Abstract
As the risk of gypsy moth outbreaks that have detrimental effects on forest ecosystem in the Northern Hemisphere increase due to climate change, a quantitative evaluation of the impact of gypsy moth defoliation is needed to support the adaptive forest management. To evaluate the host-specific impact of gypsy moth defoliation, radial growth and annual carbon accumulation were compared for one severely defoliated (Larix kaempferi (Lamb.) Carrière) and one moderate defoliated (Quercus acutissima Carruth.) host, in defoliated and non-defoliated site using tree-ring analysis. Finally, the resilience indices of radial growth variables were calculated to assess the ability of sampled trees to withstand defoliation. Gypsy moth defoliation mainly decreased latewood width and caused reduction in annual carbon absorption more than 40% for both tree species. However, L. kaempferi, showed the reduced growth until the year following defoliation, while Q. acutissima, showed no lagged growth depression and rapid growth recover. The findings show how each species reacts differently to gypsy moth defoliation and highlight the need of managing forests in a way that takes resilient tree species into account.
Similar content being viewed by others
Introduction
Outbreaks of insect pests are a natural disturbance, which may cause economic and ecological impacts by changing the structure and function of forest ecosystems1. Massive outbreaks of insect pests that affect the growth of host trees may increase tree mortality, causing a reduction of forest carbon storage2,3. For example, extensive outbreak of mountain pine beetle (Dendroctonus ponderosae Hopkins) in coniferous forests in British Columbia, Canada, changed the forests from carbon sinks to sources4. Under the influence of climate change, it is expected that large insect outbreaks will become more frequent and severe in the future, and the resulting loss of forest function is likely to also increase5. Detecting and evaluating the damage caused by insect outbreaks are essential for managing and mitigating their impact on forest functioning in a changing environment.
The gypsy moth (Lymantria dispar L.), which is native to Eurasia, is one of the most widespread and polyphagous defoliators that have detrimental effects on forest ecosystem6. Among its subspecies, European gypsy moth (EGM) has been a concern for over a century due to its rapid spread in North America7. Trees in genera Quercus, Populus, and Salix are known to be the most preferred hosts for this subspecies, and the eco-physiological impacts of EGM defoliation have been extensively investigated8,9. Asian gypsy moth (AGM) in Far East Asia also has a broad host spectrum, including conifer species such as Larix spp. and various deciduous species in the genera Quercus, Fagus and Betula10,11,12. However, the impact of AGM defoliation has received limited attention than EGM, despite its capacity to cause damage to numerous hosts. Particularly in Korea, gypsy moth is regarded as a sporadic pest, likely due to the regulation of populations by natural enemies13. Nonetheless, there have been periodic outbreaks in Japan and Russia10,14, with intensive defoliation having a negative impact on the growth and reproduction of multiple hosts11,15.
Gypsy moth defoliation causes multi-level effects, from impacts within tree tissues (xylogenesis) to changes in ecosystem functioning8. The larvae hatch in spring and actively feed on foliage during early- and mid-summer, which results in insufficient carbohydrate accumulation for xylem formation16. Many studies have reported that damage by spring defoliators cause trees to produce abnormal cells in the latewood that have small lumens and thin cell walls. This effect leads to growth rings with narrow latewood areas, the so-called light ring, which typical of wood formed after spring defoliation17,18. Defoliation also reduces the number of cells in tree-rings, leading to radial growth reduction during or after the defoliation19,20. This decrease of radial growth results in an overall decline in forest productivity, and eventually can affect the forest’s carbon dynamics21,22.
The impact of defoliation is host species-dependent and seems to be related to host preference of gypsy moth9,23. For EGM, the radial growth loss in preferred species such as Quercus rubra L. and Populus grandidentata Michx. was higher than that in the non-preferred species20. Some other species such as Fraxinus americana L. and Pinus strobus L. were rarely defoliated and thus suffered little from outbreaks9,20,24. However, this correlation between proportional reduction of radial growth and host preference has not always been observed. For example, Populus tremuloides Michx., which is another highly preferred host for EGM, showed no or little change in radial growth after defoliation20. Similarly, EGM caused severe damage to a resistant species, Pinus radiata D. Don, in northwestern Spain, with a radial growth reduction of up to 74%25. These findings emphasized the need for study on the host-specific growth response to AGM to better understand the impact of gypsy moth defoliation.
Despite being native to the Korean Peninsula, the gypsy moth (AGM) outbreaks were rarely reported26,27. However, a recent nationwide gypsy moth outbreak occurred in Korea that severely damaged about 4300 ha of forest, raising concerns over the effects of defoliation on forest ecosystems28. While their feeding preferences are not well-known, a recent study identified 46 species of coniferous and broad-leaved species suffering from severe to low canopy feeding damage28. Such defoliation of the gypsy moth could alter forest structure and composition in Korea and affect forest functions by damaging multiple hosts.
Our goal was to examine the impacts of gypsy moth defoliation on radial growth and carbon accumulation of major host species of gypsy moth in central region of Korea. The detailed objectives were (1) to evaluate the extent and duration of damage caused by gypsy moth defoliation and (2) to assess the ability to resist and recover from gypsy moth defoliation depending on host species. To do this, we studied two host species—Larix kaempferi and Quercus acutissima—in both defoliated and non-defoliated sites. Trees in the non-defoliated site served as a control against the potentially confounding effect of climate25. In central Korea, L. kaempferi is the one of the major plantation species and was the severely defoliated host in the recent outbreak of AGM29, while Q. acutissima, a moderate defoliated host, is a major component of secondary forests in urban forest areas and has the highest carbon absorption capacity among Quercus species in central Korea28,30.
