Abstract
This study demonstrates the successful, facile, and cost-effective preparation of magnetic Fe3O4 nanoparticles (MNPs) via green procedure using Cordia myxa leaf extracts for efficient adsorption of methylene blue (MB) as a model of organic pollutant. The formation of Fe3O4 NPs was confirmed by a range of spectroscopy and microscopy techniques including FT-IR, XRD, FE-SEM, TEM, EDS, VSM, TGA, and BET-BJH. The synthesized spherical nanoparticles had a high specific surface area of 115.07 m2/g with a mesoporous structure. The formed Fe3O4 MNPs exhibited superparamagnetic behavior with saturation magnetization of 49.48 emu/g. After characterization, the adsorptive performance of the synthesized MNPs toward MB was evaluated. To achieve the maximum removal efficiency, the effect of key parameters such as adsorbent dosage (MNPs), initial adsorbate concentration, pH, and contact time on the adsorption process was evaluated. A maximum adsorption capacity of 17.79 mg/g was obtained, after one-hour incubation at pH 7.5. From the pHPZC of 7.1 of the synthesized adsorbent, the electrostatic attraction between MB and Fe3O4 NPs plays an important role in the adsorption process. The adsorption experimental data showed the closest match with the pseudo-second-order kinetic and Langmuir isotherm. The prepared Fe3O4 NPs were easily recovered by an external magnet and could be reused several times. Therefore, the synthesized MNPs seem to be excellent adsorbents for the removal of MB from aqueous solution.
Similar content being viewed by others
Introduction
The release and permeation of toxic organic compounds and synthetic dyes to surface water can cause serious problems and harm to the environment and living things. Dye effluents are released from numerous dye-utilizing industries such as paper, textile, painting, cosmetics, plastics, printing, rubber, and pharmaceutical manufacturing1,2,3,4,5,6. The complex chemical structure of synthetic dyes especially the existence of aromatic rings in their molecular structure causes high durability without any volatility in high temperature, high solubility, stability, and non-biodegradability7,8. These dyes are very poisonous and may result in genetic mutation, cancer, allergic reactions, skin problems, respiratory tract diseases, heart problems, and toxicity effects of neurotoxicity, which pose serious harm to human health5. Thus, it is recommended that dye wastewater should be treated and organic dyes be correctly disposed of before being released into the aquatic environment to minimize its negative impacts and maintain ecosystems6,9. Due to the importance of the removal of dye from wastewater, finding more efficient, cheaper, and safer ways to remove dyes from industrial wastewater has become a focal point for environmental researchers10,11. Till now, several approaches have been applied for removing these hazardous dyes from the water/wastewater such as adsorption, precipitation, chemical oxidation, biological removal, membrane filtration, and coagulation–flocculation4,9. Among them, the adsorption technique is the most preferable treatment method for a wide range of water contaminants due to its low operational cost, simplicity of design, efficiency, and higher selectivity, regeneration, and designability5. In addition to these advantages of the absorption method, the use of simple, economical, and environmentally friendly adsorbents is one of the important issues in these technologies. Currently, several inexpensive adsorbents are available, such as clays12, zeolites13, plant waste materials14, and magnetic nanoparticles15.
Metal oxide nanoparticles have recently attracted much attention because of their interesting physical, chemical, and catalytic characteristics 16,17,18. Among them, Fe3O4 nanoparticles (MNPs) have received special attention due to their magnetic and microwave absorbing properties, their low toxicity, high stability, low cost, and simple production10,19 These properties have made Fe3O4 MNPs suitable for application in various areas, such as in the chemical, physical, biological, medicinal, and material sciences. For instance, MNPs are utilized for data storage, drug delivery systems, catalysts, magnetic resonance imaging, and tissue repair. Also, the MNPs have attracted considerable interest in water treatment due to their high magnetic response-ability, nanoscale particle size consequently large surface area, superb adsorption, high magnetization, surface functionality, and biocompatibility20,21. Considering the importance of MNPs, a wide variety of chemical and physical procedures have been applied for the preparation of Fe3O4 MNPs, such as solvothermal synthesis22, hydrolysis23, sonochemical synthesis24,25, hydrothermal synthesis26, electrochemical synthesis 27, inverse emulsion polymerization28, surfactant-templated synthesis29, sol–gel technology30, laser ablation31, and co-precipitation32. However, such methods faced limitations of toxic chemicals/reagents, sophisticated procedures, temperature requirements, and energy consumption, and therefore they affect the environment33,34. To circumvent the problems of conventional synthesis procedures, biosynthesis of nanoparticles has recently been introduced as an efficient alternative to physicochemical methods since it is affordable, simple, and environmentally friendly, without requiring hazardous chemicals and producing toxic by-products 35,36. The biosynthesis of Fe3O4 MNPs can be performed by numerous organisms such as yeasts37, algae38, bacteria39, fungi40, and plants8,20,41. Meanwhile, plant extracts are usually easy to handle, and the process can easily be scaled up, with good control of the synthesis and stabilization of MNPs33. Plant extracts contain phytochemicals such as terpenoids, flavonoids, alkaloids, and phenolic compounds, which act as reducing agents as well as stabilizing the MNPs42,43. Depending on which plant extracts are used, the produced nanoparticles may differ in size, morphology, dispersity, and capping materials, and hence may differ in their physical, chemical, and biological activities44. Therefore, it is of interest to study the effects of different plant extracts on the characteristics of the obtained nanoparticles.
