Abstract
Insect Growth Regulator (IGR) novaluron is an alternative to synthetic neuro-inhibitory insecticides. Present study was designed to assess appropriate dosages of novaluron for dengue vector control. Larvae of Aedes aegypti and Ae. albopictus were exposed to a concentration series of novaluron (Rimon EC10) for two fixed exposure periods of 7-days and 14-days to determined LC50 and LC99 values. Inhibition of adult emergence (IE50 and IE99) was determined by a 14-day exposure. Semi-field experiments were conducted by exposing cohorts of Ae. aegypti larvae to IE99, 2 × IE99 and 10 × IE99 novaluron concentrations in water storage buckets (10 L) and plastic barrels (200 L). For the 7-day exposure, LC50 values were 0.047–0.049 ppm and LC99 were 0.144–0.151 ppm. For 14-day exposure, these values were 0.002–0.005 ppm and 0.006–0.01 ppm respectively. For both species, IE99 was 0.001 ppb under semi-field conditions, and was effective for nearly 2 months. Novaluron concentration 0.01 ppb was effective up to 3 months, with an IE of 89–95%. Authorities should critically review a reduction of the presently recommended field dosage of 200 ppm novaluron by × 100 or more. This would provide the same efficacy but mitigate environmental pollution, development of vector resistance, and financial losses.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Introduction
Dengue fever is a mosquito-borne tropical disease caused by the dengue virus. It has been estimated that 3.9 billion people in the world are at risk of the disease. In Sri Lanka, dengue fever (DF) has become a serious public health concern especially with the alarming increase of cases in the recent past (Epidemiology reports1. The disease is transmitted through the bites of the mosquitoes Aedes aegypti and Ae. albopictus2. Two transmission periods of the disease in Sri Lanka is parallel to the two monsoon seasons, showing the close association between dengue transmission and the rain fall. The most intensive transmission period June to August coincides with the southwest monsoon, whereas the less intense October to December period coincides with the northeast monsoon3,4.
Vector control plays the major role in reducing the disease burden especially in the absence of an effective vaccine against dengue. Suppression of vector densities is commonly attempted through various methodologies such as use of insecticides, source reduction, and implementation of new regulations for vector elimination5. Although the general public is urged to destroy or remove all possible indoor and outdoor Aedes breeding sites, it has not always been effective due to inadequacy of resources and poor community support6. Use of insecticides has emerged as a predominant and efficient approach in the control of Aedes vector populations, particularly during disease outbreaks7. Among the WHO recommended insecticides, pyrethroids with low mammalian toxicity and a higher efficacy are widely used in vector control programmes5,8. For more than two decades, Sri Lanka has been using pyrethroids as adulticides and temephos (organophosphate) as a larvicide for Aedes mosquito control. Constant application and indiscriminate use have led to emergence of insecticide resistance in Aedes populations. Several studies have reported high incidence of resistance to neuroinhibitory insecticides in Sri Lankan dengue vectors9,10,11,12,13,14,15. Also, the application of temephos as a larvicide is especially a concern due to its toxic effect on non-target aquatic life16,17. Furthermore, research has shown that diversifying control methods, such as incorporating biological control agents18 or insect growth regulators19 can provide sustainable and long-term solutions for managing dengue vector populations while reducing dependence on neuroinhibitory insecticides. Therefore dengue vector control programmes are looking for effective alternatives to minimize the use of neuroinhibitory insecticides.
Insect growth regulators (IGR) have been introduced as potential alternatives to control dengue vector populations20. Among IGRs, novaluron is considered as an active agent for insect larval control worldwide (WHO, 200721). This benzoylphenyl urea compound affects larval and pupal stages of the Orders Coleoptera, Diptera and Hemiptera22,23,24,25. Novaluron has been successfully used to control Aedes mosquito larvae26. Although the mechanism of action of novaluron has not been extensively investigated, the general mechanism of benzoylphenyl urea action has been well documented27,28,29. Benzoylphenyl urea changes the elasticity and rigidity of the endocuticle in insect immature stages by changing cuticular composition. This mainly affects molting stages of insects, causing death by abnormal endocuticular deposition and interrupted molting28. At sub lethal dosages, novaluron has a low risk to the environment, including its effect to mammals, birds and aquatic insect species other than mosquito larvae30,31.
