Abstract
The term chronic critical illness describes patients suffering from persistent organ dysfunction and prolonged mechanical ventilation. In severe cases, COVID-19 led to chronic critical illness. As this population was hardly investigated, we evaluated the health-related quality of life, physical, and mental health of chronically critically ill COVID-19 patients. In this prospective cohort study, measurements were conducted on admission to and at discharge from inpatient neurorehabilitation and 3, 6, and 12 months after discharge. We included 97 patients (61 ± 12 years, 31% women) with chronic critical illness; all patients required mechanical ventilation. The median duration of ICU-treatment was 52 (interquartile range 36–71) days, the median duration of mechanical ventilation was 39 (22–55) days. Prevalences of fatigue, anxiety, and depression increased over time, especially between discharge and 3 months post-discharge and remained high until 12 months post-discharge. Accordingly, health-related quality of life was limited without noteworthy improvement (EQ-5D–5L: 0.63 ± 0.33). Overall, the burden of symptoms was high, even one year after discharge (fatigue 55%, anxiety 42%, depression 40%, problems with usual activities 77%, pain/discomfort 84%). Therefore, patients with chronic critical illness should receive attention regarding treatment after discharge with a special focus on mental well-being.
Trial registration: German Clinical Trials Register, DRKS00025606. Registered 21 June 2021—Retrospectively registered, https://drks.de/search/de/trial/DRKS00025606.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Advances in intensive care have substantially improved survival rates of critically ill patients1. However, these advances have also led to a growing population of patients suffering from persistent organ dysfunction and prolonged dependence on mechanical ventilation, a condition termed chronic critical illness (CCI)2. The underlying pathophysiology of CCI was suggested to be based on persistent inflammation, immunosuppression, and protein catabolism3,4. CCI can develop in all patients requiring treatment for acute medical, surgical, neurologic, or cardiac critical illness. It occurs especially often in older patients with sepsis, mechanical ventilation and underlying comorbid conditions2. As a consequence, CCI contributes to long-term mortality, extraordinary health-care costs, reduced long-term physical, psychological and cognitive functions, and diminished health-related quality of life5,6,7,8,9,10. The encompassing long-term disability of ICU survivors with impairments in physical function, psychological health, and cognition was previously described as post-intensive care syndrome (PICS)11.
The COVID-19 pandemic caused millions of infections worldwide. The disease caused by the severe acute respiratory syndrome coronavirus -2 (SARS-CoV-2) typically manifests as pneumonia and can lead to critical symptoms requiring treatment on ICU and mechanical ventilation12,13,14,15. Reconvalescence of ICU survivors after COVID-19 disease was shown to be protracted and large numbers suffered from health limitations even months after the infection. Within the first three months after discharge from ICU, around 90% still suffered from health state limitations16,17,18. Symptoms may even be prominent 1 year after ICU treatment, with physical, mental and cognitive impairments reported in 74%, 26% and 16% of the patients respectively19. In accordance with the reduced health state, health-related quality of life was also shown to be reduced in COVID-19 ICU survivors14,20, 21. Additionally, ICU admission and (duration of) mechanical ventilation were found to be predictors for a low health-related quality of life21,22,23.
Until now, only few studies investigated CCI in COVID-19 populations. Interestingly, reported mortality rates in COVID-19 CCI populations (90-day mortality = 28%24; 1-year mortality = 6.6%25) were substantially lower than previously described mortality rates in general CCI populations (1-year mortality = 44%6–54%5). Although mortality and survival are highly relevant, long-term outcome, prospect of life and quality of life became more and more important in intensive care medicine26. As treatment of CCI patients is highly resource-intensive, specific data on long-term trajectories are required for decision-making processes regarding resource allocation, critical care capacity and therapeutic options. Therefore, the outcome of COVID-19 patients with CCI is of high relevance, especially, as up to 50% of the investigated COVID-19 patients (being treated on ICU) were chronically critically ill24,25. Additionally, as health limitations are common in COVID-19 ICU survivors even after short durations of ICU therapy14,16, substantial and enduring health deficits can be expected in COVID-19 CCI patients6,24. However, up to now, there are no studies about the long-term outcome beyond the scope of pure survival.
Therefore, the objective of this study was to determine the physical and mental health and the health-related quality of life of chronically critically ill COVID-19 patients 3, 6 and 12 months after discharge from hospital.
Methods
Study population and setting
For this observational prospective cohort study, patients were recruited at the Schoen Clinic Bad Aibling, a center for inpatient neurorehabilitation in Germany with a focus on critically affected patients (ICU, early neurorehabilitation). Adult patients (≥ 18 years) with laboratory-confirmed COVID-19 (evaluated by real-time reverse transcriptase PCR) were eligible after the infectious stage and after being admitted to neurorehabilitation. Exclusion criteria were (1) insufficient (German) communication skills to complete the questionnaires and (2) patients receiving palliative care. For the analysis presented in this manuscript, only chronically critically ill COVID-19 patients were included. An American consensus-derived definition was applied to determine CCI27, whereby a minor adaption according to9 was used. This definition consists of at least 8 days in an ICU and one of six eligible clinical conditions (prolonged acute mechanical ventilation (≥ 96 h), tracheotomy, sepsis, severe wounds, stroke (including ischemic stroke and intracerebral hemorrhage), and traumatic brain injury). For the diagnosis of these conditions, the criteria of9 were applied.