From above, the main goal of this work was the synthesis of Fe3O4 MNPs with an easy and green approach using aqueous leaf extract of Cordia myxa through a simple co-precipitation route without the need for hazardous chemicals and investigation of its adsorption efficiency in the removal of MB. MB is a cationic dye classified as a toxic dye and could result in some health and environmental problems8. C. myxa, commonly known as Assyrian plum or Sebesten, is native to an area stretching from tropical Africa through the Middle East18,45. It possesses painkilling, laxative, anti-inflammatory, antimicrobial, immunomodulatory, antiparasitic, and insecticidal properties46,47. It contains various phytochemicals such as glycosides, flavonoids, tannins, sterols, saponins, phenolic acids, terpenoids, and alkaloids45. In this introduced synthesis of Fe3O4 MNPs, aqueous leaf extract of C. myxa, containing phenolics and antioxidants, was used as a reducing and stabilizing agent. After characterization by various biophysical techniques, the synthesized Fe3O4 MNPs were applied as a sorbent, due to the easy collection and effective separation of adsorbents using an external magnetic field, for removing methylene blue (MB) as an organic model dye in aqueous media. Moreover, the adsorption and kinetics isotherms were identified. The prepared MNPs showed excellent MB adsorption capacity, and they could easily be recycled from the target reactor by utilizing an external magnetic field.
Materials and methods
Chemicals and materials
Ferric chloride hexahydrate (FeCl3.6H2O) and ferrous sulfate heptahydrate (FeSO4.7H2O), methylene blue (MB), all with analytical grades, and sodium hydroxide (NaOH, > 97.0%) were purchased from Merck chemical company (Germany). All chemicals used in synthesis or application steps were of analytical reagent grade and utilized as received. In the synthesis procedures, the distilled was of deionized grade. All glassware was thoroughly cleaned with aqua regia and rinsed with deionized water.
Collection of the plant and preparation of the extract
This study complies with relevant institutional, national, and international guidelines and legislation. Fresh and healthy Cordia myxa L. leaves were collected from Bandar Abbas, Hormozgan province, Iran (57° 33′ E 227° 30′ N, 1050 m). The guidelines for collecting plants botany 440/540 (available at https://herbarium.eku.edu/) were used for plant collection. Dr. Mansoore Shamili (Horticulture Department, University of Hormozan, email: shamili@ut.ac.ir) identified the plant species. Accession number 386 was assigned to the plant sample in the university herbarium. Fresh Cordia myxa L. leaf extract was prepared via the green procedure which means not using any toxic or dangerous chemical additives. No cultivation of the plant was done, and there was no genetically modified organism (GMO) procedure. The collected leaves were exhaustively washed with tap water and deionized water to remove surface pollutants and dust particles. Drying the leaves was done in the shade at room temperature for 10 days. A powder was prepared from the dried leaves via an electric blender. 10.0 g of the powdered leaf was added to a 250 ml round-bottom flask containing 100 ml deionized water and refluxing was done for 60 min at 80 °C. After cooling, the mixture was filtered using Whatman No. 1 filter paper, and the final extract was kept in a refrigerator for the next characterization or studies.
Synthesis of Fe3O4 MNPs
The preparation of Fe3O4 MNPs was conducted via the following easy and environmentally-friendly procedure. In a typical reaction, 1.11 g of FeCl3.6H2O (0.004 mol) and 0.53 g of FeSO4.7H2O (0.002 mol) (amounting to a Fe3+: Fe2+ molar ratio of 2:1) were added into 100 ml deionized water, and heated to 80 ℃ under atmospheric pressure under mild stirring using a magnetic stirrer for 10 min. 5.0 ml of freshly prepared C. myxa leaves extract was added, and, under vigorous stirring for 1 h at 80 °C, 20.0 ml 1 M NaOH solution was added dropwise. During this step, the pH of the reaction was kept at pH 10–11. The color of the prepared mixture changed from yellow to completely black, which was the indicator of the construction of Fe3O4 MNPs in the precipitation reaction41. After cooling, the isolation of synthesized Fe3O4 MNPs was performed via an external magnet, and the MNPs were washed several times with ethyl alcohol. The separated Fe3O4 MNPs were dried in a vacuum oven at 90 °C for 12 h. For comparison, Fe3O4 MNPs were also synthesized in the absence of plant extract using a similar procedure. The obtained MNPs were stored in a stoppered bottle until further use.
Instrumentation and characterization
The obtained Fe3O4 MNPs were analyzed by X-ray powder diffraction (Bruker D8 Advance powder diffractometer) with Ni-filtered Cu-Kα radiation (λ = 1.5406 Å) at a setting of 40 kV/30mA with a scan rate of 0.02° per minute in the angular range (2θ) of 20 to 70°. FTIR spectra of C. myxa leaves extract and synthesized Fe3O4 MNPs were obtained in the range of 4000–550 cm−1 using a Bruker alpha FT-IR spectrometer (Germany) equipped with a Diamond attenuated total reflection (ATR) accessory at room temperature. The morphologies of the samples were observed using field emission scanning electron microscope images (FE-SEM) on a TE-SCAN MIRA3 SEM with primary electron energy of 15 kV. The chemical composition of the obtained Fe3O4 MNPs was investigated by Energy Dispersive X-ray Spectroscopy (EDS) performed in SEM. TEM studies were carried out on a Zeiss–EM10C instrument with an accelerating voltage of 100 kV. The magnetic properties of the synthesized Fe3O4 MNPs were identified at room temperature using a vibrating sample magnetometer (VSM; Meghnatis Daghigh Kavir Company LBKFB). The pore diameter and specific surface area were measured using a Brunauer–Emmett–Teller surface area analyzer (Microtracbel Corp BELSORP Mini). Nitrogen adsorption measurements were done on samples that were priory degassed at 150°C. Thermogravimetric analysis (TGA) was done with a heating program from room temperature up to 700 ºC with an increasing slope equal to 10 °C min−1 under a nitrogen atmosphere using a TGA-7 analyzer (Perkin-Elmer, USA). UV–Vis spectra were obtained at room temperature with a SCINCO-S-3100 spectrophotometer (Scinco Co., Korea) equipped with a 1.0 cm quartz cell.