Novaluron has been introduced to dengue vector control programs in many countries as an alternative to neuroinhibitory insecticides. However, there are still unanswered questions regarding its optimum field dosage. Although the WHO recommended discriminating dosage for novaluron is 10–50 ppb for dengue vector species25, many researchers, after evaluating the efficacy of the compound, have concluded different effective concentrations for field applications based on their findings26,32,33,34,35. It has been observed that novaluron acts as an emergence inhibitor at lower dosages but as a larvicide at higher concentrations. However, the LC50, LC90 and EI 100% concentration are inconsistent among the published literature. Although the application of higher dosages results larval death32, it has a negative impact on the environment and non-target organisms36,37. Dosages which inhibit larval emergence may be sufficient for the strategy to control the vector mosquitoes. Therefore, a proper understanding on larvicidal and emergence inhibition dosages of novaluron is needed for better usage of this IGR in vector control programmes. The present study was designed to undertake laboratory and semi-field trials to evaluate the efficacy and residual effect of novaluron (Rimon EC [emulsifiable concentrate] against immature stages of Ae. aegypti and Ae. albopictus.
Methods
Mosquito larvae and novaluron
During the period of January 2020 to April 2020, mosquito eggs were collected using ovitraps from Bandaranayakepura in Kurunegala Medical Officer of Health (MOH) area, where novaluron had not been used for vector control programs. Collected mosquito eggs were brought to the laboratory of Entomological Surveillance Unit, Office of the Regional Director of Kurunegala and transferred to hatching trays where they were allowed to hatch. Third instar larvae were used for all bioassay experiments with novaluron. Formulated emulsifiable novaluron (100 g/L, Rimon EC10) was a gift from Makhteshim Chemical Works Ltd, Israel.
Bioassay experiments
Determination of LC50 and LC99 values against novaluron
Novaluron bioassays were performed according to the guidelines of WHO5. Stock solution (10,000 ppm) of novaluron was prepared in tap water by 10 × dilution of 100 g/L, Rimon EC10 formulated novaluron. Desired concentrations of novaluron for the bioassay experiments were prepared by mixing the calculated volumes of the stock solution and tap water. Batches of 25 third instar larvae of Ae. aegypti and Ae. albopictus were exposed separately to eight concentrations (i.e. 0.0001 ppm, 0.001 ppm, 0.01 ppm, 0.05 ppm, 1 ppm, 2 ppm, 3 ppm, 4 ppm) of novaluron. A control experiment was conducted along with each experiment using tap water alone. Assay with each novaluron concentration was replicated 4 times. The number of dead immature stages and the number emerged as adults were counted at 24-h intervals during a 14-day period. Mortality data, obtained for a minimum of four concentrations giving mortality between 0 and 100%, at the end of 7th day (0.0001 ppm–0.05 ppm) or at the end of the 14th day (0.00001 ppm–0.05 ppm) were used separately for probit-regression analysis using SPSS to determine LC50 and LC99 values. All bioassays were undertaken at the insectary under controlled conditions 26 ± 2 °C and 80 ± 5% RH.
Determination of IE50 and IE99 values against novaluron
Insectary-reared 25 third instar larvae of Ae. aegypti and Ae. albopictus were exposed separately to concentrations in the range 0.00005 ppb-0.005 ppb novaluron. Adult emergence was assessed by exuviae counts and corresponding flying adults for a period of 14 days. The effect of novaluron was expressed in terms of percentage inhibition of adult emergence (IE %) which is the percentage of larvae that do not develop successfully into emerging adults compared to the control5. In recording IE% for each concentration, moribund and dead larvae and pupae, and partial adults that could not completely separate from the pupal cases, were considered as “affected”. Four concentrations giving IE% between 0 and 100% were used for probit-regression analysis using SPSS to determine IE50 and IE99 values for one week period and for 2-week period separately.
