Abstract
The role of inflammation in disease promotion is significant, yet the precise association between a newly identified inflammatory biomarker and insulin resistance (IR) and mortality remains uncertain. We aim to explore the potential correlation between systemic immune-inflammation index (SII) and these factors. We used data from 2011 to 2016 of National Health and Nutrition Examination Survey, and multivariate logistic regression and restricted cubic spline were employed. Subgroup and interaction analysis were conducted to recognize the consistency of the results. The association between SII and mortality was described by survival analysis. 6734 participants were enrolled, of whom 49.3% (3318) exhibited IR and 7.02% experienced mortality. Multivariate logistic regression revealed that individuals in the highest quartile (Q4) of SII had a significantly increased risk of IR compared to those in the lowest quartile (Q1). We then identified a linear association between SII and IR with an inflection point of 407, but may be influenced by gender. Similarly, compared to Q1, people whose SII at Q4 showed a higher all-cause and cardiovascular mortality. It showed a significant association between SII and both all-cause and cardiovascular mortality, but the results need to be interpreted with caution.
Similar content being viewed by others
Introduction
Insulin resistance (IR) is a multifaceted metabolic disorder characterized by intricate pathophysiological mechanisms, widely acknowledged as a prominent etiological factor in various diseases1. The development of IR is closely intertwined with internal environmental factors, including obesity2, substance metabolism3, and inflammation4, which collectively contribute to pathological alterations within the body. Among them, inflammation plays a crucial role in the pathophysiology of insulin resistance, obesity, and obesity-mediated insulin resistance5,6. However, obtaining traditional inflammation factor (such as TNF-a, interleukin-1) data is costly in clinical settings and poses challenges for scientific research and dissemination.
Systemic immune-inflammation index (SII), a new inflammatory biomarker based on platelet count × Neutrophil count/lymphocyte count calculation7,8, was proved to reflect the degree of systemic inflammation9. It reported that SII is independently associated with multisystem diseases, such as cardiovascular disease10, diabetic nephropathy11, rheumatic immune disease12,13, cancer14,15,16, osteoporosis17, metabolic diseases18. Different from traditional inflammatory factor, blood routine examination is convenient and inexpensive, and has higher practicability in disease research. Therefore, clarifying the relationship between systemic immune-inflammation index and insulin resistance could prove instrumental in advancing both scientific knowledge and clinical practice, leading to improved decision-making and reduced medical costs associated with treatment decisions. However, at present, there remains uncertainty surrounding this connection.
Therefore, we conducted a cohort study using the National Health and Nutrition Examination Survey (NHANES) database to investigate the impact of novel inflammatory markers on insulin resistance while exploring the effect of SII on mortality.
Results
Baseline characteristics of the participants
A total of 6734 participants were included in this study, of whom 49.2% were male, with an average age of 49.5 years. The prevalence of IR was 49.3% (3318), and the mean SII concentration was 496.9. The clinical characteristics of the participants were shown in Table 1, from which we can find that except for sex, smoking, renal failure, and stroke, the differences in other variables were statistically significant (p < 0.05). Participants with IR tended to be older, non-Hispanic white, alcohol drinkers, BMI ≥ 30, and abdominal obesity.
The relationship between SII and IR
Weighted multivariate logistic regression analysis was performed in Table 2, with SII dividing into quartiles (Q1–Q4). Compared to Q1, participants in Q4 were associated with an increased risk of IR, and the relationship was statistically significant in all 3 models. This association was significant in model 1 (OR 1.73; 95% CI 1.44–2.80), model 2 (OR 1.75; 95% CI 1.44–2.10), and model 3 (OR 1.42; 95% CI 1.14–1.77).
After adjusting for multiple variables, we found a linear relationship between SII and IR by using restricted cubic splines (p = 0.37). As shown in Fig. 1, we can observe the threshold effect: that is, when LnSII was lower than 6.01 (SII = 407.5), the risk of IR is almost unchanged or even has a tendency to decrease; when LnSII exceeds 6.01, the risk of IR increases rapidly. Further stratification analyses (Fig. 2) showed that the intercept of the fitting curve was greater than 1 for man. Similar threshold effect was observed in age-stratified analysis. There was no relationship between SII and IR in non-abdominal obese subjects, while the inflection point of SII in abdominal obesity participants was 6.02 (SII = 411.6).
Results of restricted cubic spline according to sex, age, and abdominal obesity. (A)The smooth curve fitting result between systemic immune-inflammation index and insulin resistance in different genders. (B) The smooth curve fitting result between systemic immune-inflammation index and insulin resistance in different age. (C) The smooth curve fitting result between systemic immune-inflammation index and insulin resistance according to abdominal obesity.
The relationship between SII and mortality
The NHANES dataset encompassed the collection of death follow-up data spanning from 1999 to 2019. Within this study, the mean duration of follow-up was determined to be 70 months. Notably, the rates of all-cause mortality, cardiovascular mortality, and cerebrovascular mortality were observed to be 7.02%, 1.86%, and 0.34%, respectively. To accurately assess the impact of SII on mortality, survival analysis was conducted, wherein adjustments were made for intermediate factors. The selection of mediating variables was guided by a directed acyclic graph (DAG), which is visually presented in Supplementary Fig. 1. After excluding the potential mediating factors (insulin resistance, cancer, liver disease, and renal failure), the results of multivariate Cox regression models (as shown in Table 3) indicate a significant association between SII at the Q4 level and both all-cause mortality and cardiovascular mortality, when compared to the Q1 level. The hazard ratios for these associations were 1.33 (95% CI 1.03–1.72) and 1.79 (95% CI 1.07–3.00), respectively. However, no significant association was found between SII and cerebrovascular death (HR 1.54, 95% CI 0.43–5.61). However, due to the limited number of cerebrovascular death events (N = 23), the results need to be interpreted with caution. According to the findings depicted in Fig. 3, the Kaplan–Meier curves indicated a progressive increase in the all-cause mortality risk for individuals at the Q4 level as time progresses. Conversely, within the Q4 group, there was no apparent rise in the risk of cardiovascular death and brain death. To address model heterogeneity, we carried out a systematic examination of Model 1, 2, and 3 through progressive analyses, which demonstrated consistency across these iterations. Supplementary Table 1 presents these results, confirming the robustness of our survival estimates.
Subgroup and interaction analysis
Subgroup analysis revealed that the association of SII and IR was not consistent in some groups. Overall, the relationship was statistically significant for participants who were female, younger than 60 years, non-Hispanic white, non-Hispanic black, and owned an abdominal obesity (p > 0.05). Furthermore, the interaction test showed that except for sex (p = 0.007), age, race, BMI, and abdominal obesity had no significant effect on the association (Table 4, interaction all p > 0.05).
Discussion
Our study harnesses the rich resources of the NHANES dataset to explore potential links between SII and clinical outcomes, specifically focusing on its relation to insulin resistance. Utilizing easily accessible and cost-effective blood tests, our findings may lead to informed decision-making, improving patient outcomes and reducing healthcare expenses associated with management strategies.
. In this cross-sectional study, we observed a positive linear association between higher SII and increased risk of insulin resistance. Notably, the risk of insulin resistance significantly escalated when SII exceeded 407.5. Further analysis using restricted cubic splines revealed that males and individuals younger than 60 years old were more susceptible to developing insulin resistance. Additionally, similar threshold effects were observed in individuals with abdominal obesity, while those without abdominal obesity exhibited lower susceptibility to insulin resistance, thus emphasizing the multifaceted benefits of weight management. Multiple Cox regression revealed a significant association between SII and both all-cause mortality and cardiovascular mortality. However, survival analysis demonstrated a lack of significant correlation between the SII and cardiovascular death, necessitating cautious interpretation of these results. Subgroup analysis indicated an interaction effect between sex and SII; however, further prospective studies are warranted for confirmation. Furthermore, a multivariate logistic regression excluding sex demonstrated that compared to the first quartile (Q1), individuals in the fourth quartile (Q4) had a higher odds ratio of 1.48 (95% CI 1.19–1.84) for developing insulin resistance—this finding was consistent with our previous result (OR 1.42; 95% CI 1.14–1.77).
To the best of our knowledge, previous studies have demonstrated a correlation between insulin resistance and inflammation. Initial investigations reported elevated levels of fibrinogen and other acute phase reactants in the bloodstream of individuals with insulin resistance19,20. It is noteworthy that several pro-inflammatory cytokines have been implicated in IR. For instance, Hotamisligil and Karasik were the first to identify TNF-α as an inducer of insulin resistance21,22. Animal experiment have shown that both classical IL-6 signal and IL-6 trans-signal can promote inflammation and insulin resistance23. An observational study revealed a positive association between high levels of IL-1 receptor antagonist and IR among African-Americans24. In target organs such as the liver and muscle tissue, macrophage polarization plays a pivotal role in the progression of IR25, thereby influencing both metabolism and inflammation through interactions with macrophages26. Notably, our stratified analysis on abdominal obesity indicated an intercept greater than 1 when SII was zero, suggesting that obesity may independently contribute to the risk of developing insulin resistance. Furthermore, previous research has consistently highlighted the significant role played by chronic inflammation in obesity-related insulin resistance27,28,29, which aligns with our findings. A study investigating racial differences suggested that adiposity mediates the relationship between inflammatory markers and IR specifically among African Americans30. Consistent with this notion, intensive lifestyle interventions aimed at weight management have been shown to reduce markers associated with inflammation and coagulation within diabetes prevention programs31, further emphasizing their importance for promoting overall health.
Extensive research spanning over 6 decades has been dedicated to investigating the mechanism underlying insulin resistance, yet the fundamental pathogenic signal remains elusive32. Inflammation-induced insulin resistance may be influenced significantly by Jun N-terminal kinase (JNK) and IκB kinase-β (IKKβ)/NF-κB. Classical receptor-mediated mechanisms involving proinflammatory cytokines such as TNF-α, IL-1, Toll, and AGE receptors activate JNK and IKKβ/NF-κB4. Cai33 reported that lipid accumulation leads to inflammation by activating NF-kB and inducing cytokine production, resulting in both local and systemic insulin resistance (IR). Additionally, the IKKβ/NF-κB axis has been identified as the target for salicylates and statins in the treatment of IR34. Extensive research has demonstrated a strong association between obesity-induced inflammation and IR35,36. The disorder of lipid metabolism results in the generation of inflammatory factors in various insulin target organs, which plays a crucial role in the development of IR28 and is considered a key characteristic of chronic inflammation associated with obesity21,37. Winer38 observed that diet-induced obesity led to a reduction in the expression of Ym1, arginase 1, and Il10 genes, causing a shift in the polarization state of macrophages from an anti-inflammatory M2 state to a proinflammatory M1 state in ATMs. Similarly, Patsouris found that the elimination of CD11c-positive cells resulted in a decrease in the expression of pro-inflammatory genes (such as macrophage and pro-inflammatory cytokine IL-6) and an increase in the expression of anti-inflammatory genes (such as anti-inflammatory cytokine IL-10) in ATMs, leading to the restoration of insulin sensitivity in obese insulin resistant mice39. Adiponectin, the most abundant protein secreted by adipose tissue, demonstrates significant anti-inflammatory effects through the activation of AMPK and PPAR-α signaling pathways via adiponectin receptor 1 (AdipoR1) and AdipoR2 respectively40,41,42. The downregulation of gene adiponectin occurs when there is a disruption in adipose tissue metabolism, resulting in reduced fatty acid oxidation and glucose uptake in muscle. Conversely, this disruption promotes gluconeogenesis in liver tissue, which is a secondary effect of IR. Amitan demonstrated that leptin, a substance secreted by adipocytes, can mitigate body weight and normalize blood glucose levels by binding to the leptin receptor (LepRb) and JAK2/STAT343. Furthermore, certain antidiabetic agents possess anti-inflammatory properties that can enhance insulin resistance in individuals with diabetes. In a study involving older individuals, a physiological dose of metformin (100 M) was found to inhibit Th17 inflammation in CD4 T cells through an autophagy-dependent mechanism44. Furthermore, an animal experiment revealed that empagliflozin induces fat utilization and browning, reduces inflammation and insulin resistance in diet-induced obese mice by polarizing M2 macrophages45.
DAG was employed to mitigate the influence of mediating variables. However, the association between SII and cardiovascular death exhibited inconsistency when examined through proportional hazards regression model and survival analysis. This inconsistency may be attributed to the limitation of considering only a single variable in the survival analysis, neglecting potential confounding factors. Nevertheless, the Kaplan–Meier curve visualization demonstrated an upward trend in mortality risk for individuals in the Q4 level, warranting cautious interpretation of the findings. Although our results showed no statistically significant correlation between SII and cerebrovascular death, the limited sample size of cerebrovascular deaths in this study (n = 23) precludes a definitive conclusion regarding the association between SII and cerebrovascular mortality. To properly interpret these findings, future research with larger sample sizes is required. It is worth noting that our study fully accounted for the robust association between SII and all-cause mortality, aligning with the findings of a prior study. This study examined the link between SII and long-term mortality in patients with stroke-associated pneumonia, revealing a substantial association between SII and mortality46.
The subgroup analysis indicated that gender may have had an impact on the assessment of IR by the SII. This could be attributed to the potential influence of estrogen deficiency, as the average age of the participants in our study was 50 years. Estrogen has been found to inhibit β-cell apoptosis47, decrease pro-inflammatory signaling48, and enhance insulin function49. Studies have demonstrated that both animals and humans lacking endogenous estrogen production display insulin resistance, which can be mitigated through estrogen supplementation50,51,52. Furthermore, genetic studies have also confirmed that women are more prone to developing insulin resistance. The results of the animal experiment indicate that there is a higher expression of metabolic genes in male mice53. Additionally, the expression of genes involved in regulating protein synthesis in fuel metabolism, such as glucose and lipid oxidation, was found to be higher in males compared to females, suggesting that males may possess a greater capacity for substrate utilization54. Furthermore, the presence of polymorphisms in the scavenger receptor class B, member I (SCARB1), a gene associated with diabetes and regulated by estrogen, was found to be linked to insulin resistance, particularly in women55.
One notable advantage of this study is its extensive sample size and appropriate adjustment for covariates. Additionally, the inclusion of subgroup and sensitivity analyses enhances the reliability and comprehensiveness of the study findings. Nevertheless, it is important to acknowledge the limitations of our study. Firstly, the determination of the temporal order between SII and IR remains inconclusive. Secondly, the diagnostic process of the diseases relied on self-reported information provided by the respondents, thereby introducing the possibility of recall bias. Lastly, due to the inherent temporal aspect of disease recognition, potential influencing factors may not have been adequately accounted for.
Materials and methods
Study design
The NHANES is a national cross-sectional survey directed by the national center for health statistics of the Centers for Disease Control and Prevention to assess the health and nutritional status of adults and children in the United States. The survey examines a nationally representative sample of about 5000 people each year, and data are collected from a home interview and standardized physical mobile examination centers (MECs) released in 2-year cycles. The study was approved by the ethics review committee of the National Center for Health Statistics (NCHS) and obtained the written informed consent of each participant.
Study population
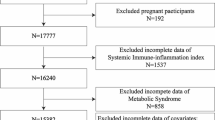
We used NHANES data from 2011 to 2016, which included 29,902 participants. Our study exclusions were as follows (Fig. 4): (1) individuals with missing fasting insulin or glucose measurements; (2) individuals with missing data of platelets, neutrophils, and lymphocyte; (3) adults aged less than 20 years old; (4) pregnant or lactating. A total of 6734 individuals were ultimately included in this study.
Definition of systemic immune-inflammation
NHANES uses several methods to monitor the quality of the analyses performed in the MECs, and the results are measured in duplicate and averaged. The NHANES quality assurance and quality control (QA/QC) protocols meet the 1988 Clinical Laboratory Improvement Act mandates. The SII level was determined by multiplying the platelet count by the neutrophil count/lymphocyte count56,57,58. SII was ln-transformed when conducting regression analysis because of the right-skewed distribution of data.
Definition of insulin resistance
Because of the limitations of the implementation condition of the euglycemic–hyperinsulinemic clamp method, previous studies used homeostatic model assessment (HOMA-IR) to assess IR, with the formula: HOMA-IR = fasting serum insulin (uIU/mL) × fasting plasma glucose (mmol/L)/22.559,60,61. Evidence suggests that it is a strong correlation between IR estimation using HOMA-IR and the gold standard euglycemic-hyperinsulinemic clamp method62. IR was defined as a HOMA-IR ≥ 2.5, and non-insulin resistant as a HOMA-IR < 2.563,64.
Covariates
The covariates included in our study that may affect the RI include: age, sex, race (Hispanic American, Non-Hispanic White, Non-Hispanic Black, Non-Hispanic Asian and Other/Multi-Racial), educational level (below 12th grade, high school graduate, some college/AA degree or college graduate or above), marital status (married, widowed, divorced/separated, never married or living with partner), ratio of family income to poverty level (< 1.3, 1.3–4.9, ≥ 5), BMI (< 25.0 kg/m2, 25.0–29.9 kg/m2, ≥ 30 kg/m2), smoking status, alcohol consumption, diabetes mellitus, hypoglycemic agent consumption, hypertension, hyperlipemia, liver disease, renal failure, coronary heart disease (CHD), chronic heart failure (CHF), stroke, cancer, abdominal obesity (AO), hemoglobin (HB), glycosylated hemoglobin (HBA1c) and fasting blood-glucose (FBG). Trained staff used standardized techniques to measure waist circumference for all adults aged 20 years or older. Abdominal obesity was defined as a waist circumference more than 102 cm in men and 88 cm in women. Smoking status was obtained from the question “have you smoked at least 100 cigarettes in your entire life?” and individuals who answered “yes” would be enrolled in the study. Participants who drank more than 12 times in their lifetime were enrolled.
Hypertension was defined as the respondents' self-reported use of antihypertensive medications or an elevated mean of three blood pressure measurements: systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg. Diabetes mellitus (DM) was defined as: (1) self-reported DM; (2) FBG ≥ 7.0 mmol/l, (3) 2-h postprandial blood glucose ≥ 11.1 mmol/L; (4) HBA1C ≥ 6.5 mmol/L; (5) taking hypoglycemic agents. Hyperlipemia was defined as total cholesterol ≥ 200 mg/dL, triglycerides ≥ 150 mg/dL, or low-density lipoprotein ≥ 130 mg/dL, and those who reported using cholesterol-lowering drugs were also classified as having hyperlipidemia. For the remaining comorbidities, participants who self-reported affirmations were considered to have the disease. For example, in the item “the doctor once told you that you had a stroke”, based on the responses, those who answered “1” were thought they had a stroke.
Statistical analysis
Continuous variables were described as the mean ± SD and were compared by student’s t-test or Mann–Whitney U test, while categorical variables were described as percentages and were compared using chi-square test. Because the SII was unevenly distributed and clearly skewed to the right, we log-normalized the data for analysis. SII was divided into quartiles from lowest (Q1) to highest (Q4) to enhance the sensitivity. Multivariate logistic regression analysis was used to estimate the prevalence odds ratios (ORs) and 95% confidence intervals (CIs) between SII and IR. With model 1 including no covariates, model 2 was adjusted for age, sex and race and model 3 was further adjusted for educational level, PIR, marital status, drinking status, smoking status, BMI, hypertension, diabetes mellitus, hypoglycemic agent, hyperlipemia, liver disease, renal failure, CHF, CHD, stroke, cancer, abdominal obesity, HB, HBA1C and FBG. The restricted cubic splines were used to explore the non-linear relationships and inflection points. Multivariate tests were constructed by controlling for variables and fitting a smooth curve. The association between SII and mortality was analyzed by multiple Cox regression and survival analysis. Finally in sensitivity analyses, we performed subgroup and interaction analysis. Due to the complexity sampling survey of the NHANES database, we used appropriate weights for the analysis (WTMEC2YR/3). The statistical analyses were conducted using EmpowerStats (version 4.1), R studio (Version 4.3.1) and STATA (version 17.0). All reported probabilities (P values) were two-sided with P < 0.05 considered statistically significant.
Ethics approval
The protocol for the research project has been approved by a suitably constituted Ethics Committee of the institution within which the work was undertaken and that it conforms to the provisions of the Declaration of Helsinki (as revised in Fortaleza, Brazil, October 2013).
Approval of the research protocol
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of the National Centre for Health Statistics (Protocol Number: #2011-17, #2018-01).
Informed consent
All participants provided informed consent before enrollment.
Data availability
The datasets analysed during the current study are available in the [NHANES] repository, [https://www.cdc.gov/nchs/nhanes/index.htm].
References
Li, M. et al. Trends in insulin resistance: Insights into mechanisms and therapeutic strategy. Signal Transduct. Target. Ther. 7(1), 216 (2022).
Wu, H. & Ballantyne, C. M. Metabolic inflammation and insulin resistance in obesity. Circ. Res. 126(11), 1549–1564 (2020).
Yang, Q., Vijayakumar, A. & Kahn, B. B. Metabolites as regulators of insulin sensitivity and metabolism. Nat. Rev. Mol. Cell Biol. 19(10), 654–672 (2018).
Shoelson, S. E., Lee, J. & Goldfine, A. B. Inflammation and insulin resistance. J. Clin. Investig. 116(7), 1793–1801 (2006).
Antuna-Puente, B., Feve, B., Fellahi, S. & Bastard, J. P. Adipokines: The missing link between insulin resistance and obesity. Diabetes Metab. 34(1), 2–11 (2008).
Guilherme, A., Virbasius, J. V., Puri, V. & Czech, M. P. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Biol. 9(5), 367–377 (2008).
Hu, B. et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 20(23), 6212–6222 (2014).
Tong, Y. S., Tan, J., Zhou, X. L., Song, Y. Q. & Song, Y. J. Systemic immune-inflammation index predicting chemoradiation resistance and poor outcome in patients with stage III non-small cell lung cancer. J. Transl. Med. 15(1), 221 (2017).
Sylman, J. L. et al. The predictive value of inflammation-related peripheral blood measurements in cancer staging and prognosis. Front. Oncol. 8, 78 (2018).
Li, H. et al. Physical activity attenuates the associations of systemic immune-inflammation index with total and cause-specific mortality among middle-aged and older populations. Sci. Rep. 11(1), 12532 (2021).
Guo, W. et al. Systemic immune-inflammation index is associated with diabetic kidney disease in Type 2 diabetes mellitus patients: Evidence from NHANES 2011–2018. Front. Endocrinol. 13, 1071465 (2022).
Tang, Y. et al. Systemic immune-inflammation index and bone mineral density in postmenopausal women: A cross-sectional study of the national health and nutrition examination survey (NHANES) 2007–2018. Front. Immunol. 13, 975400 (2022).
Zhang, J. et al. Systemic immune-inflammation index is associated with decreased bone mass density and osteoporosis in postmenopausal women but not in premenopausal women. Endocr. Connect. 12(2), e220461 (2023).
Huang, H. et al. Prognostic value of preoperative systemic immune-inflammation index in patients with cervical cancer. Sci. Rep. 9(1), 3284 (2019).
Geng, Y. et al. Systemic immune-inflammation index predicts prognosis of patients with esophageal squamous cell carcinoma: A propensity score-matched analysis. Sci. Rep. 6, 39482 (2016).
He, L., Xie, X., Xue, J., Xie, H. & Zhang, Y. Association of the systemic immune-inflammation index with all-cause mortality in patients with arteriosclerotic cardiovascular disease. Front. Cardiovasc. Med. 9, 952953 (2022).
Liu, B., Wang, J., Li, Y. Y., Li, K. P. & Zhang, Q. The association between systemic immune-inflammation index and rheumatoid arthritis: Evidence from NHANES 1999–2018. Arthritis Res. Ther. 25(1), 34 (2023).
Mahemuti, N. et al. Association between systemic immunity-inflammation index and hyperlipidemia: A population-based study from the NHANES (2015–2020). Nutrients 15(5), 1177 (2023).
Fearnley, G. R., Vincent, C. T. & Chakrabarti, R. Reduction of blood fibrinolytic activity in diabetes mellitus by insulin. Lancet (London, England) 2(7111), 1067 (1959).
Ogston, D. & McAndrew, G. M. Fibrinolysis in obesity. Lancet (London, England) 2(7371), 1205–1207 (1964).
Hotamisligil, G. S., Shargill, N. S. & Spiegelman, B. M. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science (New York, NY) 259(5091), 87–91 (1993).
Feinstein, R., Kanety, H., Papa, M. Z., Lunenfeld, B. & Karasik, A. Tumor necrosis factor-alpha suppresses insulin-induced tyrosine phosphorylation of insulin receptor and its substrates. J. Biol. Chem. 268(35), 26055–26058 (1993).
Xu, E. et al. Temporal and tissue-specific requirements for T-lymphocyte IL-6 signalling in obesity-associated inflammation and insulin resistance. Nat. Commun. 8, 14803 (2017).
Charles, B. A. et al. The roles of IL-6, IL-10, and IL-1RA in obesity and insulin resistance in African–Americans. J. Clin. Endocrinol. Metab. 96(12), E2018–E2022 (2011).
Charo, I. F. Macrophage polarization and insulin resistance: PPARgamma in control. Cell Metab. 6(2), 96–98 (2007).
Asghar, A. & Sheikh, N. Role of immune cells in obesity induced low grade inflammation and insulin resistance. Cell. Immunol. 315, 18–26 (2017).
Li, H. et al. Macrophages, chronic inflammation, and insulin resistance. Cells 11(19), 3001 (2022).
Esser, N., Legrand-Poels, S., Piette, J., Scheen, A. J. & Paquot, N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract. 105(2), 141–150 (2014).
Lumeng, C. N. & Saltiel, A. R. Inflammatory links between obesity and metabolic disease. J. Clin. Investig. 121(6), 2111–2117 (2011).
Doumatey, A. P. et al. Relationships among obesity, inflammation, and insulin resistance in African Americans and West Africans. Obesity (Silver Spring, Md) 18(3), 598–603 (2010).
Haffner, S. et al. Intensive lifestyle intervention or metformin on inflammation and coagulation in participants with impaired glucose tolerance. Diabetes 54(5), 1566–1572 (2005).
Ye, J. Mechanism of insulin resistance in obesity: A role of ATP. Front. Med. 15(3), 372–382 (2021).
Cai, D. et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat. Med. 11(2), 183–190 (2005).
Hundal, R. S. et al. Mechanism by which high-dose aspirin improves glucose metabolism in type 2 diabetes. J. Clin. Investig. 109(10), 1321–1326 (2002).
Defronzo, R. A. Banting lecture. From the triumvirate to the ominous octet: A new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 58(4), 773–795 (2009).
Roden, M. & Bernroider, E. Hepatic glucose metabolism in humans–its role in health and disease. Best Pract. Res. Clin. Endocrinol. Metab. 17(3), 365–383 (2003).
Feuerer, M. et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat. Med. 15(8), 930–939 (2009).
Winer, D. A. et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat. Med. 17(5), 610–617 (2011).
Patsouris, D. et al. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 8(4), 301–309 (2008).
Kwon, H. & Pessin, J. E. Adipokines mediate inflammation and insulin resistance. Front. Endocrinol. 4, 71 (2013).
Li, S., Shin, H. J., Ding, E. L. & van Dam, R. M. Adiponectin levels and risk of type 2 diabetes: A systematic review and meta-analysis. JAMA 302(2), 179–188 (2009).
Hotta, K. et al. Circulating concentrations of the adipocyte protein adiponectin are decreased in parallel with reduced insulin sensitivity during the progression to type 2 diabetes in rhesus monkeys. Diabetes 50(5), 1126–1133 (2001).
Amitani, M., Asakawa, A., Amitani, H. & Inui, A. The role of leptin in the control of insulin-glucose axis. Front. Neurosci. 7, 51 (2013).
Bharath, L. P. et al. Metformin enhances autophagy and normalizes mitochondrial function to alleviate aging-associated inflammation. Cell Metab. 32(1), 44-55.e6 (2020).
Xu, L. et al. SGLT2 inhibition by empagliflozin promotes fat utilization and browning and attenuates inflammation and insulin resistance by polarizing M2 macrophages in diet-induced obese mice. EBioMedicine 20, 137–149 (2017).
Xie, M. et al. Systemic immune-inflammation index and long-term mortality in patients with stroke-associated pneumonia. J. Inflamm. Res. 16, 1581–1593 (2023).
Le May, C. et al. Estrogens protect pancreatic beta-cells from apoptosis and prevent insulin-deficient diabetes mellitus in mice. Proc. Natl. Acad. Sci. U.S.A. 103(24), 9232–9237 (2006).
Evans, M. J., Lai, K., Shaw, L. J., Harnish, D. C. & Chadwick, C. C. Estrogen receptor alpha inhibits IL-1beta induction of gene expression in the mouse liver. Endocrinology 143(7), 2559–2570 (2002).
Brussaard, H. E., Gevers Leuven, J. A., Frölich, M., Kluft, C. & Krans, H. M. Short-term oestrogen replacement therapy improves insulin resistance, lipids and fibrinolysis in postmenopausal women with NIDDM. Diabetologia 40(7), 843–849 (1997).
Morishima, A., Grumbach, M. M., Simpson, E. R., Fisher, C. & Qin, K. Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J. Clin. Endocrinol. Metab. 80(12), 3689–3698 (1995).
Jones, M. E. et al. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc. Natl. Acad. Sci. U.S.A. 97(23), 12735–12740 (2000).
Takeda, K. et al. Progressive development of insulin resistance phenotype in male mice with complete aromatase (CYP19) deficiency. J. Endocrinol. 176(2), 237–246 (2003).
Améen, C. et al. Effects of gender and GH secretory pattern on sterol regulatory element-binding protein-1c and its target genes in rat liver. Am. J. Physiol. Endocrinol. Metab. 287(6), E1039–E1048 (2004).
Mittendorfer, B. Insulin resistance: Sex matters. Curr. Opin. Clin. Nutr. Metab Care 8(4), 367–372 (2005).
McCarthy, J. J. et al. Polymorphisms of the scavenger receptor class B member 1 are associated with insulin resistance with evidence of gene by sex interaction. J. Clin. Endocrinol. Metab. 94(5), 1789–1796 (2009).
Albany, C. Systemic immune-inflammation index in germ-cell tumours: Search for a biological prognostic biomarker. Br. J. Cancer 118(6), 761–762 (2018).
Xie, R. et al. Association between SII and hepatic steatosis and liver fibrosis: A population-based study. Front. Immunol. 13, 925690 (2022).
Qin, Z. et al. Systemic immune-inflammation index is associated with increased urinary albumin excretion: A population-based study. Front. Immunol. 13, 863640 (2022).
Matthews, D. R. et al. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28(7), 412–419 (1985).
Owei, I., Umekwe, N., Provo, C., Wan, J. & Dagogo-Jack, S. Insulin-sensitive and insulin-resistant obese and non-obese phenotypes: Role in prediction of incident pre-diabetes in a longitudinal biracial cohort. BMJ Open Diabetes Res. Care 5(1), e000415 (2017).
Cho, S. K., Huh, J. H., Yoo, J. S., Kim, J. W. & Lee, K. J. HOMA-estimated insulin resistance as an independent prognostic factor in patients with acute pancreatitis. Sci. Rep. 9(1), 14894 (2019).
Wallace, T. M., Levy, J. C. & Matthews, D. R. Use and abuse of HOMA modeling. Diabetes Care 27(6), 1487–1495 (2004).
Vardeny, O. et al. Insulin resistance and incident heart failure the ARIC study (atherosclerosis risk in communities). JACC Heart Fail. 1(6), 531–536 (2013).
da Silva, R. C., Miranda, W. L., Chacra, A. R. & Dib, S. A. Metabolic syndrome and insulin resistance in normal glucose tolerant brazilian adolescents with family history of type 2 diabetes. Diabetes Care 28(3), 716–718 (2005).
Acknowledgements
We thank the staff at the National Center for Health Statistics of the Centers for Disease Control.
Funding
This work was supported by the National Natural Science Foundation of China [Grant Numbers 30670870].
Author information
Authors and Affiliations
Contributions
XD collated and analyzed the data and was a major contributor in writing the manuscript; ML was mainly responsible for the project design and epidemiological analysis; DL drafted the manuscript and provided funding; JH contributed to data reduction and analysis mainly; YF analyzed data and interpreted the result. All authors meet the following conditions 1, 2, 3, 4 and 5: (1) Made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas. (2) Have drafted or written, or substantially revised or critically reviewed the article. (3) Have agreed on the journal to which the article will be submitted. (4) Reviewed and agreed on all versions of the article before submission, during revision, the final version accepted for publication, and any significant changes introduced at the proofing stage. (5) Agree to take responsibility and be accountable for the contents of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Deng, X., Liu, D., Li, M. et al. Association between systemic immune-inflammation index and insulin resistance and mortality. Sci Rep 14, 2013 (2024). https://doi.org/10.1038/s41598-024-51878-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-51878-y
- Springer Nature Limited