Point of zero charge (PZC)
Determination of the point of zero charge (pHPZC) for the synthesized Fe3O4 MNPs was done using the pH drift method48. It is well-known that the pHPZC is the pH at which the surface charge of the synthesized Fe3O4 MNPs is equal to zero. To a series of 100 ml falcon tubes, 50.0 ml of 0.01 M NaCl (as an inert electrolyte) was added, for adjusting the ionic strength throughout the experiments. Next, the pH values of the solutions (pHinitial) were brought to a pH value in the range between 2.0 and 10.0 with intervals of one by adding either HCl or NaOH. 0.010 g of the synthesized Fe3O4 MNPs was then added to each falcon tube and they were closed. The resulting mixtures were allowed to equilibrate for 12 h in a shaker kept at room temperature. After 12 h, the synthesized Fe3O4 MNPs were magnetically separated from the solution and the pHs of the remaining solutions were measured (pHfinal). The plot of ∆pH (pHfinal−pHinitial) versus pHinitial was drawn and the point of pHfinal−pHinitial = 0 was considered as the pHPZC value49.
Batch experiments
To evaluate the adsorption of methylene blue by the synthesized Fe3O4 MNPs, 15 mg of Fe3O4 MNPs were stirred for 2 h in 30.0 ml of MB solution (10 mg L−1 in distilled water) in a shaker at room temperature. The reaction was monitored by UV–Vis spectrophotometer at time intervals of 10 min by taking 3.0 ml of the mixture and separating the Fe3O4 MNPs from the reaction solution through an external magnet. The MB concentration remaining in the reaction mixture was determined by UV–Vis spectrophotometry at a wavelength of 664 nm. The precision of UV–Vis measurements before and during work was checked with a spectrophotometer, and the RSD of absorbance was not higher than 3%. The efficiency of the Fe3O4 MNPs in MB removal was obtained using Eq. (1)50:
where A0 is the initial absorbance of MB in the solution and At is its absorbance at time t.
Effects of several experimental factors on the adsorption efficiency of MB by Fe3O4 MNPs including pH (3.0, 5.0, 6.5, 7.5, 9.0, and 11.0), amount of Fe3O4 MNPs (0.17, 0.25, 0.33, 0.50, 0.67, and 0.83 mg ml−1), and initial MB concentration (5.0, 10.0, 12.0, and 15.0 mg L−1) were investigated. Each experiment was performed three times and the mean ± SD was reported. The adjustment of the pH was performed using HCl or NaOH solutions as required.
The capacity of the synthesized Fe3O4 MNPs to adsorb MB was calculated using Eq. (2)41,51:
where qt is the adsorption capacity per gram of magnetic adsorbent (synthesized Fe3O4 MNPs) at desired time t. C0 and Ct denote the dye concentrations (mg L−1) in the aqueous phase at the start of the experiment and at the desired time (t), respectively. The volume of the dye solution (L) is also shown by V in Eq. (2) and m denotes the mass (g) of the magnetic adsorbent. It is worth mentioning that at t in equilibrium contact time, Ct is equal to Ce and qt is equal to qe respectively.
Results and discussion
Powder XRD analysis
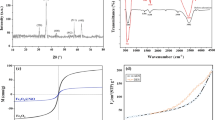
The crystallinity of the synthesized Fe3O4 MNPs was characterized by X-ray powder diffraction (XRD) (Fig. 1a). XRD analysis showed six major Bragg diffraction peaks at 30.24°, 35.64°, 43.42°, 53.52°, 57.26° and 63.68° (2θ), which correspond to the (220), (311), (400), (422), (511) and (440) crystal indices (corresponds to the standard XRD pattern of Fe3O4 from JCPDS 75-0033)52. These diffraction peaks are very similar to the peaks of the cubic spinel structure of Fe3O4 crystals52,53. No other distinct peaks of metal hydroxides or α-Fe2O3 (hematite) were observed, indicating the pure crystalline phase of the synthesized Fe3O4 MNPs and confirming the complete formation of Fe3O4 MNPs. The average crystallite size of the as-prepared Fe3O4 MNPs was estimated from the full width at half-maximum (FWHM) of the (311) reflection peak using Debye–Scherrer’s equation (Eq. 3)35:
In Debye–Scherrer’s equation, 0.89 is the shape factor, D denotes the average particle size and λ is the wavelength of the Cu-Kα irradiation. β shows the full width at half maximum intensity of the obtained diffraction peak and θ is the diffraction angle for the (311) peak of the Fe3O4 MNPs. The calculated crystallite size of the Fe3O4 MNPs was ~ 25.3 nm. To ensure the ability of the chemical method used to prepare MNPs without using the plant extract, the XRD of Fe3O4 MNPs prepared in the absence of C. myxa leaf extract is also represented in Fig. 1b. According to the results, the indicative peaks of MNPs in the XRD pattern show the construction of desired particles.
FE-SEM, EDS, and TEM of Fe3O4 MNPs
The size and shape of synthesized Fe3O4 MNPs were established by FE-SEM. As can be seen in Fig. 2a, the synthesized nanoparticles were spherical in shape and uniform in size with a size range of 21–32 nm, which was similar to the XRD result.
The composition of the Fe3O4 MNPs was established by EDS in SEM, showed that iron, oxygen, carbon, and nitrogen are the four main elements with weight percentages of 58.22, 31.86, 7.58 and 2.34%, respectively (Fig. 2b). The elements of carbon and nitrogen were derived from the phytochemicals found in C. myxa leaf extract. The presence of these elements proves that the prepared nanoparticles are coated with phytochemicals54. Also, the source of excess oxygen can be from flavonoids and phenolics of C. myxa leaf extract or the physical adsorption of oxygen from air on the surface of synthesized Fe3O4 MNPs. Furthermore, TEM images of the prepared nanoparticles confirmed the formation of spherical particles (Fig. 2c).
FTIR analysis
FT-IR analysis was performed to identify possible functional groups of the C. myxa leaf extract on the surface of prepared magnetic nanoparticles. The FT-IR spectrum of C. myxa leaf extract (Fig. 3a) displays peaks at 3500 to 3000 (centered at 3250), 2919, 1723, 1583, 1385, 1260, and 1062 cm−1, corresponding to free OHs and OH group forming hydrogen bonds, aliphatic C–H stretching vibrations, aromatic ring C=C stretching vibrations, amide C=O stretching vibrations, nitrogen N–O bending vibrations, C–OH stretching vibrations, and C–N stretching vibrations of amine groups, respectively3,4,55,56. These peaks indicate the presence of flavonoids and phenolics in C. myxa leaf extract, which could reduce metal ions to metal nanoparticles and stabilize the formed nanoparticles57. In Fig. 3b, the peak at 586 cm−1 is the characteristic Fe–O peak, confirming the successful formation of Fe3O4 MNPs3,4,10,56. Furthermore, the differences between the FT-IR spectrum of the C. myxa leaves extract and the synthesized Fe3O4 MNPs indicate that the iron cations interact with the phytochemicals (Fig. 3b). The shifted peaks at about 3372, 2926, 1587, 1356, 1130, and 923 cm−1 correspond to the O–H functional groups, C–H stretching, C=O stretching, nitrogen N–O bending, and C–N stretching vibrations, respectively50. FT-IR results indicate that the flavonoids and phenolics in C. myxa leaf extract act as capping agents for the formed Fe3O4 MNPs and prevent their aggregation through surface adsorption via π-electron interaction in the absence of other strong capping agents. A possible mechanism for the formation of Fe3O4 MNPs can be proposed as follows58 and is also illustrated in Fig. 4 to highlight the role of functional groups that modify the surface of MNPs:

The FT-IR spectrum of the modified Fe3O4 MNPs after adsorption of MB is also shown in Fig. 3c. By comparing this spectrum with the spectrum of modified Fe3O4 MNPs (Fig. 3b), it can be observed that the C=C bond (located around 1600 cm−1) in Fig. 3c is slightly reduced compared to Fig. 3b, but this change was not significant after MB adsorption. Due to the repetition of this fact in different runs, it can be suggested that the contribution of π–π interaction is not significant in this interaction59. On the other hand, the reduction of C–O–H bond at about 1070 cm−1 has a significant loss in intensity after MB adsorption which indicates the possibility of electrostatic interactions and H-bonding during this phenomenon.
Magnetic measurements
The shape, size, and morphology of nanomaterials, which are strongly dependent on the applied synthetic method, could affect the magnetic behavior of the nanomaterials55. Therefore, the magnetic properties of the prepared Fe3O4 MNPs were investigated at room temperature via a vibrating sample magnetometer (VSM), with a field sweeping from − 15,000 to + 15,000 Oe. Figure 5 shows the superparamagnetic behavior of the synthesized Fe3O4 as the magnetic hysteresis loop shows an S-like curve60. The observed saturation magnetization (Ms) was ~ 49.48 emu/g. The remnant magnetization (Mr) and coercivity (Hc) of the synthesized Fe3O4 MNPs were 2.25 emu/g and ~ 30 Oe, respectively (upper left inset of Fig. 5). These low values of Mr and Hc indicate the superparamagnetic behavior of the synthesized MNPs53. Moreover, the sufficient saturation magnetization of the synthesized Fe3O4 MNPs allowed easy and rapid separation (within seconds) of these MNPs from the mixture by an externally applied magnet, with the solution becoming clear (bottom right inset of Fig. 5). This property is very important in the reusability of the sorbent.
Thermogravimetric analysis
To investigate the thermal stability of the prepared Fe3O4 MNPs, a thermogravimetric analysis (TGA) of Fe3O4 MNPs with and without leaf extract was done (Fig. S1a,b), supporting information). The synthesized Fe3O4 MNPs in the presence of leaf extract show three weight loss steps (Fig. S1a). In the first step (below 100 °C), the weight loss is due to the dehydration of the sample, the remaining weight of which is about 96%. The weight loss steps at 230 °C and at 420 °C can be attributed to the decomposition of adsorbed phyto-compounds of C. myxa leaf extract that act as capping agents55. At temperatures higher than 520 ºC, the phytochemicals of C. myxa leaf extract were completely degraded, and the MNPs did not show further weight loss up to 700 ºC. For the MNPs caped with C. myxa leaf extract, the residual weight is 86% after 520 ºC (Fig. S1a). In contrast, the residual weight of the Fe3O4 MNPs synthesized without plant extract is about 96% at 520 ºC to 700 ºC (Fig. S1b), which is close to the residual weight of the Fe3O4 MNPs synthesized with leaf extract after the first step of weight loss. These results indicate that the Fe3O4 MNPs synthesized without extract contain only adsorbed water without any capping agent.
Surface area and pore distribution
The surface area and porous nature of the synthesized MNPs were investigated by determining the adsorption–desorption isotherm at 77 K using liquid N2 as adsorbent, as shown in Fig. S2 (Supporting information). The synthesized Fe3O4 MNPs exhibited hysteresis loops with intensities associated with capillary condensation at relatively high pressures, which are characteristic of type IV isotherms with H3 type hysteresis loops, according to the IUPAC classification10,61. The calculated Brunauer–Emmett–Teller (BET) surface area of the prepared green-coated MNPs was about ~ 115.07 m2/g, which is clearly higher than that of many other Fe3O4 MNPs10. The single-point adsorption total volume at P/P0 = 0.990 was 0.3357 cm3 g−1. The values of surface area and pore volume of the synthesized Fe3O4 MNPs indicate the potential of the proposed method in preparing the Fe3O4 MNPs with superior catalytic or adsorption activity. Moreover, the pore size distribution from the Barrett-Joyner-Halenda (BJH) analysis (inset of Fig. S2), indicates the mesoporous nature of the synthesized Fe3O4 MNPs, with a wide pore size distribution. Overall, the high BET-specific surface area and the BJH pore-size distribution analysis confirmed that these one-pot synthesized mesoporous Fe3O4 MNPs have the potential to be used for the adsorption of pollutants such as dyes and toxic metals from wastewater.