Semi-field trials
Residual effect of novaluron on Ae. aegypti 3rd instar larvae under semi-field conditions were assessed according to procedure described by WHO24,25 and WHOPE38, in two different types of water storage containers,water storage buckets (10 L and plastic barrels (200 L, the two most popular forms of water-storage containers used by house holders in the area. Aliquots of novaluron stock solution were added to the test containers with tap water to obtain required concentrations. Novaluron dosage 0.001 ppb, which gave IE99 for a 14-day period during the laboratory experiments, was used as the standard and, water storage buckets and plastic barrels were prepared with three dosages; 0.001 ppb (IE99), 0.002 ppb (2 × IE99) and 0.01 ppb (10 × IE99). Sentinel cages were used to place larvae (25 third instar larvae in each cage) in each water storage bucket/plastic barrel. This facilitated the observations on inhibition of emergence and mortality counts.
Sentinel cages were prepared by using 250 mL transparent plastic cups. Bottom of each cup was replaced with a plastic mosquito mesh. After placing larvae, the cups were covered with lids prepared by the same mesh. Each cup was emersed in water in the bucket or the barrel and its positions was stabilized using a wire linked to barrel/ bucket rim. After adding larvae to a sentinel cup, the water storage container was covered by a net to prevent mosquito egg laying. Control experiments were conducted without novaluron. Experimental set up included thirteen buckets (three test concentrations each with 4 replicates, and a control) and thirteen barrels, and were placed in an outdoor undisturbed area with a covered roof. Larval food was added once a week and water loss was replenished weekly.
A larval cohort (batch of 25) was introduced to the sentinel cage in the fresh novaluron solution filled bucket/barrel at the beginning of each experiment. After a 7-day exposure period, larvae of the first cohort were removed and a second cohort was introduced. Third cohort replaced the second cohort after another 7 days. Thereafter, each cohort was replaced by a new cohort once in a 14-day period. Before introducing a new cohort (batch of 25), all the dead/survive larvae from the previous cohort were removed. Assessment of larval survivorship was recorded. Adult emergence was assessed by counting pupal skins and emerged adults. After the count, remaining pupal skins were removed by a syringe or a fish net before the next cohort was introduced. Observations and recordings were continued until all the immature stages in the control emerged into adults.
Data analysis
Dose response curves were generated by using cumulative mortality in each concentration within the specific time period. Percentage of inhibition of emergence (IE%) was calculated using the following equation.
\(\% {\text{IE}} = 100 - \left( {{\text{T}}/{\text{C}}*100} \right)\)
where T is the number of emerged adults in tested units, C is the number of emerged adults in control unit5,26, 39.
Mortality and IE data obtained at each concentration was subjected to probit-regression analysis to determine LC50 and LC90 values and, IE50 and IE99 values using SPSS (v.9.0) program for Windows.
Residual effect in semi-field data was analyzed by one-way ANOVA. Kruskal–Wallis test was employed to compare adult emergence inhibition and adult emergence rates across all stand-alone and combination treatments. This non-parametric test was well-suited for comparing medians, particularly in cases where a non-normal distribution was observed, a condition not accommodated by the ANOVA test. A significance level of p < 0.05 was considered statistically significant.
Results
At the end of the 7-day period, larvae in control groups exhibited 100% emergence rate. The cumulative mortality of Ae. aegypti and Ae. albopictus 3rd instar larvae treated with eight different concentrations of novaluron under laboratory conditions are shown in Fig. 1. At the highest concentrations used (3 ppm and 4 ppm) Ae. aegypti larvae started to die within the first 24-h exposure whereas at the lowest concentration (0.0001 ppm) the mortality started only after 7 days. The highest concentration 4 ppm gave 100% mortality after 5 days of exposure and the concentrations 1.0 ppm and below did not result 100% mortality even after 14 days of exposure. Results showed that Ae. albopictus is slightly more resistant to novaluron. During a 24-h exposure period, Ae. albopictus larval mortalities were 3% and 10% lower than Ae. aegypti larval mortalities at the concentrations 3 ppm and 4 ppm, respectively. Concentrations 2 ppm and below could not achive 100% mortality at the end of the 14 days period (Figs. 1 and 2).
During the 7 days and 14 days exposure period, pupation of larvae of both species occurred only in the concentrations 0.05 ppm and below. At these concentrations, deformities could be observed both in larvae (elongated thorax with pigmentation and with a capsuled head) and pupae (incomplete molting resulting intermediate stage between larva and pupa). In the concentrations above 1.0 ppm, larval mortality without pupation occurred (Figs. 3 and 4). Aedes aegypti showed a statistically significant (p = 0.002, F = 10.78) higher pupal mortality compared to that of Ae. albopictus.