Patients received approximately 100 min of multi-disciplinary neurorehabilitation therapies per day, including physio-, occupational-, dysphagia-, and breathing therapies, as well as neuropsychology. Duration of neurologic rehabilitation was distinct for every patient.
Part of this study population were described previously in a study on the clinical course during neurorehabilitation20 and a study about severe post-COVID-19 condition28.
The study was approved by the medical ethics committee of the Ludwig Maximilian University Munich according to the Declaration of Helsinki (project No. 20-0478). Written informed consent was obtained from all participants (or their legal guardians). The study was registered at the German Clinical Trials Register (DRKS00025606).
Study visits and outcomes
Patients were included after admission to neurological rehabilitation (after discharge from ICU and after weaning from mechanical ventilation). Each of the 5 study visits (at study inclusion, at discharge from neurorehabilitation, and 3, 6, and 12 months after discharge) comprised a comprehensive set of questionnaires, functional tests, and questions about personal living conditions. The visits at study onset (visit 1 = V1) and at discharge (visit 2 = V2) from inpatient rehabilitation took place in person, visits 3, 4, and 5 (V3–V5) after discharge were conducted via structured telephone interviews and questionnaires sent by post. The study visits were conducted by trained and experienced study staff.
ICU treatment characteristics, complications, and pre-existing diseases were extracted from the medical records. To describe pre-existing diseases, a comorbidity index based on the Elixhauser classification system was used29. In order to investigate critical illness polyneuropathy and –myopathy, sensory and motor nerve conduction studies and needle electromyography (if applicable) were conducted after study inclusion.
This analysis focuses on the following assessments:
-
The Fatigue Severity Scale-7 (FSS-7) is used to evaluate fatigue. The seven-item version has better psychometric properties than the nine–item version30. Score: 1–7. The cut-off ≥ 4 was interpreted as indicative of fatigue31.
-
The Hospital Anxiety and Depression Scale (HADS) is a valid and reliable tool to measure anxiety and depression and was repeatedly used in critically ill patients32. Score: 0–21 each for anxiety and depression. A score of > 7 in each category was interpreted as clinically significant33.
-
The EuroQol-5 dimensions-5 level (EQ-5D-5L) was used to measure health-related quality of life34. The index value for the German population ranges from − 0.205 (0 = health state equivalent to death; negative values = health state worse than death) to 1.000 (best health state)35. Patients who died after V1 were assigned a score of 0 in all further study visits. Additionally, the visual analogue scale (included in the EQ-5D-5L; 0–100) was used. 100 indicates the best imaginable state of health.
-
The generic World Health Organization Disability Assessment Schedule 2.0 (WHODAS-12) measures health and disability and comprises the categories cognition, mobility, self-care, getting along, life activities, and participation. It is reliable, widely used and has good internal consistency36. The total score was converted into a percentage ((sum/48)*100): no (0–4%); mild (5–24%); moderate (25–49%); severe (50–95%); and complete (96–100%) disability.
-
Frailty (Clinical Frailty Scale, score 1–9; 9 = deathly ill37), overall disability (modified Rankin Scale, score 0–6; 6 = death38) and dyspnea (modified Medical Research Council dyspnea scale, score 0–4; 4 = severest dyspnea39. For frailty and overall disability, a preclinical value was recorded retrospectively at visit 1.
Statistical analysis
Categorical variables are presented as absolute values and percentages, continuous variables as mean ± standard deviation or median (quartile 1–quartile 3).
For the comparison of symptoms between different study visits, Friedman-Test was used as data were either non-parametric or did not follow normal distribution (as checked visually by means of QQ-plots). Effect sizes were calculated based on the Wilcoxon signed-rank test as Z statistic divided by square root of the sample size (r = Z/√N) for the comparisons of different study visits (in all patients with available data pairs).
Correlation between fatigue, depression, anxiety and health-related quality of life (index value) was calculated by spearman´s rank correlation coefficient and interpreted according to40.
Linear mixed-effect models for repeated measures were used to investigate the impact of preclinical health states and ICU treatment characteristics over time. Models were calculated separately for each of the outcomes, i.e. health related quality of life, fatigue, anxiety and depression. Variable selection was done based on literature research and expert knowledge. Multicollinearity was assessed for the independent variables using generalized variance inflation factors. The final model included age (included either annually or in decades for enhanced interpretability), sex, duration of mechanical ventilation (included either in days or with z-standardized values for enhanced interpretability), preclinical frailty, comorbidities (Elixhauser Comorbidity Index), obesity, diabetes, and ECMO treatment as fixed effects and a random intercept. A random intercept can be interpreted as individual variations of the referring outcome at baseline. Assumptions (normality of residuals, linearity and homogeneity of residual variance) were inspected visually for systematic violations. The adjusted intraclass-correlation coefficient (ICC) (the proportion of explained variance that can be attributed to the random effects) and the conditional R2 (the proportion of the explained variance of the full model, taking the fixed and random effects into account) were reported for each model. As a sensitivity analysis, models with additional random slopes were investigated. A random slope can be interpreted as individual variation in change of the outcome over time. Likelihood-ratio tests were used to compare models with random intercepts alone and models with additional random slopes. Although including a random slope significantly improved the model over a random intercept model, the conditional R2 and ICC were > 0.9 and therefore indicated a risk for overfitting. The results of the models with random slopes were included in Supplementary Table 2.
Statistical analyses were performed using R version 4.1.1 and IBM SPSS Statistics 19. The linear mixed-effect models was fitted using the ‘lme’ function of the ‘nlme’ package. The ICC and the R2 were calculated using the ‘icc’ and the ‘r2_nakagawa’ function of the ‘performance’ package. A p-value ≤ 0.05 was considered significant. Missing data was not replaced.
Ethics approval and consent to participate
The study was approved by the medical ethics committee of the Ludwig Maximilian University Munich according to the Declaration of Helsinki (project No. 20-0478). Written informed consent was obtained from all participants (or their legal guardians).
Results
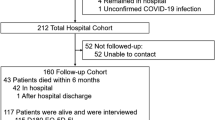
A total of 349 patients were screened between April 2020 and January 2022. 130 were enrolled in the study from June 2020 until January 2022 and 97 were included in this analysis (Fig. 1). The median length of stay on ICU was 52 days (Table 1). Patients were admitted from 33 different ICUs and 52% patients were treated on two or three different ICUs. All patients required prolonged mechanical ventilation (median = 39 days), which led to the definition of CCI. The last telephone interview was conducted on February 1, 2023. Seven patients (7.2%) died over the course of the study. Patients frequently had suffered from sepsis (87.6%), acute respiratory distress syndrome (81.4%) and critical illness polyneuropathy and –myopathy (84.2%).
Table 2 shows the results of the outcomes from visit 1–5 (see Supplementary Table 1 for numbers of available data for every assessment and every time point). The general health state improved over time regarding the overall disability, frailty and dyspnea, as illustrated by the large effect sizes from visit 1–2 (which indicates the positive effect of the neurological rehabilitation) and visit 1–5. However, values for fatigue, anxiety and depression at visits 3–5 were higher than at visit 1 (shortly after discharge from ICU) and their prevalence increased since that time. Health-related quality of life improved since study onset, although the maximum value was found to be at discharge from rehabilitation (index value 0.73 ± 0.20).
The largest changes were found to be between visit 2 (at discharge) and visit 3 (3 months after discharge), especially regarding the prevalence of fatigue, anxiety, and depression. This is also illustrated in Fig. 2, in which the shape of the violin plots clearly changes from visit 2 to visit 3. Corresponding to this symptom deterioration, health-related quality of life decreased significantly from visit 2 to visit 3. Especially the frequency of problems regarding usual activities, anxiety and depression increased within this timeframe (Fig. 3). Overall, this symptom deterioration is also displayed by moderate to large effect sizes (r = 0.32–0.53).
Violin plots including boxplots for comparing probability distributions of fatigue (a), anxiety (b) and depression (c) over the time course of visits 1–5. The remarkable change of data between visits 2 and 3 (i.e. the distribution) is clearly visualized by the plots’ change of shape and the increased medians and interquartile ranges within the boxplots.
Between visit 3–5, the burden of symptoms stayed mostly unchanged (small effect sizes for most outcomes, except for the Clinical Frailty Scale and modified Rankin Scale). Three months after discharge, the majority of patients suffered from pain or discomfort (83.1%) and faced most problems regarding usual activities (80.5%) and walking around (75.3%). The frequency of problems remained high until visit 5 (Fig. 3). Approximately one third suffered from severe disability, whereas approximately 50% had no or only mild disability according to WHODAS-12 (Fig. 3). Although severity of dyspnea decreased over time, nearly two thirds still had problems with breathing (62.9%, data not shown) at visit 5.
Correlations of fatigue with depression (rs = 0.55, p < 0.001), anxiety (rs = 0.60, p < 0.001) and health-related quality of life (rs = − 0.33, p < 0.001) were fair to moderate.
Results of the linear mixed-effect models are displayed in Table 3. In the final model, time since disease onset (β = 0.09–0.18, p < 0.01), mechanical ventilation (β = − 0.06, p = 0.01 (z-standardized)) and preclinical frailty (β = − 0.07, p = 0.02) were significant predictors for health-related quality of life. Time was also a significant predictor for the outcomes fatigue (β = 1.02–1.18, p < 0.0001), anxiety (β = − 1.12–1.20, p < 0.04) and depression (β = 1.09–1.50, p < 0.04). All model confirmed the significant increase of fatigue, anxiety and depression over time compared to the levels at visit 1. Additionally, a significant association of obesity with anxiety was found (β = − 2.18, p = 0.02).
Discussion
We investigated the physical and mental health, and the health-related quality of life of chronically critically ill COVID-19 patients 3, 6 and 12 months after discharge from neurorehabilitation. We showed that the overall disability, frailty and dyspnea improved after admission to neurologic rehabilitation. However, the prevalence of fatigue, anxiety, and depression increased over time, especially between discharge and the first study visit three months later, and remained on a high level until the last study visit one year after discharge. Accordingly, health-related quality of life was shown to be limited without noteworthy improvement until the last study visit. Time (since disease onset) had a significant influence on the outcomes anxiety, depression, fatigue and health-related quality of life.
Post-intensive care syndrome (PICS) and symptom prevalences
Patients after critical illness in general frequently suffer from physical, mental and cognitive symptoms in the long-term, which is described as PICS7. As an example, PICS problems were reported to be present in 56% of patients with respiratory failure or shock 12 months after hospital discharge41. PICS was also frequently described in patients after severe COVID-19 disease42 and percentages were similar, as PICS was reported in 61% of patients 13.5 months after ICU discharge43. As we did not plan to investigate PICS in our study, no cognitive evaluation was included in the outcome parameters. However, physical function and mental health were investigated by the EQ-5D-5L and the HADS, which are both recommended assessments to detect PICS44. 12 months after discharge from rehabilitation, anxiety and depression were present in 42% and 39% of patients, respectively. 68% reported problems with walking and even 84% reported pain or discomfort, wherefore we can conclude that the majority of our participants suffered from PICS, even more than one year after the infection.
Previously described long-term outcomes of COVID-19 ICU survivors included a variety of symptoms, which are summarized by the terms post COVID-19 condition (according to the WHO’s case definition) or post-COVID-19 syndrome (according to the NICE guideline on long COVID) and often meet the diagnostic criteria for PICS. However, the symptom load of the post COVID-19 condition is substantially higher in our CCI cohort (Supplementary Table 3). Heesakkers et al.19 reported anxiety and depression in 18% of patients one year after ICU (median 18.5 days on ICU), compared to ~ 40% in our cohort. These authors also reported a median frailty of 2 (vs. 4 in our cohort). Hodgson et al.14 investigated the outcome of a cohort at 6 months (median 8.3 days on ICU). In this cohort, only 5% suffered from a severe disability according to the WHODAS-12, compared to 31% in our cohort. Accordingly, health-related quality of life was substantially lower in our cohort (visual analogue scale, 61 ± 22), compared to the Hodgson-cohort (median = 70 (IQR 60–85)). These examples illustrate the diverging convalescence of patients, which is most likely due to differences in the length of ICU treatment and mechanical ventilation (median 13–14 days14,19 compared to 39 days in our cohort).
PICS and an impaired health status were also frequently described in patients after critical illness in general (non-COVID-19 diseases)7. Just like in COVID-19 patients, symptom load seems to be associated with the length of stay on ICU or the duration of mechanical ventilation in patients with general critical illness (Supplementary Table 3). In patients after sepsis (median duration on ICU 10 days), 6 months after ICU admission, anxiety and depression were only reported in 26% and 21% (compared to our reports of 37% and 29%).45 Accordingly, 12 months after ICU, health-related quality of life (expressed by the visual analogue scale of the EQ-5D-5L) was 66 (44–80) in patients with a median of 8 days on ICU46 and 75 (60–89) in patients with a median of 2 days on ICU47. Both values were substantially higher compared to our cohort (59 ± 24). In patients with CCI, the quality of life is more similar to ours (Supplementary Table 3). Thomas et al. reported a value of 60 (IQR 29) in patients after 41 days on ICU48, Gardner et al. reported a value of 49 ± 7 in patients after 21 days on ICU5. According to our results in comparison with the literature it might be assumed that ICU parameters have a greater influence on CCI outcome than the disease leading to ICU admission. However, up to now, studies in patients with CCI examining patient-reported outcomes / PICS and their predictors are scarce.
Health-related quality of life
Health-related quality of life improved after ICU discharge, but then remained rather unchanged at a low level. This level is substantially lower compared to an equally aged general population in Germany (index value 0.87 ± 0.20; our cohort at visit 5 0.63 ± 0.33). This lack of improvement is contrary to published studies, in which improvements in health-related quality of life from 3 to 12 months in critically ill COVID-19 patients were reported23,49,50. As the duration of mechanical ventilation was shown to negatively influence health-related quality of life50, this might be one explanation for the low level reported in our cohort with particularly long durations of mechanical ventilation.
The peak quality of life at discharge from inpatient care in our study might be explained by the patients’ improvements of independence in activities of daily living and happiness at finally going home. Additionally, at visit 2, the patients were still in the sheltered environment of the neurorehabilitation center with offers of help, prepared meals and accessible surroundings. The return to home with its responsibilities and being on one’s own might have been challenging and therefore might cause a reduction of health-related quality of life. The same might apply for fatigue. Inability to manage activities of daily living without help and being confronted with the prior healthy living conditions might further lead to anxiety and depression.
Fatigue, anxiety and depression
Although COVID-19 ICU survivors usually improve their physical functions over time49,51, fatigue prevalence may rise, as observed in our cohort. Mazza et al. made the same observation in a cohort of hospitalized COVID-19 patients (~ 8% of ICU admissions), where fatigue increased from 22 to 34% from 1 to 12 months after COVID-1952. Likewise, the results of a meta-analysis (68 studies, hospitalized and non-hospitalized patients) indicated no significant improvement of fatigue frequency ≥ 6 months compared to < 6 months after COVID-19 infection53. Two studies with non-COVID-19 CCI patients also concluded that time after discharge had no influence on fatigue severity54,55. Regarding anxiety and depression after COVID-19, different trajectories were described56. Rosa et al. showed a slight increase of symptoms of anxiety (16–25%) and depression (15–20%) from 3 to 12 months23. Vlake et al. and Lorent et al. (median ICU stay 13/18 days) reported unimproved severities of anxiety and depression over the course from 1.5 to 6 months and 3–12 months after hospital discharge, respectively51,57. In contrast, Gramaglia et al. described a significant reduction of anxiety and depression symptoms from 4 to 12 months after discharge in their less affected cohort (~ 12% ICU admissions)58. In a review, several studies were mentioned in which disease severity was a risk factor for anxiety and depression56, which speaks for the high percentages in our CCI cohort.
Inflammatory processes
Inflammatory parameters are subject of several COVID-19 investigations. One review reported elevations in at least one measure of inflammation in 13 of 14 studies. Additionally, in 9 of 14 studies, proinflammatory markers and persistent fatigue and/or cognitive dysfunction were present53. Furthermore, it was reported that chronic fatigue, anxiety and depression of COVID-19 patients share the same pathophysiological mechanisms, which have a strong association with increased oxidative toxicity, lowered antioxidant defenses and inflammatory signs59. In addition to COVID-19, persistent inflammation is also one underlying pathophysiology of CCI and PICS3,4, 7. Furthermore, systemic inflammation is the primary cause of critical illness polyneuropathy and myopathy60, which was diagnosed in 84% of our CCI patients. Therefore, inflammatory processes might be one explanation for the extraordinarily high percentages of fatigue, anxiety and depression in our cohort of CCI patients after COVID-19.
Limitations
We are aware that our study may have several limitations. First, our study was monocentric and the sample size was rather small; additionally, some patients did not participate in every study visit, so the sample size per assessment and per study visit differs and is reduced. Thus, larger multicenter cohorts with CCI patients are needed to confirm our findings. Second, our patients were largely unvaccinated (as most got infected before vaccination got available). As vaccinations was shown to have protective effect on post-COVID-19 condition61, symptoms might differ in CCI COVID-19 populations with vaccination. Furthermore, symptoms of anxiety and depression were shown to be more frequent in patients with previous psychiatric history56, which was not assessed in our study. Additionally, we were not able to include CCI patients with non-COVID-19 diagnoses as a control group, thus we cannot conclude that the reported symptoms are specific for COVID-19.
Conclusions
Persons with CCI associated with COVID-19 suffer greatly from fatigue, anxiety, depression and low health-related quality of life, even one year after discharge from hospital. Improvements were found regarding basic functional capacities and dyspnea. In contrast, fatigue, mental health and health-related quality of life deteriorated over time or remained unchanged at an undesirable level. This analysis showed a higher burden of symptoms compared to other studies with shorter durations of ICU treatment and mechanical ventilation. Therefore, patients with CCI in COVID-19 require adapted therapies and supportive structures even several months after discharge. Special attention should be paid to this group in research and medical treatment.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CCI:
-
Chronic critical illness
- ICU:
-
Intensive care unit
- PICS:
-
Post-intensive care syndrome
References
Vincent, J.-L. & Singer, M. Critical care: Advances and future perspectives. The Lancet 376, 1354–1361. https://doi.org/10.1016/S0140-6736(10)60575-2 (2010).
Nelson, J. E., Cox, C. E., Hope, A. A. & Carson, S. S. Chronic critical illness. Am. J. Respir. Crit. Care Med. 182, 446–454. https://doi.org/10.1164/rccm.201002-0210CI (2010).
Mira, J. C. et al. Sepsis pathophysiology, chronic critical illness, and persistent inflammation-immunosuppression and catabolism syndrome. Crit. Care Med. 45, 253–262. https://doi.org/10.1097/ccm.0000000000002074 (2017).
Stortz, J. A. et al. Evidence for persistent immune suppression in patients who develop chronic critical illness after sepsis. Shock (Augusta, Ga.) 49, 249–258. https://doi.org/10.1097/shk.0000000000000981 (2018).
Gardner, A. K. et al. The development of chronic critical illness determines physical function, quality of life, and long-term survival among early survivors of sepsis in surgical ICUs. Crit. Care Med. 47, 566–573. https://doi.org/10.1097/ccm.0000000000003655 (2019).
Unroe, M. et al. One-year trajectories of care and resource utilization for recipients of prolonged mechanical ventilation: A cohort study. Ann. Intern. Med. 153, 167–175. https://doi.org/10.7326/0003-4819-153-3-201008030-00007 (2010).
Voiriot, G. et al. Chronic critical illness and post-intensive care syndrome: From pathophysiology to clinical challenges. Ann. Intens. Care 12, 58. https://doi.org/10.1186/s13613-022-01038-0 (2022).
Ohbe, H., Matsui, H., Fushimi, K. & Yasunaga, H. Epidemiology of chronic critical illness in Japan: A nationwide inpatient database study. Crit. Care Med. 49, 70–78. https://doi.org/10.1097/CCM.0000000000004723 (2021).
Kahn, J. M. et al. The epidemiology of chronic critical illness in the United States*. Crit. Care Med. 43, 282–287. https://doi.org/10.1097/CCM.0000000000000710 (2015).
Dubin, R. et al. Functional outcomes, goals, and goal attainment among chronically critically Ill long-term acute care hospital patients. Ann. Am. Thorac. Soc. 18, 2041–2048. https://doi.org/10.1513/AnnalsATS.202011-1412OC (2021).
Rawal, G., Yadav, S. & Kumar, R. Post-intensive care syndrome: An overview. J. Transl. Intern. Med. 5, 90–92. https://doi.org/10.1515/jtim-2016-0016 (2017).
Tan, E., Song, J., Deane, A. M. & Plummer, M. P. Global impact of coronavirus disease 2019 infection requiring admission to the ICU: A systematic review and meta-analysis. Chest 159, 524–536. https://doi.org/10.1016/j.chest.2020.10.014 (2021).
Roedl, K. et al. Mechanical ventilation and mortality among 223 critically ill patients with coronavirus disease 2019: A multicentric study in Germany. Aust. Crit. Care Off. J. Confed. Aust. Crit. Care Nurses 34, 167–175. https://doi.org/10.1016/j.aucc.2020.10.009 (2021).
Hodgson, C. L. et al. The impact of COVID-19 critical illness on new disability, functional outcomes and return to work at 6 months: A prospective cohort study. Crit. Care 25, 382. https://doi.org/10.1186/s13054-021-03794-0 (2021).
Gattinoni, L. et al. COVID-19 pneumonia: Pathophysiology and management. Eur. Respir. Rev. 30, 210138. https://doi.org/10.1183/16000617.0138-2021 (2021).
Martillo, M. A. et al. Postintensive care syndrome in survivors of critical illness related to coronavirus disease 2019: Cohort study from a new York City critical care recovery clinic. Crit. Care Med. 49, 1427–1438. https://doi.org/10.1097/ccm.0000000000005014 (2021).
Rousseau, A. F. et al. Post-intensive care syndrome after a critical COVID-19: Cohort study from a Belgian follow-up clinic. Ann. Intens. Care 11, 118. https://doi.org/10.1186/s13613-021-00910-9 (2021).
Daste, C. et al. Post-intensive care syndrome in patients surviving COVID-19. Ann. Phys. Rehabil. Med. 64, 101549–101549. https://doi.org/10.1016/j.rehab.2021.101549 (2021).
Heesakkers, H. et al. Clinical outcomes among patients with 1-year survival following intensive care unit treatment for COVID-19. Jama 327, 559–565. https://doi.org/10.1001/jama.2022.0040 (2022).
Wimmer, C. et al. Critical COVID-19 disease: Clinical course and rehabilitation of neurological deficits. Front. Neurol. https://doi.org/10.3389/fneur.2022.1012685 (2022).
Gamberini, L. et al. Quality of life of COVID-19 critically ill survivors after ICU discharge: 90 days follow-up. Qual. Life Res. Int. J. Qual. Life Aspects Treat. Care Rehabil. 30, 2805–2817. https://doi.org/10.1007/s11136-021-02865-7 (2021).
Malik, P. et al. Post-acute COVID-19 syndrome (PCS) and health-related quality of life (HRQoL)-A systematic review and meta-analysis. J. Med. Virol. 94, 253–262. https://doi.org/10.1002/jmv.27309 (2022).
Rosa, R. G. et al. Association between acute disease severity and one-year quality of life among post-hospitalisation COVID-19 patients: Coalition VII prospective cohort study. Intens. Care Med. https://doi.org/10.1007/s00134-022-06953-1 (2013).
Roedl, K. et al. Chronic critical illness in patients with COVID-19: Characteristics and outcome of prolonged intensive care therapy. J. Clin. Med. 11, 1049. https://doi.org/10.3390/jcm11041049 (2022).
Blayney, M. C. et al. Prevalence, characteristics, and longer-term outcomes of patients with persistent critical illness attributable to COVID-19 in Scotland: A national cohort study. Br. J. Anaesth. 128, 980–989. https://doi.org/10.1016/j.bja.2022.03.017 (2022).
Valentin, A. Intensive care medicine-survival and prospect of life. Medizinische Klinik, Intensivmedizin und Notfallmedizin 112, 584–588. https://doi.org/10.1007/s00063-017-0349-y (2017).
Kandilov AM et al. Chronically Critically Ill Population Payment Recommendations (CCIP-PR) <https://innovation.cms.gov/files/reports/chronicallycriticallyillpopulation-report.pdf> (2014).
Egger, M. et al. Severe Post-COVID-19 Condition after Mild Infection: Physical and Mental Health Eight Months Post Infection: A Cross-Sectional Study. Int. J. Environ. Res. Public Health. 21, 21. https://doi.org/10.3390/ijerph21010021 (2024).
van Walraven, C., Austin, P. C., Jennings, A., Quan, H. & Forster, A. J. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med. Care 47, 626–633. https://doi.org/10.1097/MLR.0b013e31819432e5 (2009).
Johansson, S., Kottorp, A., Lee, K. A., Gay, C. L. & Lerdal, A. Can the Fatigue Severity Scale 7-item version be used across different patient populations as a generic fatigue measure–a comparative study using a Rasch model approach. Health Qual. Life Outcomes 12, 24. https://doi.org/10.1186/1477-7525-12-24 (2014).
Valko, P. O., Bassetti, C. L., Bloch, K. E., Held, U. & Baumann, C. R. Validation of the fatigue severity scale in a Swiss cohort. Sleep 31, 1601–1607. https://doi.org/10.1093/sleep/31.11.1601 (2008).
Nikayin, S. et al. Anxiety symptoms in survivors of critical illness: A systematic review and meta-analysis. Gen. Hosp. Psychiatry 43, 23–29. https://doi.org/10.1016/j.genhosppsych.2016.08.005 (2016).
Zigmond, A. S. & Snaith, R. P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 67, 361–370. https://doi.org/10.1111/j.1600-0447.1983.tb09716.x (1983).
Janssen, M. F., Bonsel, G. J. & Luo, N. Is EQ-5D-5L better than EQ-5D-3L? A head-to-head comparison of descriptive systems and value sets from seven countries. PharmacoEconomics 36, 675–697. https://doi.org/10.1007/s40273-018-0623-8 (2018).
van Hout, B. et al. Interim scoring for the EQ-5D-5L: Mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health J. Int. Soc. Pharmacoecon. Outcomes Res. 15, 708–715. https://doi.org/10.1016/j.jval.2012.02.008 (2012).
Saltychev, M., Katajapuu, N., Bärlund, E. & Laimi, K. Psychometric properties of 12-item self-administered World Health Organization disability assessment schedule 2.0 (WHODAS 2.0) among general population and people with non-acute physical causes of disability—systematic review. Disabil. Rehabil. 43, 789–794. https://doi.org/10.1080/09638288.2019.1643416 (2021).
Pugh, R. J. et al. Reliability of frailty assessment in the critically ill: A multicentre prospective observational study. Anaesthesia 74, 758–764. https://doi.org/10.1111/anae.14596 (2019).
Cantier, M. et al. Functional outcomes in adults with tuberculous meningitis admitted to the ICU: A multicenter cohort study. Crit. Care 22, 210. https://doi.org/10.1186/s13054-018-2140-8 (2018).
Mahler, D. A. & Wells, C. K. Evaluation of clinical methods for rating dyspnea. Chest 93, 580–586. https://doi.org/10.1378/chest.93.3.580 (1988).
Akoglu, H. User’s guide to correlation coefficients. Turk. J. Emerg. Med. 18, 91–93. https://doi.org/10.1016/j.tjem.2018.08.001 (2018).
Marra, A. et al. Co-occurrence of post-intensive care syndrome problems among 406 survivors of critical illness. Crit. Care Med. 46, 1393–1401. https://doi.org/10.1097/CCM.0000000000003218 (2018).
Nakanishi, N. et al. Post-intensive care syndrome and its new challenges in coronavirus disease 2019 (COVID-19) pandemic: A review of recent advances and perspectives. J. Clin. Med. https://doi.org/10.3390/jcm10173870 (2021).
Hatakeyama, J. et al. Prevalence and risk factor analysis of post-intensive care syndrome in patients with COVID-19 requiring mechanical ventilation: A multicenter prospective observational study. J. Clin. Med. https://doi.org/10.3390/jcm11195758 (2022).
Mikkelsen, M. E. et al. Society of critical care medicine’s international consensus conference on prediction and identification of long-term impairments after critical illness. Crit. Care Med. 48, 1670–1679. https://doi.org/10.1097/ccm.0000000000004586 (2020).
Hodgson, C. L. et al. Comparison of 6-month outcomes of sepsis versus non-sepsis critically ill patients receiving mechanical ventilation. Crit. Care 26, 174. https://doi.org/10.1186/s13054-022-04041-w (2022).
Griffiths, J. et al. An exploration of social and economic outcome and associated health-related quality of life after critical illness in general intensive care unit survivors: A 12-month follow-up study. Crit. Care 17, R100. https://doi.org/10.1186/cc12745 (2013).
Cavalleri, J. et al. One-year functional decline in COVID-19 and non-COVID-19 critically ill survivors: A prospective study incorporating a Pre-ICU status assessment. Healthcare 10, 2023 (2022).
Thomas, S. & Mehrholz, J. Health-related quality of life, participation, and physical and cognitive function of patients with intensive care unit-acquired muscle weakness 1 year after rehabilitation in Germany: The GymNAST cohort study. BMJ Open 8, e020163. https://doi.org/10.1136/bmjopen-2017-020163 (2018).
Bels, J. L. M. et al. One-year outcomes of mechanically ventilated COVID-19 ICU survivors: A prospective cohort study. Am. J. Respir. Crit. Care Med. 206, 777–780. https://doi.org/10.1164/rccm.202112-2789LE (2022).
Figueiredo, E. A. B. et al. The health-related quality of life in patients with post-COVID-19 after hospitalization: A systematic review. Revista da Sociedade Bras. de Med. Trop. 55, e0741. https://doi.org/10.1590/0037-8682-0741-2021 (2022).
Lorent, N. et al. Prospective longitudinal evaluation of hospitalised COVID-19 survivors 3 and 12 months after discharge. ERJ Open Res. 8, 00004–02022. https://doi.org/10.1183/23120541.00004-2022 (2022).
Mazza, M. G. et al. Prevalence, trajectory over time, and risk factor of post-COVID-19 fatigue. J. Psychiatr. Res. 155, 112–119. https://doi.org/10.1016/j.jpsychires.2022.08.008 (2022).
Ceban, F. et al. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain Behav. Immun. 101, 93–135. https://doi.org/10.1016/j.bbi.2021.12.020 (2022).
Wintermann, G. B. et al. Self-reported fatigue following intensive care of chronically critically ill patients: A prospective cohort study. J. Intens. Care 6, 27. https://doi.org/10.1186/s40560-018-0295-7 (2018).
Morel, J. et al. Prevalence of self-reported fatigue in intensive care unit survivors 6 months-5 years after discharge. Sci. Rep. 12, 5631. https://doi.org/10.1038/s41598-022-09623-w (2022).
Schou, T. M., Joca, S., Wegener, G. & Bay-Richter, C. Psychiatric and neuropsychiatric sequelae of COVID-19—A systematic review. Brain Behav. Immun. 97, 328–348. https://doi.org/10.1016/j.bbi.2021.07.018 (2021).
Vlake, J. H. et al. Psychologic distress and quality of life after ICU treatment for coronavirus disease 2019: A multicentre, observational cohort study. Crit. Care Explor. 3, e0497–e0497. https://doi.org/10.1097/CCE.0000000000000497 (2021).
Gramaglia, C. et al. Anxiety, stress and depression in COVID-19 survivors from an Italian cohort of hospitalized patients: Results from a 1-year follow-up. Front. Psych. 13, 862651. https://doi.org/10.3389/fpsyt.2022.862651 (2022).
Al-Hakeim, H. K., Al-Rubaye, H. T., Al-Hadrawi, D. S., Almulla, A. F. & Maes, M. Long-COVID post-viral chronic fatigue and affective symptoms are associated with oxidative damage, lowered antioxidant defenses and inflammation: A proof of concept and mechanism study. Mol. Psych. 28, 564–578. https://doi.org/10.1038/s41380-022-01836-9 (2023).
Kramer, C. L. Intensive care unit-acquired weakness. Neurol. Clin. 35, 723–736. https://doi.org/10.1016/j.ncl.2017.06.008 (2017).
Byambasuren, O., Stehlik, P., Clark, J., Alcorn, K. & Glasziou, P. Effect of covid-19 vaccination on long covid: Systematic review. BMJ Med. 2, e000385. https://doi.org/10.1136/bmjmed-2022-000385 (2023).
Acknowledgements
The authors thank all the patients for their engagement and for taking the time for the interviews. Furthermore, we thank Katie Göttlinger for copy-editing the manuscript. We thank Dr. Ralf Strobl of the German Center of Vertigo and Balance Disorders and the IBE at the Ludwig-Maximilians-Universität in Munich for statistical advice.
Funding
This work was funded by the Else Kröner-Fresenius-Stiftung (2021_EKEA.78).
Author information
Authors and Affiliations
Contributions
ME: Conceptualization, Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Supervision; Validation; Visualization; Writing—original draft; Writing—review & editing. CW: Conceptualization; Investigation; Writing—review & editing. SS: Investigation; Writing—review & editing. JR: Investigation; Writing—review & editing. JB: Conceptualization; Funding acquisition; Methodology; Project administration; Supervision; Writing—review & editing. FM: Conceptualization; Methodology; Resources; Writing—review & editing. KJ: Conceptualization; Funding acquisition; Methodology; Project administration; Resources; Supervision; Writing—review & editing. All authors read and approved the submitted version and have agreed to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Egger, M., Wimmer, C., Stummer, S. et al. Reduced health-related quality of life, fatigue, anxiety and depression affect COVID-19 patients in the long-term after chronic critical illness. Sci Rep 14, 3016 (2024). https://doi.org/10.1038/s41598-024-52908-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-52908-5
- Springer Nature Limited