Results
Changes in radial growth variables
The annual mean tree-ring width (TW) values before the gypsy moth outbreak and defoliation event of 2010–2019 were 1.34 ± 0.65 mm for L. kaempferi and 1.92 ± 0.80 mm for Q. acutissima at the defoliated site and 1.53 ± 0.64 mm and 2.64 ± 0.69 mm at the non-defoliated site (Table 1). Without the age-trend correction, L. kaempferi trees at the two sites had similar annual radial growth during the pre-event period. For Q. acutissima, latewood width (LW) and TW values were higher in the non-defoliated than the defoliated site, while BAI values showed no difference between two sites. In contrast to the pre-event period, the radial growth of L. kaempferi trees was much lower at the defoliated site than for trees at the non-defoliated site during the gypsy moth defoliation event and post-event period. However, Q. acutissima trees showed lower radial growth at the defoliated site during the year of gypsy moth defoliation, but not in subsequent years in the post-event period.
When considering the detrended ring width index, the results showed a clear pattern dependent on the tree species and the radial growth variables (Fig. 1). During the pre-event period, L. kaempferi trees at the defoliated site had significantly larger EW, LW and TW index values than at the non-defoliated site (p < 0.05), whereas for Q. acutissima, there were no differences in the ring width index values between two sites. However, when the gypsy moth defoliation occurred, for both species there were differences between the two sites for all ring width indexes except EW, with lower values of both trees at the defoliated site (p < 0.05). In years after the defoliation, L. kaempferi trees at the defoliated site continued to show lower values for the ring width index. In contrast, in the post-event period, Q. acutissima trees at the defoliated site showed no difference in radial growth from trees at the non-defoliated site. Significant differences between the two sites in basal area increment were observed in both the defoliation year and the post-event period for L. kaempferi trees, and for Q. acutissima, growth was reduced at the defoliated site only during the year of defoliation.
Ring width indices and basal area increments of Larix kaempferi and Quercus acutissima in the pre-defoliation, during defoliation, and post gypsy moth defoliation event of 2020. Error bars indicate standard deviations. Asterisks above the bars indicate significant differences between sites when tested by Mann–Whitney U-test (p < 0.05). Abbreviations: EW, earlywood width; LW, latewood width; TW, tree-ring width; BAI, basal area increment.
Resistance, recovery, and resilience indices of radial growth variables
The resistance, recovery, and resilience indices, calculated from four radial growth variables, showed significant differences depending on the presence or absence of gypsy moth defoliation and on the tree species (Fig. 2). In both species, the resistance index based on BAI was consistently higher in the non-defoliated site (p < 0.05). High resistance index values for the non-defoliated site were also found for the EW, LW, and TW variables. However, there were no significant differences between species for any radial growth variables within the same site.
Resistance, recovery, and resilience indices for ring widths and basal area increments of Larix kaempferi and Quercus acutissima at sites that were either defoliated or not defoliated during the gypsy moth defoliation event of 2020. Different lowercase letters indicated the significant differences at the 0.05 level tested by two-way analysis of variance or Kruskal–Wallis test. Abbreviations: EW, earlywood width; LW, latewood width; TW, tree-ring width; BAI, basal area increment.
Recovery index values showed various patterns according to the tree species. L. kaempferi trees at the defoliated site had lower recovery index values of radial growth than that at non-defoliated site, except for the LW (p < 0.05). In contrast to L. kaempferi, for Q. acutissima the recovery indices for BAI, TW, and LW was higher at the defoliated site than at the non-defoliated site. The differences in recovery and resilience indices for the defoliation event were found to be larger for L. kaempferi than for Q. acutissima. Defoliated L. kaempferi trees had the lowest resilience index values, while for Q. acutissima the resilience index showed no difference between defoliated and non-defoliated trees.
Individual-level carbon accumulation
As shown in the results of the pairwise comparisons, before the gypsy moth outbreak of 2020, there were no significant differences in the annual carbon accumulation between the defoliated and non-defoliated sites for either tree species except in the year 2012 for L. kaempferi (Fig. 3). In the pre-event period of 2010–2019, regardless of gypsy moth defoliation, Q. acutissima exhibited higher carbon accumulation than L. kaempferi. During this period, the average carbon accumulation absorbed annually by an individual tree was 5.8 ± 3.2 kg year−1 in L. kaempferi and 12.9 ± 5.3 kg year−1 in Q. acutissima. However, annual carbon accumulation for both species was significantly reduced at the defoliated site in the year of the gypsy moth outbreak (p < 0.001). In 2020, L. kaempferi and Q. acutissima trees at the defoliated site absorbed 4.0 ± 2.7 kg year−1 and 7.5 ± 3.9 kg year−1, respectively, which was about 42.5% and 46.1% less carbon than the trees at non-defoliated site (which were 6.9 ± 3.9 kg year−1 for L. kaempferi and 13.9 ± 5.2 kg year−1 for Q. acutissima). A reduction in carbon accumulation was also found the next year for L. kaempferi and when defoliated trees accumulated just 59.5% of the amount of carbon accumulated by non-defoliated trees.
Annual carbon accumulation (kg) of Larix kaempferi and Quercus acutissima as affected in 2020 by gypsy moth defoliation. Points and error bars represent means and standard errors of individual-level annual carbon accumulation. Asterisks indicate significant differences (p < 0.05) as determined by repeated measure two-way ANOVA with Bonferroni correction.
Discussion
This study detected detrimental impacts of gypsy moth defoliation on both L. kaempferi and Q. acutissima, which are major species in central region of Korea. Impacts were reduction of radial growth and decline in carbon accumulation during and after the outbreak year. For the intense defoliated host, L. kaempferi, defoliation caused prolonged suppression of radial growth with low resilience, while the moderated defoliated host, Q. acutissima, showed rapid recovery of radial growth after gypsy moth defoliation. This host-specific growth response was also associated with the ability to accumulate carbon. The reduction of carbon accumulation in defoliated trees was greater and lasted longer in L. kaempferi, and this impact will affect the ecological functioning of forests in central Korea during and after gypsy moth outbreaks.
In this study, L. kaempferi showed a prolonged depression of all radial growth variables until the year following gypsy moth defoliation, while Q. acutissima showed no growth depression of radial growth (Table 1). Some Quercus species are known to suffer growth declines for 2–4 years after gypsy moth defoliation31, however, no such lagged effects were observed in Q. acutissima. Damage caused by defoliation can vary depending on the characteristic of defoliation, especially its duration and severity19. In particular, the more preferred host species tend to be more severely damaged during episodes of weak or moderate defoliation32. In North America, Quercus species are preferred by EGM over other host species, making them susceptible to defoliation, and they also suffer from severe damage to growth after defoliation33,34. Red oak, Q. rubra (the most preferred host of EGM) also experiences the largest and longest depression of radial growth, followed by lesser effects for the intermediate and non-preferred hosts9. While feeding on non-preferred species may increase when favored resources are scarce due to the heavy or successive defoliation32. For example, Abies balsamea (L.) Mill., which is known to be a poor host for EGM in North America, actually suffered more damage than susceptible tree species during episodes of heavy forest defoliation in New Brunswick, Canada, losing an average of 55% of the wood volume23. The recent gypsy moth outbreak in Korea, which was a one-year event and was not intensive enough to cause tree mortality, is likely to have resulted in concentrated defoliation on preferred hosts rather than less preferred hosts. These less severe defoliation conditions may explain some of the observed differences in damage on the radial growth between L. kaempferi and Q. acutissima. However, in a laboratory feeding trials, Asian gypsy moth population showed the higher larval survival and performance in Quercus velutina Lam. than Larix occidentalis Nutt.35. Additional research on feeding preferences is necessary to clearly elucidate the relationship between host preference and the damage on the radial growth.
Another reason for differential growth response could be the biotic and abiotic factors related to the gypsy moth population density and the susceptibility of host species. The intensity of defoliation can be directly related to the density of the gypsy moth population36. In this study, both species were subjected to similar environmental conditions, suggesting that the impact of abiotic factors, such as climate and soil, on controlling the gypsy moth population was considered minimal. On the other hand, biological factors, particularly predation by small mammals, are likely to have played a significant role in regulating the gypsy moth population based on forest type37. The findings which found higher predation intensity and density of small mammals in oak forests compared to L. kaempferi forests, may explain why defoliation severity was higher in L. kaempferi stands than Q. acutissima in this study10. Meanwhile, from the perspective of the susceptibility of host species, the mean sensitivity of tree-ring series was lower for Q. acutissima than for L. kaempferi (Table 2), which means that the current habitat has fewer factors limiting the radial growth of Q. acutissima trees, resulting in less annual variation in tree-ring width. Given the more constraints imposed by environmental factors on radial growth of L. kaempferi, its defensive capacity is expected to be limited38. Consequently, it is more likely to be susceptible to gypsy moth outbreaks, resulting in substantial radial growth loss when compared to Q. acutissima.
During the gypsy moth defoliation in Korea, the biggest change in radial growth was founded in the latewood width (LW) for both species (Fig. 1), as seen in previous studies20,39,40. A plausible reason for this is the temporal overlap of the latewood formation with the gypsy moth larval feeding. Gypsy moth eggs hatch in central Korea in mid-April, and larvae vigorously feed on the leaves from June to July, causing a shortage of current photosynthate as a result of defoliation27. In the study region, latewood formation starts in late-May for Quercus species and in mid-June for L. kaempferi41,42. Extensive herbivory in these time periods may limit the formation of latewood by reducing the current photosynthate, which is a more important source for latewood formation than for earlywood43. Moreover, the secondary leaf flush that was observed in August after defoliation might be another reason for the reduction of LW because leaf replacement would consume current photosynthate for both species.
In contrast, we observed a relatively small impact of gypsy moth defoliation on EW, which may occur because earlywood cells are formed before the period of gypsy moth feeding. In general, many temperate deciduous species begin cambium reactivation (leading to early wood formation) in spring before the flushing of new leaves44. For example, ring-porous Quercus species begin the formation of earlywood vessels as early as mid-March using the photosynthate from the previous summer and autumn45,46. Similarly, in central Korea the deciduous conifer, L. kaempferi initiates cambium activity in mid-April and begins to form earlywood tracheids42. Since in most deciduous trees, earlywood formation is a product of both previous and current photosynthate43,47, the presence of pre-formed earlywood (synthesized with previous year photosynthate reserves) appears to offset the impact on earlywood of defoliation by using stored photosynthate to compensate for the shortage of current photosynthate, in both study species.
Except for earlywood width (EW), defoliated trees of both species were less resistant to gypsy moth defoliation than non-defoliated trees, showing growth reduction during the year of defoliation for all radial growth variables (resistance < 1). In general, stressed trees are likely to be damaged first48. Stressful conditions such as soil compaction or pollutants at the defoliated site (which was surrounded by an urbanized area), seem to have affected the stand vigor, making trees more susceptible to defoliation49. For four radial growth variables measured, the latewood of defoliated trees showed narrower (lower width), lower resilience, lower resistance, but higher recovery following gypsy moth defoliation (Fig. 2). These findings suggest that latewood characteristics are the most informative measures for detecting impacts on the radial growth response of trees to gypsy moth defoliation and for reconstructing past outbreaks40,50.
Defoliated Q. acutissima trees showed a rate of high recovery for all growth variables in the year following defoliation (Fig. 2). This may be in part explained by the compensatory growth habit20. Quercus species exhibit compensatory growth after damage from herbivory and can return to normal growth rates immediately after defoliation51,52. Defoliated Q. serrata Murray and Q. crispula Vuk. reduce their investment in shoots while increasing the number of leaves during subsequence flushes53. Moreover, the number of flushes increases after a defoliation event53. The rapid recovery in Q. acutissima may be the result of the same compensatory strategy against herbivory as seen in the above Quercus species. This is likely because Q. acutissima exhibits an indeterminate growth habit, with secondary leaf flushing occurring after defoliation54. Although L. kaempferi also has compensatory responses, such as increasing the rate of photosynthesis by the remaining leaves55, rapid growth recovery after defoliation was not observed in this study for this species. Whether this was influenced by the severity of the defoliation requires further research.
Species such as ring-porous Quercus and the deciduous conifers of the genus Larix are known to have lower declines in growth and lower mortality rates following defoliation than do the diffuse-porous trees or evergreen conifers56. Therefore, to comprehensively assess the impacts of gypsy moth defoliation, it would be necessary to investigate impacts on a broader range of hosts. Also, climate change will affect the host preferences of gypsy moth by altering the tree host’s physiological traits57. Investigating the host-specific growth responses of more host species should help clarify the relationship between host preference and damage following gypsy moth defoliation.
In the defoliation year, a significant decrease in the carbon accumulation was observed in defoliated trees for both species (Fig. 3). Defoliation not only reduces total carbon assimilation but also changes the allocation priority within the carbon pool58. Following defoliation, trees shift carbon allocation from vegetative growth to storage as non-structural carbohydrates (NSC) causing woody biomass accumulation significantly decrease under conditions of insufficient carbon uptake58,59. This pattern of reallocation explains the subsequent decline of radial growth in defoliated L. kaempferi. In Larix gmelinii (Rupr.) Rupr., 43% of carbon in starch pool for wood formation of the current year came from old C storage60. So, declines in carbon assimilation in a defoliation year may result in a reduction of the radial growth in the following year.
Furthermore, the severity of defoliation determines the levels of carbon accumulation and mortality. As the severity of defoliation increases, carbon allocation to wood gradually decreases61. Moreover, reductions in total NSC due to severe defoliation leads to carbon depletion and increased risk of tree mortality62. To survive under carbon-limiting conditions, trees have to maintain a certain amount of NSC. If the NSC storage falls below a certain threshold, the risk of mortality is likely to increase62. In this study, the cumulative reduction in carbon assimilation was greater in the severely defoliated host L. kaempferi. This species is more likely to suffer carbon starvation and increased mortality than moderated defoliated host Q. acutissima. Therefore careful attention needs to be paid to the impact of such defoliation on carbon storage and the consequences for sustaining forest functions. For adaptive forest management, improving the individual tree’s vigor and mixing stands with resilient species should minimize the impacts of defoliation63,64. Silvicultural treatment such as thinning may also reduce forest susceptibility to defoliation-induced mortality and promote rapid recovery after defoliation events65.
Conclusions
Future bouts of gypsy moth defoliation will likely affect forest function by reducing radial growth and carbon assimilation, with the extent of the impact varying based on the defoliation severity depending on host species. This suggests that adaptive forest management should take into consideration the host-specific growth responses of tree species in order to maximize overall forest resilience to gypsy moth defoliation. Future studies need to examine more hosts species and clarify the effect of host preference on the impacts of defoliation by gypsy moth.
Materials and methods
Study site
This study was conducted in Wonju city (37° 20ʹ N, 127° 55ʹ E) in the central part of Korean peninsula (Fig. 4). The region has a temperate climate with dry winters and humid summers. Data obtained from the nearest weather station showed a mean annual temperature for 1992–2021 of 12.0 ℃, ranging from 3.1 ℃ in January and 25.4 ℃ in August66. Mean annual precipitation over the same period was 1293 mm, and 60% of the total precipitation fell between June and August in the summer season. Following unusually high winter temperatures and spring drought in 2020, gypsy moth populations dramatically increased in the area, causing serious damage to crown of multiple hosts during the mid-summer28. Among the defoliated hosts, L. kaempferi experienced the most significant damage from this gypsy moth outbreak compared to other host species. The gypsy moth damaged 22% of all the L. kaempferi plantations in the study area, and 274 ha out of total 5306 ha of larch plantations were severely defoliated29. The large gypsy moth outbreak in 2020 was thought to be a unique event, from only a single year. The gypsy moth nuclear polyhedrosis virus (NPV) and its parasitoids increased during the outbreak, and by the following year (2021) the population density of gypsy moth collapsed13,67.
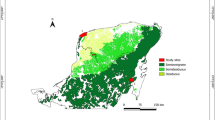
Location of study sites. The area with gypsy moth outbreaks (yellow) is modified from Choi et al.29. The blue circle indicates the defoliated site where gypsy moth (GM) was in outbreak in the summer of 2020. The red square indicates the non-defoliated site in this study. The black cross-circle indicates the location of the nearest weather station, from which all climatic data were obtained. Map was created using ArcGIS Desktop 10.2 (https://www.esri.com/en-us/arcgis/products/arcgis-pro/overview).
In November of 2021, we chose two forested areas with L. kaempferi plantations and adjacent stands of Q. acutissima, one area that was defoliated in 2020, while the other site (the control) was chosen from a similar area that had not been previously defoliated29. Both sites were located on gentle slopes of 15°–25°, with the defoliated site facing southwest (262 m above the sea level) and the non-defoliated site facing northeast (181 m above the sea level). The larch plantations and Q. acutissima stands were 31–40 years old and they were located within 300 m of each other within the same site. Before the sampling, the designation as defoliated or non-defoliated (in the previous year) was confirmed by a visual check of gypsy moth egg mass numbers at the two sites. The site defoliated in 2020 was located in a small forest stand near an urbanized area, and traces remained on tree trunks of large numbers of egg masses. No sign, however, was seen of increased tree mortality in 2021 (the year of our observations). In contrast, the non-defoliated site, which was located in a forested area about 7.5 km northwest of the defoliated site, had no-signs of large numbers of egg masses.
Tree coring and measurement of radial growth
For core sampling, 10–17 dominant or co-dominant trees in each site were selected of each of two species (L. kaempferi and Q. acutissima), and their diameter at breast height (DBH, 1.3 m above the ground) was measured. One core per each tree was extracted at this height using a 5.15 mm increment borer (Haglöf Sweden AB, Långsele, Sweden) while avoiding taking cores on the side of slope. Sample cores were stored in plastic straws and taken to the laboratory for analysis. Following standard dendrochronological methods, the cores were air-dried and glued onto wooden mounts. Then, using sandpaper with up to 400 grit, cores were sanded until the surface was clearly visible68. Under the stereomicroscope (Stemi 305, Carl Zeiss, Oberkochen, Germany), cross-dating between cores was conducted using the list method69. Cross-dated cores were scanned at 2400 dpi with an EPSON V370 scanner (Epson Corp., Nagano, Japan) and the earlywood width (EW), latewood width (LW) and tree-ring width (TW) were measured to 0.001 mm precision with a WinDENDRO 2016a (Regent Instruments, Quebec, Canada). The quality of cross-dating was evaluated using COFECHA software70.
For calculating basal area increments (BAI), which is an accurate measure of production, annual diameters were reconstructed using the current measured DBH and annual tree-ring widths, and then converted to BAI using the following formula.
where BAIt is annual basal area increment (cm2) of corresponding year t and defined as the difference of basal area at two consecutive points in year (t and t − 1). DBH (cm) is the reconstructed diameter at breast height under the assumption of circular growth in each year.
Chronology building
Chronologies were built from three ring width series (and consequent basal area increment) for each tree species and study site using dplR package in R program71,72. To remove any age or size-related trends, the ring width series were standardized with negative exponential curves or spline curves of 50% frequency response at a wavelength of 2/373. Each detrended ring width series and basal area increment was averaged with a bi-weight robust mean and upscaled to the site-level chronologies. Then, all chronologies were truncated at the year 1990 where the subsample signal strength (SSS) reaches the threshold value 0.8574 and the number of samples was greater than five (Fig. 5). Basic statistics, including inter-series correlation, mean sensitivity, Rbar, and expressed population signal (EPS), were calculated75 for the common period of 1990–2021 (Table 2).
Estimation of carbon accumulation from above-ground biomass
Annual carbon accumulation was estimated from the above-ground biomass (AGB) assuming a constant carbon concentration in all tissues. To estimate the individual-level AGB from sampled trees, simplified allometric equations were employed for each species76. Based on the estimated annual AGB, annual carbon accumulation was calculated by multiplying species-specific carbon conversion factor as followings.
where AGBt is the above-ground biomass of an individual tree (g) and DBHt is the reconstructed annual diameter (cm) of year t. The two parameters a and b are species-specific constants obtained from previous study76, namely 44.49 and 2.70 for the L. kaempferi (R2 = 0.96) and 143.38 and 2.49 for Q. acutissima (R2 = 0.94), respectively. AGBIt is annual AGB increment, calculated as the difference of AGB between two consecutive years (t and t − 1). A parameter cf is carbon conversion factor of 0.50 for L. kaempferi and 0.48 for Q. acutissima76.
Resilience indices
To evaluate the capacity of the radial growth to withstand the gypsy moth defoliation, three growth-based resilience indices were calculated77. Resistance is defined as the ability to retain the growth through the disturbance event, and it is calculated as the ratio of the growth during the gypsy moth defoliation to pre-event growth in this study. Recovery indicates how much a tree recovers the growth lost from damage after the disturbance ends. To calculate the recovery index, the post-event growth was divided by the growth during the defoliation. Resilience is defined as the ability to sustain the growth rate present that existed before to a disturbance once the disturbance has passed and was calculated as the ratio of post-event growth to pre-event growth using the following formulae77.
where G is the radial growth during the gypsy moth defoliation of 2020. Pre-G indicates the average radial growth of the 3 years preceding the defoliation year. Due to lack of growth in the year after the defoliation event, Post-G is defined only as the radial growth of the first year following the defoliation year in this study. All resistance, recovery, and resilience indices were calculated at the individual tree level using each ring width series and basal area increments, which were then averaged to obtain site level values for comparison.
Statistical analysis
Differences in radial growth between defoliated and non-defoliated trees were investigated in three time periods—pre-event, during the event, and post-event—using the Mann–Whitney U-test. Radial growth in the pre-event period was characterized using three measures, the annual mean of the raw ring widths, the ring width index, and the basal area increment from 2010 to 2019. The raw ring width, ring width index and basal area increment of the defoliation year in 2020 and that of one year after the event in 2021 were used as measures of the radial growth during the defoliation period and post-event period, respectively.
Two-way analysis of variance (ANOVA) and post-hoc Tukey's test were applied to test effects of species and defoliation event (site) on resilience indices for each radial growth variables. When normality or equal-variance assumptions were not satisfied, the non-parametric Kruskal–Wallis test was used. Multiple comparisons were made using Fisher's least significant difference (LSD) test with the Bonferroni correction.
The differences in annual carbon accumulation from the defoliation event (site) were assessed by use of repeated measures two-way ANOVA with Bonferroni correction for each species. For the period of 2010–2021, the analysis was performed using year as within-subjects variable and defoliation event (site) as between-subjects variable. To meet the assumption of normality, the outcome variable (carbon accumulation) was log-transformed before the analysis and examined for normality using Shapiro–Wilk test. The homogeneity of variance across between-subjects was tested by Levene’s test and the equality of variances of the differences between within-subjects was checked with Mauchly’s test of sphericity. All statistical analysis was conducted using R program72.
Data availability
The data from the current study are available from the corresponding author upon reasonable request.
References
Bradshaw, C. J. A. et al. Massive yet grossly underestimated global costs of invasive insects. Nat. Commun. 7, 12986. https://doi.org/10.1038/ncomms12986 (2016).
Hicke, J. A. et al. Effects of biotic disturbances on forest carbon cycling in the United States and Canada. Glob. Change Biol. 18, 7–34 (2012).
Seidl, R. et al. Invasive alien pests threaten the carbon stored in Europe’s forests. Nat. Commun. 9, 1626 (2018).
Kurz, W. A. et al. Mountain pine beetle and forest carbon feedback to climate change. Nature 452, 987–990 (2008).
Couture, J. J., Meehan, T. D., Kruger, E. L. & Lindroth, R. L. Insect herbivory alters impact of atmospheric change on northern temperate forests. Nat. Plants 1, 15016. https://doi.org/10.1038/nplants.2015.16 (2015).
Elkinton, J. S. & Liebhold, A. M. Population dynamics of gypsy moth in North America. Annu. Rev. Entomol. 35, 571–596 (1990).
Weseloh, R. M. People and the gypsy moth: A story of human interactions with an invasive species. Am. Entomol. 49, 180–190 (2003).
Twery, M. J. Effects of defoliation by gypsy moth. in Proceedings of USDA Interagency Gypsy Moth Research Review 1990 (Eds. Gottschalk, K. W., Twery, M. J. & Smith, S. I.) 27–39 (U.S. Department of Agriculture, Forest Service, 1991).
Naidoo, R. & Lechowicz, M. J. Effects of gypsy moth on radial growth of deciduous trees. For. Sci. 47, 338–348 (2001).
Liebhold, A. M., Higashiura, Y. & Unno, A. Forest type affects predation on gypsy moth (Lepidoptera: Lymantriidae) pupae in Japan. Environ. Entomol. 27, 858–862 (1998).
Nakajima, H. Refoliation of deciduous canopy trees following severe insect defoliation: Comparison of Fagus crenata and Quercus crispula. Plant Ecol. 219, 665–675 (2018).
Ohno, Y. et al. Variation in shoot mortality within crowns of severely defoliated Betula maximowicziana trees in Hokkaido, northern Japan. Ecol. Res. 23, 355–362 (2008).
Lee, J.-H. & Pemberton, R. W. Parasitoid complex of the gypsy moth (Lymantria dispar) in the increase-phase populations in Korea. J. Ecol. Environ. 32, 75–81 (2009).
Gninenko, Y. I. & Orlinskii, A. D. Outbreaks of Lymantria dispar in Russian forests during the 1990s. EPPO Bulletin 33, 325–329 (2003).
Nakajima, H. Defoliation by gypsy moths negatively affects the production of acorns by two Japanese oak species. Trees 29, 1559–1566 (2015).
Fierravanti, A., Rossi, S., Kneeshaw, D., De Grandpré, L. & Deslauriers, A. Low non-structural carbon accumulation in spring reduces growth and increases mortality in conifers defoliated by spruce budworm. Front. For. Glob. Change 2, 15. https://doi.org/10.3389/ffgc.2019.00015 (2019).
Watanabe, Y. & Ohno, Y. Severe insect defoliation at different timing affects cell wall formation of tracheids in secondary xylem of Larix kaempferi. Trees 34, 931–941 (2020).
Liang, C., Filion, L. & Cournoyer, L. Wood structure of biotically and climatically induced light rings in eastern larch (Larix laricina). Can. J. For. Res. 27, 1538–1547 (1997).
Baker, W. L. Effect of gypsy moth defoliation on certain forest trees. J. For. 39, 1017–1022 (1941).
Muzika, R. M. & Liebhold, A. M. Changes in radial increment of host and nonhost tree species with gypsy moth defoliation. Can. J. For. Res. 29, 1365–1373 (1999).
Medvigy, D., Clark, K. L., Skowronski, N. S. & Schäfer, K. V. R. Simulated impacts of insect defoliation on forest carbon dynamics. Environ. Res. Lett. 7, 045703 (2012).
Kretchun, A. M. et al. Predicted effects of gypsy moth defoliation and climate change on forest carbon dynamics in the New Jersey Pine Barrens. PLoS ONE 9, e102531. https://doi.org/10.1371/journal.pone.0102531 (2014).
Hennigar, C. R., Maclean, D. A. & Norfolk, C. J. Effects of gypsy moth defoliation on softwood and hardwood growth and mortality in New Brunswick, Canada. North. J. Appl. For. 24, 138–145 (2007).
Mauffette, Y., Lechowicz, M. J. & Jobin, L. Host preferences of gypsy moth, Lymantria dispar (L.), in southern Quebec. Can. J. For. Res. 13, 53–60 (1983).
Camarero, J. J., Álvarez-Taboada, F., Hevia, A. & Castedo-Dorado, F. Radial growth and wood density reflect the impacts and susceptibility to defoliation by gypsy moth and climate in Radiata pine. Front. Plant Sci. 9, 1582. https://doi.org/10.3389/fpls.2018.01582 (2018).
Pemberton, R. W., Lee, J. H., Reed, D. K., Carlson, R. W. & Han, H. Y. Natural enemies of the Asian gypsy moth (Lepidoptera: Lymantridae) in South Korea. Ann. Entomol. Soc. Am. 86, 423–440 (1993).
National Institute of Forest Science. Annual report of monitoring for forest insect pests and diseases in Korea (Korea Fores Service, National Institute of Forest Science, 2013).
Jung, J.-K. et al. Tree-crown defoliation caused by outbreak of forest insect pests in Korea during 2020. Korean J. Appl. Entomol. 59, 409–410 (2020).
Choi, W. I., Kim, E.-S., Yun, S.-J., Lim, J.-H. & Kim, Y.-E. Quantification of one-year gypsy moth defoliation extent in Wonju, Korea, using landsat satellite images. Forests 12, 545. https://doi.org/10.3390/f12050545 (2021).
National Institute of Forest Science. Standard carbon uptake of major forest species (ver. 1.2). (Korea Fores Service, National Institute of Forest Science, 2019).
Fajvan, M. A., Rentch, J. & Gottschalk, K. The effects of thinning and gypsy moth defoliation on wood volume growth in oaks. Trees 22, 257–268 (2008).
Campbell, R. W. & Sloan, R. J. Forest stand responses to defoliation by the gypsy moth. For. Sci. 23, a0001-z0001 (1977).
Gansner, D. A. & Herrick, O. W. Host preferences of gypsy moth on a new frontier of infestation. Research Note NE-330 (U.S. Department of Agriculture, Forest Service, Northeastern Forest Experiment Station, 1985).
Jedicka, J., Vandermeer, J., Aviles-Vazquez, K., Barros, O. & Perfecto, I. Gypsy moth defoliation of oak trees and a positive response of red maple and black cherry: An example of indirect interaction. Am. Midl. Nat. 152, 231–236 (2004).
Keena, M. A. & Richards, J. Y. Comparison of survival and development of gypsy moth Lymantria dispar L. (Lepidoptera: Erebidae) populations from different geographic areas on north American conifers. Insects. 11, 260 (2020).
Williams, D. W. et al. Oak defoliation and population density relationships for the gypsy moth (Lepidoptera: Lymantriidae. J. Econ. Entomol. 84, 1508–1514 (1991).
Jones, C. G., Ostfeld, R. S., Richard, M. P., Schauber, E. M. & Wolff, J. O. Chain reactions linking acorns to gypsy moth outbreaks and Lyme disease risk. Science 279, 1023–1026 (1998).
Herms, D. A. & Mattson, W. J. The dilemma of plants: To grow or defend. Q. Rev. Biol. 67, 283–335 (1992).
Dulamsuren, C., Hauck, M., Leuschner, H. H. & Leuschner, C. Gypsy moth-induced growth decline of Larix sibirica in a forest-steppe ecotone. Dendrochronologia 28, 207–213 (2010).
Asshoff, R., Schweingruber, F. H. & Wermelinger, B. Influence of a gypsy moth (Lymantria dispar L.) outbreak on radial growth and wood-anatomy of Spanish chestnut (Castanea sativa Mill.) in Ticino (Switzerland). Dendrochronologia 16–17, 133–145 (1999).
Kwon, S. M. & Kim, N. H. Annual ring formation of major wood species growing in Chuncheon, Korea (II)—Formation of resin canals, tyloses and latewood. J. Korean Wood Sci. Technol. 33, 1–7 (2005).
Yoo, H.-J. et al. Estimation of the optimal periods for planting and felling Larix kaempferi based on the period of its cambial activity. J. Korean Wood Sci. Technol. 49, 399–415 (2021).
Kagawa, A., Sugimoto, A. & Maximov, T. C. 13CO2 pulse-labelling of photoassimilates reveals carbon allocation within and between tree rings. Plant Cell Environ. 29, 1571–1584 (2006).
Marchand, L. J. et al. Timing of spring xylogenesis in temperate deciduous tree species relates to tree growth characteristics and previous autumn phenology. Tree Physiol. 41, 1161–1170 (2021).
Kwon, S. M. & Kim, N. H. Annual ring formation of major wood species growing in Chuncheon, Korea (I)—The period of cambium activity. J. Korean Wood Sci. Technol. 33, 1–8 (2005).
Kudo, K., Yasue, K., Hosoo, Y. & Funada, R. Relationship between formation of earlywood vessels and leaf phenology in two ring-porous hardwoods, Quercus serrata and Robinia pseudoacacia, in early spring. J. Wood Sci. 61, 455–464 (2015).
Palacio, S., Paterson, E., Sim, A., Hester, A. J. & Millard, P. Browsing affects intra-ring carbon allocation in species with contrasting wood anatomy. Tree Physiol. 31, 150–159 (2011).
Muzika, R. M. & Gottschalk, K. W. Gypsy moth role in forest ecosystems: the good, the bad, and the indifferent. in Forest Health Through Silviculture: Proceedings of the 1995 National Silviculture Workshop (Eskew, L. G.) 99–104 (US Rocky Mountain Forest and Range Experiment Station, 1995).
Czaja, M., Kołton, A. & Muras, P. The complex issue of urban trees—Stress factor accumulation and ecological service possibilities. Forests 11, 932. https://doi.org/10.3390/f11090932 (2020).
Arbellay, E., Jarvis, I., Chavardès, R. D., Daniels, L. D. & Stoffel, M. Tree-ring proxies of larch bud moth defoliation: Latewood width and blue intensity are more precise than tree-ring width. Tree Physiol. 38, 1237–1245 (2018).
Hilton, G. M., Packham, J. R. & Willis, A. J. Effects of experimental defoliation on a population of pedunculate oak (Quercus robur L.). New Phytol. 107, 603–612 (1987).
Mizumachi, E., Mori, A., Osawa, N., Akiyama, R. & Tokuchi, N. Shoot development and extension of Quercus serrata saplings in response to insect damage and nutrient conditions. Ann. Bot. 98, 219–226 (2006).
Mizumachi, E., Osawa, N., Akiyama, R. & Tokuchi, N. The effects of herbivory and soil fertility on the growth patterns of Quercus serrata and Q. crispula saplings at the shoot and individual levels. Popul. Ecol. 46, 203–211 (2004).
Gaytán, Á. et al. The co-existence of multiple oak leaf flushes contributes to the large within-tree variation in chemistry, insect attack and pathogen infection. New Phytol. 235, 1615–1628 (2022).
Vanderklein, D., Daquila, E. & Carrozza, E. White pine, Japanese larch, and bear oak respond differently to partial defoliation. Northeast. Nat. 8, 319–330 (2001).
Foster, J. R. Xylem traits, leaf longevity and growth phenology predict growth and mortality response to defoliation in northern temperate forests. Tree Physiol. 37, 1151–1165 (2017).
Hättenschwiler, S. & Schafellner, C. Gypsy moth feeding in the canopy of a CO2-enriched mature forest. Glob. Change Biol. 10, 1899–1908 (2004).
Schäfer, K. V. R., Clark, K. L., Skowronski, N. & Hamerlynck, E. P. Impact of insect defoliation on forest carbon balance as assessed with a canopy assimilation model. Glob. Change Biol. 16, 546–560 (2010).
Wiley, E., Casper, B. B. & Helliker, B. R. Recovery following defoliation involves shifts in allocation that favour storage and reproduction over radial growth in black oak. J. Ecol. 105, 412–424 (2017).
Kagawa, A., Sugimoto, A. & Maximov, T. C. Seasonal course of translocation, storage and remobilization of 13C pulse-labeled photoassimilate in naturally growing Larix gmelinii saplings. New Phytol. 171, 793–804 (2006).
Deslauriers, A., Caron, L. & Rossi, S. Carbon allocation during defoliation: Testing a defense-growth trade-off in balsam fir. Front. Plant Sci. 6, 338. https://doi.org/10.3389/fpls.2015.00338 (2015).
Plotkin, A. B. et al. Defoliated trees die below a critical threshold of stored carbon. Funct. Ecol. 35, 2156–2167 (2021).
Blanco-Rodríguez, M. Á. & Espelta, J. M. Tree species composition and management influence short-term resilience to defoliation by Lymantria dispar L. in oak forests. For. Ecol. Manage. 520, 120399 (2022).
Brown, B. J. & Ewel, J. J. Responses to defoliation of species-rich and monospecific tropical plant communities. Oecologia 75, 12–19 (1988).
Gottschalk, K. W. Silvicultural guidelines for forest stands threatened by gypsy moth. General Technical Report NE-171 (U.S. Department of Agriculture, Forest Service, Northeastern Forest Experiment Station, 1993)
Korea Meteorological Administration. Automated Synoptic Observing System dataset. https://data.kma.go.kr/data/grnd/selectAsosRltmList.do (2022).
Hwang, H.-S., Lee, Y. S., Lee, H. A., Choi, D. S. & Lee, K.-Y. Natural enemies of the Asian gypsy moth, Lymantria dispar asiatica (Lepidoptera: Erebidae) and the genetic variation analysis of L. dispar multiple nucleopolyhedrovirus. Korean J. Appl. Entomol. 60, 379–386 (2021).
Stokes, M. A. & Smiley, T. L. An Introduction to Tree-Ring Dating (University of Chicago Press, 1968).
Yamaguchi, D. K. A simple method for cross-dating increment cores from living trees. Can. J. For. Res. 21, 414–416 (1991).
Holmes, R. L. Computer assisted quality control in tree-ring dating and measurement. Tree-Ring Bull. 43, 69–78 (1983).
Bunn, A. G. A dendrochronology program library in R (dplR). Dendrochronologia 26, 115–124 (2008).
R Core Team. R: a Language and Environment for Statistical Computing http://www.R-project.org (R Foundation for Statistical Computing, 2021).
Cook, E. R. & Peters, K. The smoothing spline: A new approach to standardizing forest interior tree-ring width series for dendroclimatic studies. Tree-Ring Bull. 41, 45–53 (1981).
Buras, A. A comment on the expressed population signal. Dendrochronologia 44, 130–132 (2017).
Briffa, K. & Jones, P. Basic chronology statistics and assessment. In Methods of Dendrochronology: Applications in the Environmental Sciences (eds Cook, E. R. & Kairiukstis, L. A.) 137–152 (Kluwer Academic Publishers, 1990).
National Institute of Forest Science. Carbon emission factor of major tree species for forest greenhouse gas inventory (Korea Fores Service, National Institute of Forest Science, 2010).
Lloret, F., Keeling, E. G. & Sala, A. Components of tree resilience: Effects of successive low-growth episodes in old ponderosa pine forests. Oikos 120, 1909–1920 (2011).
Acknowledgements
We specially thank Chan Kyu Park for his assistance with field sampling. We appreciate the reviewers for their valuable comments.
Funding
This research was funded by the National Institute of Forest Science (NIFoS) (Grant number: FE0100-2018-11).
Author information
Authors and Affiliations
Contributions
J.B.J.: conceptualization, data collection, formal analysis, visualization, writing—original draft preparation, review and editing. E.S.K.: data collection. J.H.L.: data collection. W.I.C.: conceptualization, data collection, project administration, resources, supervision, writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jung, J.B., Kim, ES., Lim, JH. et al. Host-specific growth responses of Larix kaempferi and Quercus acutissima to Asian gypsy moth defoliation in central Korea. Sci Rep 14, 1477 (2024). https://doi.org/10.1038/s41598-024-51907-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-51907-w
- Springer Nature Limited