Lethal concentrations of novaluron which killed 50% (LC50) and 99% (LC99) of larvae/pupae at the end of 7-day period and at the end of 14-day periods and the calculated IE50 and IE99values for 14-day exposure periods are presented in the Table 1.
When the LC50 values for 7-day and 14-day exposure periods are compared, concentrations needed to kill 50% or 99% of Ae. aegypti population in 7 days were about 24 times higher than the respective concentrations needed for the 14 day period. For Ae. albopictus, the respective concentrations needed were about 10–15 times higher (Table 1).
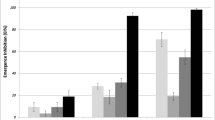
Adult emergence percentage by novaluron during the 14-day period is shown in the Figs. 5 and 6. In the controls, adult emergence of both species started from 5th day onwards. Aedes aegypti larvae started to emerge after the 6th day of exposure at the lowest 0.00005 ppb concentration and after 13 days of exposure at the highest 0.005 ppb concentration. After 14 days, adult emergence was 73 ± 0.12 at 0.00005 ppb and 3 ± 0.53 at 0.0005 ppb (Fig. 5). Corresponding values for Ae. albopictus were 62 ± 0.4 and 2 ± 0.2 respectively, showing that adult emergence inhibition for Ae. albopictus was more or less similar to that of Ae. aegypti for the tested novaluron concentrations (Fig. 6).
All three novaluron concentrations used for the semi-field experiments caused 96% adult emergence inhibition up to 2 weeks and 88–100% IE up to 4 weeks (one month) (Table 2). An inhibition of 55%-64% was observed at 0.001 ppb–0.002 ppb concentrations up to a 7-week period. Novaluron concentration 0.01 ppb was highly effective giving 89–95% IE for a 3-month and 67–68% IE for a 4-month period. No significant differences were observed in IE values (F = 0.09, p < 0.05) and adult emergence (F- 0.087, P < 0.05) between the two types of containers tested.
Discussion
Although dengue infections have been documented from Sri Lanka since the 1960s, the first significant DHF outbreak with 206 clinically diagnosed cases and 20 deaths (CFR 9.8%) occurred in Colombo in 198940. Since then, dengue outbreaks have been gradually spreading to many regions of the island, with an increase in reported cases occurring every 3 to 5 years (Ministry of Health Sri41. Increasing tendency of dengue prevalence in Sri Lanka emphasizes the urgent need of effective vector control measures. Traditional vector control methods have several limitations including vector resistance15,42, 43. Biological insecticides based on Bacillus thuringiensis (Bt) have been employed in Sri Lanka as an alternative in integrated vector control strategies with varying success42. Storage challenges and cost-effectiveness, and resistance development are major challenges against Bt insecticides26,44, 45. Insect growth regulators (IGRs) are a group of chemicals that have been recently used as an effective alternative for controlling insect vectors worldwide7. They exhibit a good margin of safety to most non-target biota, thus offering some advantages in mosquito control programmes26 and are strictly arthropod-specific and environmentally safe and show very low toxicity to birds, mammals and honey bees24,46. Novaluron 10 EC formulation has been developed under WHOPES guidance and supervision, as an IGR against insects, including mosquitoes24,25.
Previous studies have shown the efficacy of novaluron against Ae. aegypti and Ae. albopictus28, Arredondo-Jiménez et al.35, Culex quinquefasciatus26, Arredondo-Jiménez et al.35 and Anopheles species47. Novaluron is reportedly effective against Aedes larvae, although it is unclear what dosages are to be applied in vector control programs.
Present results shows that Ae. albopictus slightly more resistant to novaluron than Ae. aegypti, an observation made by some previous workers as well33,35. Both vector species exhibit 100% larval mortality within a 24-h exposure period at 3 ppm–4 ppm novaluron concentrations, which is lower than the 9.43 ppm dosage reported for the same purpose by Gunathilaka et al.32 for Aedes aegypti. Also, our results revealed that, in a 14-day exposure, a concentration of 1.0 ppm is enough to kill both Ae. aegypti and Ae. albopictus larvae before pupation. Even at 0.05 ppm, although some larvae pupated to deformed pupae, all larvae and pupae got killed during the 14-day exposure. Deformities caused by abnormal endocuticular deposition and interrupted molting are expected from novaluron exposure28. Similar deformed pupations had been observed by Mulla et al.26 at 0.25 ppb technical novaluron concentration for the 2nd instar Ae. aegypti larvae and by Fontoura et al.34 at 0.40 ppb formulated novaluron concentration for 3rd instar Ae. aegypti larvae, under field conditions.
Lethal concentrations of novaluron which kill 50% (LC50) and 99% (LC99) of larvae/pupae for 7-day period exposure was 0.047 ppm and 0.144 ppm respectively for Ae. aegypti, and 0.049 ppm and 0.151 ppm for Ae. albopictus respectively. Arredondo-Jiménez et al.35 reported LC50 and LC99 values 0.025 ppm and 0.07 ppm respectively for Ae. aegypti and, 0.035 ppm and 0.09 respectively for Ae. albopictus for the 7-day period exposure to the same formulated novaluron (Rimon 10EC). For a 24-h exposure, Gunathilaka et al.32 have reported 2.72 ppm LC50 and 9.43 ppm LC99 for Ae. aegypti. For a 14-day exposure period, Withanage et al.33 have reported × 10 lower LC50 and LC99 values (0.0002 ppm and 0.01 ppm respectively for Ae. aegypti, 0.0003 ppm and 0.004 ppm respectively for Ae. albopictus) than ours for the same formulated novaluron.
Novaluron, which is an insect growth regulator, inhibits adult emergence and the immature stages ultimately die after a prolonged immature period. We could observe this effect of novaluron clearly at lower concentrations. Under laboratory conditions, 50% adult emergence inhibition came from 0.0003 ppb for Ae. aegypti and from 0.0004 ppb for Ae. albopictus whereas 99% adult emergence inhibition was achieved by both species at 0.001 ppb. Present study indicated a higher efficacy of novaluron than that has been reported previously i.e., 550 ppb IE99 for both vectors35, 0.14 ppb IE50 and 0.22 ppb IE9934 and, 0.09 ppb IE50 and 0.1 ppb IE99 for Ae. aegypti48 for a 14-day exposure period.
During our study, residual effect of novaluron was evaluated under semi-field conditions. Number of days during which the emergence inhibition is more than 80% is considered as the duration of effectiveness38. According to this definition, even 0.001 ppb was effective nearly for 2 months. Novaluron concentration 0.01 ppb was effective for 3 months, with an IE of 89–95%. Fontoura et al.34 and Arredondo-Jiménez et al.35 have reported that formulated novaluron (Rimon 10EC) is effective for a 4 month period at concentrations 0.22 ppb and 0.55 ppm respectively which are nearly × 10 and × 10,000 times respectively higher than the 0.01 ppb concentration we demonstrated for 3 months.
Our data demonstrates that 0.02 ppm concentration of formulated novaluron (Rimon 10EC) can be effectively used as a larvicide against dengue vectors. It will kill larvae before pupation. A concentration 0.01 ppb is effective for 3 months as an adult emergence inhibitor. However, the present WHO recommended dosage is 0.01–0.05 ppm24,25. Gunathilaka et al.32 have recommended 20 ppm dosage of novaluron for vector control against Sri Lankan Aedes mosquitoes. This concentration gave 100% mortality within 24 h of application. In Sri Lanka, the label of the commercially available Rimon EC10 bottle states that the recommended dosage to be used for larval breeding sites is 200 ppm which is an extraordinarily high value. Authorities should take this matter seriously and reduce the recommended dosages for field application to an appropriate level considering scientific data coming from research studies so that environmental pollution, effect on non-target insect larvae, resistance development and financial loss can be minimized.
Our study highlights that novaluron can contribute significantly to vector control strategies by offering an effective, environment friendly and a cost-effective alternative to traditional insecticides. Also, our results challenge the current guidelines on novaluron usage, advocating for lower dosages. The work presented here was limited to laboratory and semi-field conditions. Field studies to validate these findings in real-world settings are needed to confirm the findings. Also, long-term impact on mosquito populations and potential resistance development and management, which are crucial for assessing the sustainability of this vector control strategy, require further research. A broader environmental impact analysis to fully validate and support the argument for changing current vector control practices is also a necessity.
Conclusion
Formulated novaluron (Rimon 10EC) 0.02 ppm concentration can be effectively used as a larvicide against dengue vectors. A concentration 0.01 ppb is effective for 3 months as an adult emergence inhibitor. The recommended dosages for the field application of novaluron 200 ppm is extraordinarily high, and the authorities should reconsider their recommendation to minimize environmental pollution, the effect on non-target insect larvae, resistance development, and financial loss.
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
Epidemiolog Unit, Sri Lanka. http://www.epid.gov.lk/web/index.php?option=com_cases and deaths&Itemid=448&lang=en#. (2022).
World Health Organization. Dengue Fact Sheet. https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (2020)
Surendran, S. N. et al. Salinity tolerant Aedes aegypti and Ae. albopictus—infection with dengue virus and contribution to dengue transmission in a coastal peninsula. J. Vector Borne Dis. 55, 26 (2018).
Herath, J. M. M. K., Abeyasundara, H. T. K., De Silva, W. A. P. P., Weeraratne, T. C. & Karunaratne, S. H. P. P. Weather-based prediction models for the prevalence of dengue vectors Aedes aegypti and Ae. albopictus. J. Trop. Med. https://doi.org/10.1155/2022/4494660 (2022).
World Health Organization (WHO). Monitoring and managing insecticide resistance in Aedes mosquito populations; Interim guidance for entomologists (2016).
Sirisena, P. & Noordeen, F. Dengue control in Sri Lanka—improvements to the existing state of the art in the island, Sri Lankan. J. Infect. Dis. 6, 2–16 (2016).
World Health Organization (WHO). Manual for monitoring insecticide resistance in mosquito vectors and selecting appropriate interventions (2022).
Amelia-Yap, Z. H. et al. Pyrethroid resistance in the dengue vector Aedes aegypti in Southeast Asia: Present situation and prospects for management. Parasit. Vectors 11, 332 (2018).
Dalpadado, R., Gunathilaka, N., Amarasinghe, D. & Udayanga, L. A challenge for a unique dengue vector control programme: Assessment of the spatial variation of insecticide resistance status amongst Aedes aegypti and Aedes albopictus populations in Gampaha District, Sri Lanka. Bio Med. Res. Int. https://doi.org/10.1155/2021/6619175 (2021).
Fernando, H. S. D. et al. First report of V1016G and S989P knockdown resistant (kdr) mutations in pyrethroid-resistant Sri Lankan Aedes aegypti mosquitoes. Parasite Vectors 11, 526 (2018).
Fernando, H. S. D. et al. Resistance to commonly used insecticides and underlying mechanisms of resistance in Aedes aegypti (L.) from Sri Lanka. Parasites Vectors 13, 407. https://doi.org/10.1186/s13071-020-04284-y (2020).
Hegoda, W. K. D. L., Fernando, H. S. D. & de Silva, B. G. N. K. Detecting of knockdown resistance (kdr) F1534C allele in the dengue vector Aedes aegypti in peri-urban areas of Colombo South, Sri Lanka. J. Entomol. Zool. Stud. 5, 1926–1929 (2017).
Karunaratne, S. H. P. P., Weeraratne, T. C., Perera, M. D. B. & Surendran, S. N. Insecticide resistance and efficacy of space spraying and Larviciding in the control of dengue vectors Aedes aegypti and Aedes albopictus in Sri Lanka. Pestic. Biochem. Physiol. 107, 98–105 (2013).
Nugapola, N.W.N.P., de Silva, W.A.P.P., Weeraratne, T.C. & Karunaratne, S. H. P. P. kdr type mutations and enhanced GST based insecticide resistance in dengue vector mosquitoes Aedes aegypti and Aedes albopictus. Int. J. Trop. Insect Sci. 41, 10 (2020).
Karunaratne, S. H. P. P. & Surendran, S. N. Mosquito control: A review on the past, present and future strategies. J. Natl. Sci. Found. Sri Lanka. 50, 277–292 (2022).
Brown, M. D., Thomas, D. & Kay, B. H. Acute toxicity of selected pesticides to the Pacific blue-eye, Pseudomugil signifer (Pisces). J. Am. Mosq. Control Assoc. 14, 463–466 (1998).
Marina, C. F. et al. Efficacy and non-target impact of spinosad, Bti and temephos larvicides for control of Anopheles spp. in an endemic malaria region of southern Mexico. Parasites Vectors 7, 55. https://doi.org/10.1186/1756-3305-7-55 (2014).
Boyce, R. et al. Bacillus thuringiensis israelensis (Bti) for the control of dengue vectors: systematic literature review. Trop. Med. Int. Health. 18(5), 564–577 (2013).
Marcombe, S. et al. Alternative insecticides for larval control of the dengue vector Aedes aegypti in Lao PDR: Insecticide resistance and semi-field trial study. Parasites & Vectors 11, 616. https://doi.org/10.1186/s13071-018-3187-8 (2018).
Belinato, T. A., Martins, A. J., Lima, J. B. P. & Valle, D. Effect of triflumuron, a chitin synthesis inhibitor, on Aedes aegypti, Aedes albopictus, and Culex quinquefasciatus under laboratory conditions. Parasite & Vectors 06, 83 (2013).
World Health Organization (WHO). Novaluron in drinking-water: use for vector control in drinking-water sources and containers. WHO/SDE/WSH/07.01/11. (2007).
Cutler, G. C., Scott-Dupree, C. D., Tolman, J. H. & Harris, C. R. Toxicity of the insect growth regulator novaluron to the non-target predatory bug Podisus maculiventris (Heteroptera: Pentatomidae). Biol. Control 38, 196–204 (2006).
Djeghader, N. E. H., Djeghader, N. E., Aïssaoui, L., Amira, & Boudjelida, H. Impact of a chitin synthesis inhibitor, novaluron, on the development and the reproductive performance of mosquito Culex pipiens. World Appl. Sci. 29, 954–960 (2014).
World Health Organization (WHO). Guidelines for Laboratory and Field Testing of Mosquito Larvicides. https://www.who.int/publications/i/item/WHO-CDS-WHOPES-GCDPP-2005.13 (2005).
World Health Organization. Evaluations. Part I, Part I. in Joint meeting of the FAO panel of experts on pesticide residues in food and environment. Food and Agriculture Organization of the United Nations, World Health Organization, Rome (2005).
Mulla, T., Su, M. S. & Zaim, M. Laboratory and field evaluations of novaluron, a new insect growth regulator (IGR), against Culex mosquitoes. J. Am. Mosq. Control Assoc. 19, 408–418 (2003).
Grosscurt, A. C. & Andersen, S. O. VII effects of diflubenzuron on some chemical and mechanical properties of the elytra of Leptinotarsa decemlineata. Proceedings of the Koninklijke Nederlandse Akademie Van Wetenschappen 83, 143–150 (1980).
Swale, D. R. et al. Development of an autodissemination strategy for the deployment of novel control agents targeting the common malaria mosquito, Anopheles quadrimaculatus say (Diptera: Culicidae). PLoS Negl. Trop. Dis. 12(4), e0006259 (2018).
Fiaz, M. et al. Behavioral and ultrastructural effects of novaluron on Aedes aegypti larvae. Infect. Genet. Evol. 93, 104974 (2021).
Dhadialla, T. S., Retnakaran, A. & Smagghe, G. Insect growth- and development-disrupting insecticides. In Insect Development Morphogenesis Molting and Metamorphosis (ed. Gilbert, L. I.) 679–740 (Academic Press, 2009).
Tunaz, H. & Nedim, U. Insect growth regulators for insect pest control. Turk. J. Agric. For. 28(6), 377 (2004).
Gunathilaka, N. et al. Field-based evaluation of novaluron EC10 insect growth regulator, a chitin synthesis inhibitor against dengue vector breeding in leaf axils of pineapple plantations in Gampaha District, Sri Lanka. Parasites Vectors 13, 228. https://doi.org/10.1186/s13071-020-04109-y (2020).
Withanage, G. P., Sameera, D., Viswakula, Y. S. G. & Gunawardene, M. D. H. Use of novaluron-based autocidal gravid ovitraps to control Aedes dengue vector mosquitoes in the District of Gampaha, Sri Lanka. Biomed. Res. Int. 29, 9567019 (2020).
Fontoura, N. G., Bellinato, D. F., Valle, D. & Lima, J. B. P. The efficacy of a chitin synthesis inhibitor against field populations of organophosphate-resistant Aedes aegypti in Brazil. Mem. Inst. Oswaldo Cruz 107, 387–395 (2012).
Arredondo-Jiménez, J. I. & Valdez-Delgado, K. M. J. I. Effect of Novaluron (Rimon 10 EC) on the mosquitoes Anopheles albimanus, Anopheles pseudopunctipennis, Aedes aegypti, Aedes albopictus, and Culex quinquefasciatus from Chiapas, Mexico. Med. Vet. Entomol. 20, 377–387 (2006).
Hwang, J. M., Bae, J. W., Jung, E. J., Lee, W. J. & Kwon, W. S. Novaluron has detrimental effects on sperm functions. Int. J. Environ. Res. Public Healt. 19(1), 61 (2021).
Santorum, M. et al. Bombyx mori (Lepidoptera: Bombycidae) and its impact on egg production. Environ. Pollut. 249, 82–90 (2019).
WHO Pesticide Evaluation Scheme (WHOPES). Pesticides and their application for the control of vectors and pests of public health importance. http://whqlibdoc.who.int/hq/2006/WHO_CDS_NTD_WHOPES_GCDPP_2006.1_eng.pdf (2006).
Mulla, M. S., Darwazeh, H. A. & Norland, R. L. Insect growth regulators: Evaluation procedures and activity against mosquitoes. Mosq. News. 10, 329–332 (1974).
Munasinghe, D. R., Amarasekera, P. J. & Fernando, C. F. An epidemic of dengue-like fever in Ceylon (chikungunya—a clinical and haematological study. Ceylon Med. J. 11(4), 129–142 (1966).
Ministry of Health, Sri Lanka. Annual Health Bulletin Sri Lanka (2000).
National Dengue Control Unit (NDCU). Sri Lanka. Guidelines for Aedes Vector Surveillance and Control.http://www.dengue.health.gov.lk/web/phocadownload/guidlines_for_aedes_vector_surveillance_and_control_in_sri_lanka_new.pdf. (2016).
Noordeen, F. Dengue control in Sri Lanka—Challenges and prospects for improving current strategies. Sri Lankan J. Infect. Dis. 6, 2–16 (2016).
Rao, D. R. et al. Development of a high level of resistance to Bacillus sphaericus in a field population of Culex quinquefasciatus from Kochi, India. J. Am. Mosq. Control Assoc. 11, 1–5 (1995).
Tissera, H. A. et al. Use of Bacillus thuringiensis israelensis in integrated vector control of Aedes sp. in Sri Lanka: A prospective controlled effectiveness study. Trop. Med. Int. Health. 23, 229–235. https://doi.org/10.1111/tmi.13015 (2018).
World Health Organization (WHO). WHO specifications and evaluations for public health pesticides.https://extranet.who.int/pqweb/sites/default/files/vcp-documents/WHOVC-SP_Novaluron_2004.pdf (2004).
Ngonzi, A. J. et al. Susceptibility status of major malaria vectors to novaluron, an insect growth regulator South-Eastern Tanzania. Pan Afr. Med. J. 41, 273 (2022).
Farnesi, L. C. et al. Physiological and morphological aspects of Aedes aegypti developing larvae: Effects of the chitin synthesis inhibitor novaluron. PLoS ONE 7(1), e30363 (2012).
Acknowledgements
Assistance given by the staff of the Entomological Surveillance Unit, Office of Regional Director of Health Services, Kurunegala and the Department of Zoology, University of Peradeniya, Sri Lanka is acknowledged.
Funding
This work was supported by the Office of Regional Director of Health Services, Kurunegala and the University of Peradeniya, Sri Lanka.
Author information
Authors and Affiliations
Contributions
S.H.P.P.K., W.A.P.P.S. and T.C.W. designed the research work. J.M.M.K.H. carried out experiments and collected data and wrote the first draft of the manuscript. All authors analysed data and reviewed the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Herath, J.M.M.K., De Silva, W.A.P.P., Weeraratne, T.C. et al. Efficacy of the insect growth regulator novaluron in the control of dengue vector mosquitoes Aedes aegypti and Ae. albopictus. Sci Rep 14, 1988 (2024). https://doi.org/10.1038/s41598-024-52384-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-52384-x
- Springer Nature Limited