Adsorption of methylene blue
Next, the synthesized Fe3O4 MNPs were used to remove MB, as a model of an organic dye pollutant, from aqueous solution. The effects of various parameters on the adsorption capacity of the Fe3O4 MNPs were followed: including the pH of the solution, the amount of sorbent, the dye concentration, and the adsorption time.
pH dependence studies
The pH of the solution is a key factor in the adsorption of dye from water because pH affects the surface charge of the adsorbent as well as the structure and ionization value of the dye molecules62. Using an initial dye concentration of 10 mg L−1 and 10 mg MNPs in 30.0 ml dye solution (0.33 mg ml−1) the removal efficiency of MB by Fe3O4 MNPs was studied at pHs from 3.0 to 11.0. According to Fig. 6a, the removal efficiency increases with the increase of initial pH solution (from pH 3.0 to 7.5) and remains almost constant at higher pHs. Similar remarks have been observed in the adsorption of methylene blue by other adsorbents63,64,65. This result can be explained by the pHPZC value of the adsorbent. In pHPZC, the electric charge density on the sorbent surface immersed in the electrolyte solution is zero. At pH < pHPZC, the net surface charge of adsorbent is positive and the adsorption of anions dominates, while, at pH > pHPZC, the net surface charge is negative and thus allows the trapping of cations66. The pHPZC of the synthesized Fe3O4 MNPs in the presence of C. myxa leaf extract was found to be 7.1 (see Fig. S3, Supporting information). MB is a cationic dye with pKa = 3.8 and has a permanent positive charge in the studied pH range. Hence, at pH lower than pHPZC, the adsorption of MB onto Fe3O4 MNPs decreases due to the positive charge of adsorbent and electrostatic repulsion. In addition, the H+ concentration, which is high at lower pH, competes with the positively charged MB for vacant adsorption sites. Thus, at low pH, the adsorption is very low. However, at pH higher than pHPZC, the surface charge of MNPs is negative, due to the deprotonation of carboxyl groups and adsorption of OH− on the surface of the adsorbent, and the adsorption of MB increases due to electrostatic attraction between adsorbent and MB. These results confirm that electrostatic attraction plays a key role in the adsorption process. The maximum adsorption is achieved at a pH close to the pHPZC of the adsorbent63,67, which explains the optimum pH (= 7.5).
Adsorption efficiency of the synthesized Fe3O4 MNPs versus time as a function of (a) solution pH (other experimental conditions: MNPs dosage = 0.33 mg ml−1 and MB conc. = 10.0 mg L−1), (b) concentration of synthesized Fe3O4 MNPs (at pH = 7.5 and MB conc. = 10.0 mg L−1), and (c) initial MB concentration (at pH = 7.5 and MNPs dosage = 0.50 mg ml−1). Inset: The color change of MB solution with the concentration of 12.0 mg L−1 before and after the adsorption process (at the optimum pH of 7.5 and adsorbent dosage of 0.50 mg ml−1).
Effect of the amount of Fe3O4 MNPs
Since the adsorption of MB takes place at the Fe3O4–H2O interface, the amount of adsorbent has a great influence on the adsorption capacity. According to Fig. 6b, increasing the amount of adsorbent from 0.17 to 0.50 mg ml−1 increases the removal of MB (10 mg L−1) from 51 to 88%. This is related to the increased surface area of the adsorbent and access to a large number of adsorption sites for MB68. The removal efficiency remains constant at higher amounts of adsorbent (0.67 and 0.83 mg ml−1).
Effect of the initial concentration of MB
Figure 6c shows the influence of the initial dye concentration on the removal efficiency of MB at a fixed dosage of Fe3O4 MNPs (0.50 mg ml−1) and at pH 7.5. Enhancing the concentration of MB from 5.0 to 12.0 mg L−1 did not affect the removal efficiency. However, a further increase of the MB concentration to 15.0 mg L−1 clearly reduced the removal efficiency. This can be justified by the saturation of the MB binding sites: at a given dosage of MNPs, the number of MB binding sites is constant. Hence, with the increase in MB concentration, the adsorption of MB molecules becomes a competitive process, which leads to a decrease in removal percentage.
Reusability of the synthesized Fe3O4 MNPs
For any adsorbent, it is desirable that its adsorption capacity remains more or less constant during regenerated and reuse. This property makes the use of the adsorbent economically sustainable, which is especially important for commercial and industrial applications. Therefore, in each cycle, after the adsorption process, the utilized Fe3O4 MNPs are magnetically separated from the solution, washed with ethanol to remove the adsorbed MB, dried, and reused in the next cycle. From Fig. 7, it can be concluded that the removal efficiency remained more or less constant after three successive runs. After the 4th cycle, only about 11% of its removal efficiency was lost, indicating the stability and reusability of the Fe3O4 MNPs.
Kinetics of dye adsorption
Most adsorption processes are time-dependent. In this study, equilibrium was reached after about 60 min, after which the adsorption efficiency remained almost constant (Fig. 6). Kinetic models are used to investigate different mechanisms controlling the adsorption of the dye molecules from the aqueous solutions; that is, these models help to describe the adsorption rate of solutes from the solute-solution interface. In fact, adsorption kinetics provide valuable information for the design of the adsorption process for practical applications. To investigate the kinetic parameters of the adsorption process in more detail, three kinetic models were considered: pseudo-first-order or Lagergren model (shown in Eq. 4)69,70, pseudo-second-order (shown in Eq. 5)71, and intraparticle diffusion (shown in Eq. 6)72,73.
The parameter qe (mg/g) is the adsorption capacity in the equilibrium and qt is the adsorption capacity at time t. qe and qt show the amount of MB adsorbed on the magnetic adsorbent. The k1 (min-1), k2 (mg/g min), and kid (mg/g min0.5) are rate constants for pseudo-first-order kinetics, pseudo-second-order kinetics, and intraparticle diffusion, respectively69,71,72. The C (mg/g) in Eq. (6) is a constant value related to the thickness of the boundary layer. Kinetic studies of the adsorption process were performed with an initial concentration of MB of 12 mg L-1, Fe3O4 MNPs dosage of 0.50 mg ml-1, and pH 7.5. All the kinetic parameters were calculated by fitting the experimental data to different kinetic models and are presented in Table 1 and Fig. 8. The highest R2 value (above 0.99) from fitting the experimental data to the pseudo-second-order kinetic shows that this model describes the process in the best way compared to the others. Moreover, the qe value calculated from the second-order model is close to the experimental value and demonstrates a smaller deviation compared to the first-order model, which further confirming that the adsorption mechanism is second order. The verification of this model suggests that both adsorbent and adsorbate concentrations are associated with the rate-determining step of the adsorption process along with chemisorption, via valence forces through the exchange or sharing of electrons between the dye and nanoparticles, chelation, coordination and/or complexation74,75.
Adsorption isotherm
The analysis of the adsorption equilibrium models can provide useful information about the adsorption mechanism, surface properties, and affinity of the applied adsorbent76. In order to understand the interaction behavior between adsorbate (MB dye) and adsorbent (green synthesized Fe3O4 MNPs), isotherm experiments were conducted at room temperature and the obtained results were studied with Langmuir and Freundlich models as two common. The Langmuir isotherm is suitable for monolayer adsorption on a surface containing a finite number of identical sites. This model assumes uniform adsorption energies on the surface and there is no transmigration of the adsorbate in the plane of the surface. During the adsorption process, an active site adsorbs a dye molecule and then does not allow any additional adsorption on the occupied active site76,77,78. The linearized Langmuir isotherm is expressed as74:
where qe is the equilibrium adsorption capacity as denoted previously, Ce (mg L−1) is the equilibrium concentration of MB in solution, qmax (mg g−1) is the maximum adsorption capacity, and b (L mg−1) is the Langmuir constant.
The Freundlich model assumes that with an increase in the concentration of the adsorbate, the adsorbate concentration on the adsorbent surface also increases and, correspondingly, the sorption energy decreases exponentially with the completion of adsorption sites of adsorbent. On the other hand, this isotherm was used to describe the adsorption characteristics of multilayer and heterogeneous surfaces with unequally available adsorption sites that have different adsorption energies76,77. The Freundlich adsorption isotherm is given as74:
where n and kf are the Freundlich adsorption isotherm constants related to the adsorption intensity and the adsorption capacity, respectively.
The obtained Freundlich and Langmuir constants from regression analysis are represented in Table 2. Based on the R2 values, the Langmuir equation describes the adsorption of MB onto Fe3O4 MNPs better than the Freundlich equation. Herein, the qmax of the MNPs was found to be 17.79 (± 0.06) mg g−1 in three repeated sets of experiences. Based on the obtained results, it is clear that the modification of MNPs with the phytochemical compounds enhanced the adsorption capability of adsorbent. To show the importance of modifying the surface of MNPs with the C. myxa leaf extract in the adsorption capacity, the qmax of bare MNPs was calculated in similar conditions and it was found that in spite of weak correlation Langmuir model, the qmax was significantly lower than qmax of modified MNPs (< 65%). The important role of functional groups obtained from the extract is schematically shown in Fig. 9.
Comparison with other adsorption studies using Fe3O4 MNPs
The commercial applicability of an adsorbent depends on its adsorption capacity, specific surface area, availability, and compatibility with the environment and the user. Table 3 shows a comparison of the adsorption capacity of various green synthesized Fe3O4 MNPs reported in the literature79,80,81,82 for the removal of MB from aqueous solutions. Based on Table 3, Fe3O4 MNPs prepared with C. myxa leaf extracts have better or comparable adsorption capacity compared with other MNPs, with the exception of MNPs prepared with Cress seed mucilage. Therefore, the synthesized Fe3O4 MNPs produced with C. myxa leaf extract appear to be a sustainable adsorbent for the removal of MB from aqueous solutions. It is clear that qmax is one of the most important criteria for the application of nanosorbents in real applications. On the other hand, by comparing the required pH of our suggested MNP with previous green synthesized ones, it can be observed from Table 3 that the pH of the current work is not too alkaline or acidic which makes it a good applied sorbent for application without serious need of pH adjustment. The highlight point of the current work was the using C. myxa leaf to enhance the surface properties of Fe3O4 MNPs which changes its adsorption ability in comparison with the previous similar reports.
Conclusions
Here, we reported a green, one-pot synthesis of Fe3O4 MNPs using C. myxa leaf extract. The resulting spherical particles had a size of 21–32 nm with a saturation magnetization value of about ~ 49.48 emu/g. XRD analysis of Fe3O4 MNPs confirmed their cubic spinel structure. FT-IR spectroscopy confirmed the presence of C. myxa phyto-compounds in the nanoparticles, contributing to their stabilization. BET revealed the mesoporous nature of the synthesized Fe3O4 MNPs with a high surface area (~ 115.07 m2/g) that could act as an effective adsorbent to remove MB from aqueous media. The maximum removal efficiency (88.8%) was recorded at the following optimum operation conditions; adsorbent dosage = 0.50 mg ml−1, solution pH = 7.5, and contact time = 60 min. with a maximum adsorption capacity of 17.79 mg/g at pH 7.5, after 60 min with pseudo-second-order kinetics. The maximum adsorption capacity of 17.79 mg/g was recorded from the Langmuir model. The second-order isotherm model shows the best fit with the experimental data. It was demonstrated that modification and capping of MNPs with the phytochemical compounds present in C. myxa leaf extract increases the adsorption capability of the adsorbent. The Fe3O4 MNPs were easily recovered from the solution by an external magnet and could be successfully be reused several times and no significant decrease in removal performance was observed. The non-toxic and magnetically separable green synthesized Fe3O4 MNPs could be applied as a cost-effective adsorbent with possible wide application in wastewater treatment technologies and removal of organic water pollutants. However, the study of the thermodynamics of the adsorption process was not within the scope of this work but its investigation in future studies can enhance our knowledge about the adsorption property of the proposed nanosorbent. The presented work was not the best in comparison with the previous studies but can obtain an alternative way to decrease the dye pollution in aqueous media using stable and green-stabilized MNPs which are simply and easily prepared.
Data availability
Most of details and data have been included in the manuscript and other details can be available via a reasonable request to the corresponding author (F. Samari).
References
Paredes-Quevedo, L. C., González-Caicedo, C., Torres-Luna, J. A. & Carriazo, J. G. Removal of a textile azo-dye (basic red 46) in water by efficient adsorption on a natural clay. Water Air Soil Pollut. 232, 4 (2021).
Dawood, S., Sen, T. K. & Phan, C. Synthesis and characterisation of novel-activated carbon from waste biomass pine cone and its application in the removal of Congo red dye from aqueous solution by adsorption. Water Air Soil Pollut. 225, 1818 (2014).
Reghioua, A. et al. Magnetic chitosan-glutaraldehyde/zinc Oxide/Fe3O4 nanocomposite: Optimization and adsorptive mechanism of remazol brilliant blue r dye removal. J. Polym. Environ. 29, 3932–3947 (2021).
Reghioua, A. et al. Parametric optimization by Box-Behnken design for synthesis of magnetic chitosan-benzil/ZnO/Fe3O4 nanocomposite and textile dye removal. J. Environ. Chem. Eng. 9, 105166 (2021).
Jawad, A. H., Sahu, U. K., Jani, N. A., ALOthman, Z. A. & Wilson, L. D. Magnetic crosslinked chitosan-tripolyphosphate/MgO/Fe3O4 nanocomposite for reactive blue 19 dye removal: Optimization using desirability function approach. Surfaces Interfaces 28, 101698 (2022).
Abdulhameed, A. S. et al. Statistical modeling and mechanistic pathway for methylene blue dye removal by high surface area and mesoporous grass-based activated carbon using K2CO3 activator. J. Environ. Chem. Eng. 9, 105530 (2021).
Moradihamedani, P. Recent advances in dye removal from wastewater by membrane technology: A review. Polym. Bull. 79, 2603–2631 (2022).
Mohammadpour, A. et al. Green synthesis, characterization, and application of Fe3O4 nanoparticles for methylene blue removal: RSM optimization, kinetic, isothermal studies, and molecular simulation. Environ. Res. 225, 115507 (2023).
Katheresan, V., Kansedo, J. & Lau, S. Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 6, 4676–4697 (2018).
Prasad, C., Yuvaraja, G. & Venkateswarlu, P. Biogenic synthesis of Fe3O4 magnetic nanoparticles using Pisum sativum peels extract and its effect on magnetic and Methyl orange dye degradation studies. J. Magn. Magn. Mater. 424, 376–381 (2017).
dos Santos, D. K. et al. Removal of methyl violet dye by adsorption process on hydrogen titanate nanotubes: Experimental-theoretical study. Water Air Soil Pollut. 233, 166 (2022).
Liang, X. et al. Bentonite/carbon composite as highly recyclable adsorbents for alkaline wastewater treatment and organic dye removal. Microporous Mesoporous Mater. 241, 107–114 (2017).
Humelnicu, I., Băiceanu, A., Ignat, M.-E. & Dulman, V. The removal of Basic Blue 41 textile dye from aqueous solution by adsorption onto natural zeolitic tuff: Kinetics and thermodynamics. Process. Saf. Environ. Prot. 105, 274–287 (2017).
Jain, S. N. & Gogate, P. R. Adsorptive removal of acid violet 17 dye from wastewater using biosorbent obtained from NaOH and H2SO4 activation of fallen leaves of Ficus racemosa. J. Mol. Liq. 243, 132–143 (2017).
Juang, R.-S. et al. Synthesis of magnetic Fe3O4/activated carbon nanocomposites with high surface area as recoverable adsorbents. J. Taiwan Inst. Chem. Eng. 90, 51–60 (2018).
Rabbani, M., Rafiee, F., Ghafuri, H. & Rahimi, R. Synthesis of Fe3O4 nonoparticles via a fast and facile mechanochemicl method: Modification of surface with porphyrin and photocatalytic study. Mater. Lett. 166, 247–250 (2016).
Kerour, A., Boudjadar, S., Bourzami, R. & Allouche, B. Eco-friendly synthesis of cuprous oxide (Cu2O) nanoparticles and improvement of their solar photocatalytic activities. J. Solid State Chem. 263, 79–83 (2018).
Samari, F., Parkhari, P., Eftekhar, E., Mohseni, F. & Yousefinejad, S. Antioxidant, cytotoxic and catalytic degradation efficiency of controllable phyto-synthesised silver nanoparticles with high stability using Cordia myxa extract. J. Exp. Nanosci. 14, 141–159 (2019).
Zokhtareh, R., Rahimnejad, M., Najafpour-Darzi, G. & Karimi-Maleh, H. A novel sensing platform for electrochemical detection of metronidazole antibiotic based on green-synthesized magnetic Fe3O4 nanoparticles. Environ. Res. 216, 114643 (2023).
Yaghoobi, M., Asjadi, F. & Sanikhani, M. A facile one-step green hydrothermal synthesis of paramagnetic Fe3O4 nanoparticles with highly efficient dye removal. J. Taiwan Inst. Chem. Eng. 144, 104774 (2023).
Kurnia Sari, E. et al. Green synthesize of magnetically separable and reusable Fe3O4/cdots nanocomposites photocatalyst utilizing Moringa oleifera extract and watermelon peel for rapid dye degradation. Carbon Resour. Convers. https://doi.org/10.1016/j.crcon.2023.04.003 (2023).
Zong, Y. et al. One-pot, template- and surfactant-free solvothermal synthesis of high-crystalline Fe3O4 nanostructures with adjustable morphologies and high magnetization. J. Magn. Magn. Mater. 423, 321–326 (2017).
Iida, H., Takayanagi, K., Nakanishi, T. & Osaka, T. Synthesis of Fe3O4 nanoparticles with various sizes and magnetic properties by controlled hydrolysis. J. Colloid Interface Sci. 314, 274–280 (2007).
Ghanbari, D., Salavati-Niasari, M. & Ghasemi-Kooch, M. A sonochemical method for synthesis of Fe3O4 nanoparticles and thermal stable PVA-based magnetic nanocomposite. J. Ind. Eng. Chem. 20, 3970–3974 (2014).
Azizabadi, O., Akbarzadeh, F., Danshina, S., Chauhan, N. P. S. & Sargazi, G. An efficient ultrasonic assisted reverse micelle synthesis route for Fe3O4@Cu-MOF/core–shell nanostructures and its antibacterial activities. J. Solid State Chem. 294, 121897 (2021).
Chen, Z., Du, Y., Li, Z., Yang, K. & Lv, X. Controllable synthesis of magnetic Fe3O4 particles with different morphology by one-step hydrothermal route. J. Magn. Magn. Mater. 426, 121–125 (2017).
Karimzadeh, I., Dizaji, H. R. & Aghazadeh, M. Development of a facile and effective electrochemical strategy for preparation of iron oxides (Fe3O4 and γ-Fe2O3) nanoparticles from aqueous and ethanol mediums and in situ PVC coating of Fe3O4 superparamagnetic nanoparticles for biomedical applications. J. Magn. Magn. Mater. 416, 81–88 (2016).
Hong, R. Y. et al. Preparation and characterization of Fe3O4/polystyrene composite particles via inverse emulsion polymerization. J. Alloys Compd. 476, 612–618 (2009).
Cui, H., Li, D. & Zhang, Z. Preparation and characterization of Fe3O4 magnetic nanoparticles modified by perfluoropolyether carboxylic acid surfactant. Mater. Lett. 143, 38–40 (2015).
Zhang, Y., Chai, C. P., Luo, Y. J., Wang, L. & Li, G. P. Synthesis, structure and electromagnetic properties of mesoporous Fe3O4 aerogels by sol–gel method. Mater. Sci. Eng. B 188, 13–19 (2014).
Muniz-Miranda, M., Gellini, C., Giorgetti, E. & Margheri, G. Bifunctional Fe3O4/Ag nanoparticles obtained by two-step laser ablation in pure water. J. Colloid Interface Sci. 489, 100–105 (2017).
Radoń, A., Drygała, A., Hawełek, Ł & Łukowiec, D. Structure and optical properties of Fe3O4 nanoparticles synthesized by co-precipitation method with different organic modifiers. Mater. Charact. 131, 148–156 (2017).
Li, W.-H. & Yang, N. Green and facile synthesis of Ag–Fe3O4 nanocomposites using the aqueous extract of Crataegus pinnatifida leaves and their antibacterial performance. Mater. Lett. 162, 157–160 (2016).
Ebrahimi, A., Samari, F., Eftekhar, E. & Yousefinejad, S. Rapid and efficient colorimetric sensing of clindamycin and Fe3+ using controllable phyto-synthesized silver/silver chloride nanoparticles by Syzygium cumini fruit extract. J. Anal. Sci. Technol. 13, 10 (2022).
Prasad, C., Gangadhara, S. & Venkateswarlu, P. Bio-inspired green synthesis of Fe3O4 magnetic nanoparticles using watermelon rinds and their catalytic activity. Appl. Nanosci. 6, 797–802 (2016).
Kamali, M., Samari, F. & Sedaghati, F. Low-temperature phyto-synthesis of copper oxide nanosheets: Its catalytic effect and application for colorimetric sensing. Mater. Sci. Eng. C 103, 109744 (2019).
Rajesh Kumar, S., Jayavignesh, V., Selvakumar, R., Swaminathan, K. & Ponpandian, N. Facile synthesis of yeast cross-linked Fe3O4 nanoadsorbents for efficient removal of aquatic environment contaminated with As(V). J. Colloid Interface Sci. 484, 183–195 (2016).
El-Kassas, H. Y., Aly-Eldeen, M. A. & Gharib, S. M. Green synthesis of iron oxide (Fe3O4) nanoparticles using two selected brown seaweeds: Characterization and application for lead bioremediation. Acta Oceanol. Sin. 35, 89–98 (2016).
Zhang, N. et al. Bacteria-inspired fabrication of Fe3O4—Carbon/graphene foam for lithium-ion battery anodes. Electrochim. Acta 223, 39–46 (2017).
Ding, C., Cheng, W., Sun, Y. & Wang, X. Novel fungus-Fe3O4 bio-nanocomposites as high performance adsorbents for the removal of radionuclides. J. Hazard. Mater. 295, 127–137 (2015).
Weng, X. et al. Removal of doxorubicin hydrochloride using Fe3O4 nanoparticles synthesized by euphorbia cochinchinensis extract. Chem. Eng. J. 353, 482–489 (2018).
Chankaew, C. et al. Kaffir lime leaf extract mediated synthesis, anticancer activities and antibacterial kinetics of Ag and Ag/AgCl nanoparticles. Particuology 40, 160–168 (2018).
Salgado, P., Márquez, K., Rubilar, O., Contreras, D. & Vidal, G. The effect of phenolic compounds on the green synthesis of iron nanoparticles (FexOy-NPs) with photocatalytic activity. Appl. Nanosci. 9, 371–385 (2019).
Nasiri, J., Rahimi, M., Hamezadeh, Z., Motamedi, E. & Naghavi, M. R. Fulfillment of green chemistry for synthesis of silver nanoparticles using root and leaf extracts of Ferula persica: Solid-state route vs. solution-phase method. J. Clean. Prod. 192, 514–530 (2018).
Al-Ati, T. Assyrian plum (Cordia myxa L.). in Postharvest Biology and Technology of Tropical and Subtropical Fruits 116–126e (Elsevier, 2011). https://doi.org/10.1533/9780857092762.116.
Matias, E. F. F. et al. The genus cordia: Botanists, ethno, chemical and pharmacological aspects. Rev. Bras. Farmacogn. 25, 542–552 (2015).
Ghohestani, E., Samari, F. & Yousefinejad, S. An efficient removal of methylene blue and lead(II) from aqueous solutions by green synthesized iron oxide/pillared bentonite nanocomposite. