positively correlated with τPCr (both at on-kinetics and recovery) and with Qmax. These discoveries may indicate that the acetylcarnitine content within skeletal muscle influences mitochondrial inertia and acts as a trigger for PCr kinetics during exercise.
It has already been shown, and here we re-confirm, that acetylcarnitine content and CrAT protein activity were lower in T2DM and obese individuals. This supports the notion that reduced CrAT protein activity-and therefore, a low capacity to form acetylcarnitine from Acetyl-CoA and carnitine-might underlie metabolic inflexibility and impaired insulin sensitivity1. Importantly, the CrAT enzyme also functions in the reverse direction to supply Acetyl-CoA from acetylcarnitine when energy demand suddenly increases.
In addition, we found relation between basal acetylcarnitine levels and VO2max. This connection may be based on the facts that rate of oxidative phosphorylation from human skeletal muscle is in very close correlation with VO2max58,59. Myoglobin transports oxygen to the mitochondria where it can be used as the last electron acceptor within oxidative phosphorylation, allowing for ATP synthesis to occur. During transitions from low to high exercise workloads, Krebs cycle flux must increase to keep pace with the high ATP demands of muscle contraction. Here CrAT functions to sustain high rates of oxidative ATP regeneration by acting as a channel for acetyl group transfer60. Elevated levels of acetylcarnitine might therefore indicate better mitochondrial efficiency, leading to improved energy production during aerobic activities, which could be in good agreement with better aerobic fitness.
Limitaition is, that all correlations are shown from both groups (lean/active and overweight/sedentary) and analysis of individual groups may be important. However, this correlation anaylsis detected trends but did not reach significancy through variables probably owing to the point that individual groups contained 10 and 9 subjects only.
Nevertheless, from our results, we cannot draw general conclusions about exact changes and the exercise effects on skeletal acetylcarnitine content in the insulin-resistant and diabetic population. This is because our study included only a limited number of volunteers and was aimed mainly at the methodological aspects to allow testing the dynamic data acquisition and exploring the behavior of in vivo skeletal muscle acetylcarnitine, as well as the relation to phosphocreatine during submaximal plantar flexion exercise using interleaved 1H/31P MRS at 7 T. Further studies on the influence of food intake, diet composition, sex, age, and different pathophysiological conditions with a larger number of volunteers with a broader range of metabolic conditions and physical fitness are necessary for detailed analysis.
In conclusion, we are the first to show in vivo changes of skeletal muscle acetylcarnitine during acute exercise and immediate exercise recovery with a submaximal aerobic workload using interleaved 1H/31P MRS at 7 T that can be tolerated by a broad range of volunteer and patient populations. Moreover, we report on between-group differences in changes of acetylcarnitine metabolism over the rest/exercise/recovery periods, as well as significantly lower Qmax and slower τPCr (both at on-kinetics and recovery) in an overweight/sedentary group compared to a lean/active group. Furthermore, we report cross-linked relationships between τPCr on-kinetics, τPCr recovery, Qmax, VO2max and in vivo skeletal muscle acetylcarnitine.
Data availability
Anonymized MR (Magnetic Resonance) data acquired at MR scanner are copied from the scanner and are stored at the secured internal servers provided by the beneficiary with access restricted to the researchers involved in the study. All persons who receive access to encrypted and non-encrypted data are subject to the data protection basic regulation (DSGVO) as well as the Austrian adaptation regulations in the respectively valid version. Those datasets are available from the corresponding author on reasonable request. Non-encrypted data will not be available to protect study participant privacy.
Abbreviations
- CrAT:
-
Carnitine acetyltransferase
- CK:
-
Creatine kinase
- TE:
-
Echo time
- GM:
-
Gastrocnemius muscle
- GPC:
-
Glycerolphoshocholine
- GPE:
-
Glycerolphosphoethanolamine
- IGT:
-
Impaired glucose tolerance
- Pi:
-
Inorganic phosphate
- VO2max :
-
Maximal oxygen uptake
- MVC:
-
Maximal voluntary contraction force
- Qmax :
-
Maximum oxidative capacity
- NADP:
-
Nicotinamide adenine dinucleotide phosphate
- NGT:
-
Normal glucose tolerance
- NA:
-
Number of averages
- PE:
-
Phopshoethanolamine
- PCr:
-
Phosphocreatine
- PDEs:
-
Phosphodiesters
- PMEs:
-
Phosphomonoesters
- PC:
-
Phosphocholine
- 31P MRS:
-
Phosphorus magnetic resonance spectroscopy
- 1H MRS:
-
Proton magnetic resonance spectroscopy
- TR:
-
Repetition time
- τPCr on -kinetics :
-
The time constant of PCr decay at the onset of exercise
- τPCr recovery :
-
The time constant of PCr resynthesis
- T2DM:
-
Type 2 diabetes mellitus
- VOI:
-
Volume-of-interest
References
Lindeboom, L. et al. Long-echo time MR spectroscopy for skeletal muscle acetylcarnitine detection. J. Clin. Invest. 124(11), 4915–4925. https://doi.org/10.1172/JCI74830 (2014).
Klepochová, R. et al. Detection and alterations of acetylcarnitine in human skeletal. Invest. Radiol. 52(7), 412–418. https://doi.org/10.1097/RLI.0000000000000355 (2017).
Muoio, D. M. et al. Muscle-specific deletion of carnitine acetyltransferase compromises glucose tolerance and metabolic flexibility. Cell Metab. 15(5), 764–777. https://doi.org/10.1016/J.CMET.2012.04.005 (2012).
Noland, R. C. et al. Carnitine insufficiency caused by aging and overnutrition compromises mitochondrial performance and metabolic control. J. Biol. Chem. 284(34), 22840. https://doi.org/10.1074/JBC.M109.032888 (2009).
Stephens, F. B., Constantin-Teodosiu, D. & Greenhaff, P. L. New insights concerning the role of carnitine in the regulation of fuel metabolism in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 5(2), 431–444. https://doi.org/10.1152/ajpendo.00277.2001 (2007).
Muoio, D. M. Metabolic inflexibility: When mitochondrial indecision leads to metabolic gridlock. Cell. 159(6), 1253. https://doi.org/10.1016/J.CELL.2014.11.034 (2014).
Sugden, M. C. & Holness, M. J. Recent advances in mechanisms regulating glucose oxidation at the level of the pyruvate dehydrogenase complex by PDKs. Am. J. Physiol. Endocrinol. Metab. 284(5), 47 (2003).
Seiler, S. E. et al. Obesity and lipid stress inhibit carnitine acetyltransferase activity. J. Lipid Res. 55(4), 635. https://doi.org/10.1194/JLR.M043448 (2014).
Friolet, R., Hoppeier, H. & Krähenbühl, S. Relationship between the coenzyme A and the carnitine pools in human skeletal muscle at rest and after exhaustive exercise under normoxic and acutely hypoxic conditions. J. Clin. Investig. 94(4), 1490–1495. https://doi.org/10.1172/JCI117488 (1994).
Harris, R. C., Foster, C. V. L. & Hultman, E. Acetylcarnitine Formation During Intense Muscular Contraction in Humans. (1987). www.physiology.org/journal/jappl.
Lennon, D. L., Shrago, E. R., Madden, M., Nagle, F. J. & Hanson, P. Dietary carnitine intake related to skeletal muscle and plasma carnitine concentrations in adult men and women. Am. J. Physiol. Endocrinol. Metab. 43, 13 (1986).
Watt, M. J., Heigenhauser, G. J. F., Stellingwerff, T., Hargreaves, M. & Spriet, L. L. Carbohydrate ingestion reduces skeletal muscle acetylcarnitine availability but has no effect on substrate phosphorylation at the onset of exercise in man. J. Physiol. 544(3), 949–956. https://doi.org/10.1113/jphysiol.2002.026757 (2002).
Wall, B. T. et al. Chronic oral ingestion of l-carnitine and carbohydrate increases muscle carnitine content and alters muscle fuel metabolism during exercise in humans. J. Physiol. 589(4), 963–973. https://doi.org/10.1113/jphysiol.2010.201343 (2011).
Roepstorff, C. et al. Malonyl-CoA and carnitine in regulation of fat oxidation in human skeletal muscle during exercise. Am. J. Physiol. Endocrinol. Metab. 288, 133–142. https://doi.org/10.1152/ajpendo.00379.2004.-Intracellular (2005).
Valkovič, L., Klepochová, R. & Krššák, M. Multinuclear magnetic resonance spectroscopy of human skeletal muscle metabolism in training and disease. Muscle Cell Tissue Curr. Status Res. Field. https://doi.org/10.5772/intechopen.77107 (2018).
Klepochová, R. et al. Muscle-specific relation of acetylcarnitine and intramyocellular lipids to chronic hyperglycemia: A pilot 3-T 1 H MRS study. Obesity. 28(8), 1405–1411. https://doi.org/10.1002/oby.22846 (2020).
Ren, J., Lakoski, S., Haller, R. G., Sherry, A. D. & Malloy, C. R. Dynamic monitoring of carnitine and acetylcarnitine in the trimethylamine signal after exercise in human skeletal muscle by 7T 1H-MRS. Magn. Reson. Med. 69(1), 7–17. https://doi.org/10.1002/MRM.24249 (2013).
Chance, B., Im, J., Nioka, S. & Kushmerick, M. Skeletal muscle energetics with PNMR: Personal views and historic perspectives. NMR Biomed. 19(7), 904–926. https://doi.org/10.1002/NBM.1109 (2006).
Kemp, G. J., Ahmad, R. E., Nicolay, K. & Prompers, J. J. Quantification of skeletal muscle mitochondrial function by 31P magnetic resonance spectroscopy techniques: A quantitative review. Acta Physiol. 213(1), 107–144. https://doi.org/10.1111/apha.12307 (2015).
Fiedler, G. B. et al. Skeletal muscle ATP synthesis and cellular H(+) handling measured by localized (31)P-MRS during exercise and recovery. Sci. Rep. 6, 32037. https://doi.org/10.1038/srep32037 (2016).
Mancilla, R. F. et al. Skeletal muscle mitochondrial inertia is associated with carnitine acetyltransferase activity and physical function in humans. JCI Insight. https://doi.org/10.1172/JCI.INSIGHT.163855 (2023).
Roussel, M., Bendahan, D., Mattei, J. P., LeFur, Y. & Cozzone, P. J. 31P Magnetic resonance spectroscopy study of phosphocreatine recovery kinetics in skeletal muscle: The issue of intersubject variability. Biochim. Biophys. Acta Bioenerg. 1457(1–2), 18–26. https://doi.org/10.1016/S0005-2728(99)00111-5 (2000).
Krššák, M. et al. Proton magnetic resonance spectroscopy in skeletal muscle: Experts ’ consensus recommendations. NMR Biomed. https://doi.org/10.1002/nbm.4266 (2020).
Meyerspeer, M. et al. 31P magnetic resonance spectroscopy in skeletal muscle: Experts’ consensus recommendations. NMR Biomed. 34(5), e4246. https://doi.org/10.1002/NBM.4246 (2021).
Lopez Kolkovsky, A. L., Carlier, P. G., Marty, B. & Meyerspeer, M. Interleaved and simultaneous multi-nuclear magnetic resonance in vivo: Review of principles, applications and potential. NMR Biomed. https://doi.org/10.1002/nbm.4735 (2022).
Niess, F. et al. Interleaved multivoxel 31P MR spectroscopy. Magn. Reson. Med. 77(3), 921–927. https://doi.org/10.1002/mrm.26172 (2017).
Niess, F. et al. Interleaved 31P MRS/1H ASL for analysis of metabolic and functional heterogeneity along human lower leg muscles at 7T. Magn. Reson. Med. 83(6), 1909. https://doi.org/10.1002/MRM.28088 (2020).
Meyerspeer, M. et al. Simultaneous and interleaved acquisition of NMR signals from different nuclei with a clinical MRI scanner. Magn. Reson. Med. 76(5), 1636–1641. https://doi.org/10.1002/mrm.26056 (2016).
Lopez Kolkovsky, A. L., Marty, B., Giacomini, E., Meyerspeer, M. & Carlier, P. G. Repeatability of multinuclear interleaved acquisitions with nuclear Overhauser enhancement effect in dynamic experiments in the calf muscle at 3T. Magn. Reson. Med. 86(1), 115–130. https://doi.org/10.1002/mrm.28684 (2021).
Goluch, S. et al. A form-fitted three channel phosphorus 31P, two channel 1H transceiver coil array for calf muscle studies at 7 T. Magn. Reson. Med. 73(6), 2376–2389. https://doi.org/10.1002/mrm.25339 (2015).
Klepochová, R. et al. Muscle-specific relation of acetylcarnitine and intramyocellular lipids to chronic hyperglycemia: A pilot 3T 1H MRS study. Obesity. 28(8), 1405–1411. https://doi.org/10.1002/oby.22846 (2020).
Klepochová, R. et al. Differences in muscle metabolism between triathletes and normally active volunteers investigated using multinuclear magnetic resonance spectroscopy at 7T. Front. Physiol. 9, 1–13. https://doi.org/10.3389/fphys.2018.00300 (2018).
Seiler, S. E. et al. Carnitine acetyltransferase mitigates metabolic inertia and muscle fatigue during exercise. Cell Metab. 22(1), 65–76. https://doi.org/10.1016/j.cmet.2015.06.003 (2015).
Meyerspeer, M. et al. Semi-LASER localized dynamic 31P magnetic resonance spectroscopy in exercising muscle at ultra-high magnetic field. Magn. Reson. Med. 65(5), 1207–1215. https://doi.org/10.1002/MRM.22730 (2011).
Scheenen, T. W. J., Klomp, D. W. J., Wijnen, J. P. & Heerschap, A. Short echo time 1H-MRSI of the human brain at 3T with minimal chemical shift displacement errors using adiabatic refocusing pulses. Magn. Reson. Med. 59(1), 1–6. https://doi.org/10.1002/mrm.21302 (2008).
Bottomley, P. A., Foster, T. B. & Darrow, R. D. Depth-resolved surface-coil spectroscopy (DRESS) for in Vivo 1H, 31P, and 13C NMR. J. Magn. Reson. 59(2), 338–342. https://doi.org/10.1016/0022-2364(84)90179-3 (1984).
Valkovič, L. et al. Skeletal muscle alkaline Pi pool is decreased in overweight-to-obese sedentary subjects and relates to mitochondrial capacity and phosphodiester content. Sci. Rep. Nat. 2016, 1–9. https://doi.org/10.1038/srep20087 (2015).
Valkovič, L., Chmelík, M. & Krššák, M. In-vivo 31P-MRS of skeletal muscle and liver: A way for non-invasive assessment of their metabolism. Anal. Biochem. https://doi.org/10.1016/j.ab.2017.01.018 (2017).
Jeneson, J. A. L., Westerhoff, H. V., Brown, T. R., Van Echteld, C. J. A. & Berger, R. Quasi-linear relationship between Gibbs free energy of ATP hydrolysis and power output in human forearm muscle. Am. J. Physiol. 268(6), 37. https://doi.org/10.1152/AJPCELL.1995.268.6.C1474 (1995).
Niess, F. et al. Dynamic multivoxel-localized 31 P MRS during plantar flexion exercise with variable knee angle. Am. J. Physiol. https://doi.org/10.1002/nbm.3905 (2018).
Meyerspeer, M. et al. Comparing localized and nonlocalized dynamic 31P magnetic resonance spectroscopy in exercising muscle at 7T. Magn. Reson. Med. 68(6), 1713–1723. https://doi.org/10.1002/mrm.24205 (2012).
Egan, B. & Zierath, J. R. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 17(2), 162–184. https://doi.org/10.1016/j.cmet.2012.12.012 (2013).
Gollnick, P. D., Sjodin, B., Karlsson, J., Jansson, E. & Saltin, B. Human soleus muscle: A comparison of fiber composition and enzyme activities with other leg muscles. Pflugers Arch. 348(3), 247–255. https://doi.org/10.1007/BF00587415 (1974).
Henriksson-Larsten, K. B., Lexell, J. & Sjostrom, M. Distribution of Different Fibre Types in Human Skeletal Muscles. I. Method for the Preparation and Analysis of Cross-Sections of Whole Tibialis Anterior. Vol 15 (1983).
Sahlin, K. Muscle Carnitine Metabolism During Incremental Dynamic Exercise in Humans, Vol 138 (1990).
Stephens, F. B., Constantin-Teodosiu, D., Laithwaite, D., Simpson, E. J. & Greenhaff, P. L. An acute increase in skeletal muscle carnitine content alters fuel metabolism in resting human skeletal muscle. J. Clin. Endocrinol. Metab. 91(12), 5013–5018. https://doi.org/10.1210/jc.2006-1584 (2006).
Blaak, E. E. Basic disturbances in skeletal muscle fatty acid metabolism in obesity and type 2 diabetes mellitus. Proc. Nutr. Soc. 63(2), 323–330. https://doi.org/10.1079/pns2004361 (2004).
Colberg, S. R., Simoneau, J. A., Thaete, F. L. & Kelley, D. E. Skeletal Muscle Utilization of Free Fatty Acids in Women with Visceral Obesity.
Kelley, D. E. et al. Skeletal Muscle Fatty Acid Metabolism in Association with Insulin Resistance, Obesity, and Weight Loss. (1999). http://www.ajpendo.org.
Blaak, E. E., Van Aggel-Leijssen, D. P. C., Wagenmakers, A. J. M., Saris, W. H. M. & Van Baak, M. A. Impaired oxidation of plasma-derived fatty acids in type 2 diabetic subjects during moderate-intensity exercise. Diabetes. 49(12), 2102–2107. https://doi.org/10.2337/DIABETES.49.12.2102 (2000).
Gnoni, A., Longo, S., Gnoni, G. V. & Giudetti, A. M. Carnitine in human muscle bioenergetics: Can carnitine supplementation improve physical exercise?. Molecules. https://doi.org/10.3390/molecules25010182 (2020).
Stephens, F. B. Does skeletal muscle carnitine availability influence fuel selection during exercise?. Proc. Nutr. Soc. 77, 11–19. https://doi.org/10.1017/S0029665117003937 (2018).
Brass, E. P. Supplemental carnitine and exercise. Am. J. Clin. Nutr. 72(2), 618S-623S. https://doi.org/10.1093/ajcn/72.2.618S (2000).
Lin, A. et al. Minimum reporting standards for in vivo magnetic resonance spectroscopy (MRSinMRS): Experts’ consensus recommendations. NMR Biomed. 34(5), e4484. https://doi.org/10.1002/nbm.4484 (2021).
Mcmahon, S. & Jenkins, D. Factors Affecting the Rate of Phosphocreatine Resynthesis Following Intense Exercise.
Phielix, E. et al. Lower intrinsic ADP-stimulated mitochondrial respiration underlies in vivo mitochondrial dysfunction in muscle of male type 2 diabetic patients. Diabetes. 57(11), 2943–2949. https://doi.org/10.2337/DB08-0391 (2008).
Holloway, G. P. et al. Age-associated impairments in mitochondrial ADP sensitivity contribute to redox stress in senescent human skeletal muscle. Cell Rep. 22(11), 2837–2848. https://doi.org/10.1016/J.CELREP.2018.02.069 (2018).
Mootha, V. K. et al. PGC-1α-Responsive Genes Involved in Oxidative Phosphorylation are Coordinately Downregulated in Human Diabetes, Vol 34, 1–10 (2003).
Tonkonogi, M. & Sahlin, K. Rate of oxidative phosphorylation in isolated mitochondria from human skeletal muscle: Effect of training status. Acta Physiol. Scand. 161(3), 345–353. https://doi.org/10.1046/j.1365-201X.1997.00222.x (1997).
Hayden, C. M. T., Nagarajan, R., Smith, Z. H., Gilmore, S. & Kent, J. A. Post-contraction [acetylcarnitine] reflects inter-individual variation in skeletal muscle ATP production patterns in vivo. Am. J. Physiol. Regul. Integr. Compar. Physiol. https://doi.org/10.1152/ajpregu.00027.2023 (2023).
Acknowledgements
The authors would like to thank all the participants for their patience and cooperation. This study was supported by Austrian Science Foundation (FWF KLI 904 & KLI 1122 to M.K., KLI1106 to F.N.) Part of this study was presented at the Joint Annual Meeting ISMRM-ESMRMB & ISMRT 31st Annual Meeting, 2022, London (Klepochova et al. Skeletal muscle acetylcarnitine during submaximal exercise and recovery: interleaved 1H/31P MRS 7T pilot study). All authors and authors’ institutions have no conflicts of interest to disclose.
Funding
This study was supported by Austrian Science Foundation (FWF KLI 904 & KLI 1122 to M.K. and KLI1106 to F.N).
Author information
Authors and Affiliations
Contributions
R.K.—conception and design of study, acquisition of data, analysis and/or interpretation of data, drafting and revising the manuscript, final approval of the version of the manuscript to be published. F.N.—methodological help, data processing script programming, revising the manuscript, final approval of the version of the manuscript to be published. M.M.—methodological help, technical help, revising the manuscript, final approval of the version of the manuscript to be published. D.S.—administration help, revising the manuscript, final approval of the version of the manuscript to be published. I.J.—general support, part of a collaboration group, writing assistance, drafting and revising the manuscript, final approval of the version of the manuscript to be published. S.T.—drafting the manuscript, revising the manuscript critically for important intellectual content, final approval of the version of the manuscript to be published. J.U.—drafting the manuscript, revising the manuscript critically for important intellectual content, final approval of the version of the manuscript to be published. B.U.—drafting the manuscript, revising the manuscript critically for important intellectual content, final approval of the version of the manuscript to be published. A.K.-W.—drafting the manuscript, revising the manuscript critically for important intellectual content, final approval of the version of the manuscript to be published. M.L.—drafting the manuscript, revising the manuscript critically for important intellectual content, final approval of the version of the manuscript to be published. M.K.—conception and design of study, analysis and/or interpretation of data, drafting and revising the manuscript critically for important intellectual content, final approval of the version of the manuscript to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Klepochová, R., Niess, F., Meyerspeer, M. et al. Correlation between skeletal muscle acetylcarnitine and phosphocreatine metabolism during submaximal exercise and recovery: interleaved 1H/31P MRS 7 T study. Sci Rep 14, 3254 (2024). https://doi.org/10.1038/s41598-024-53221-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-53221-x
- Springer Nature Limited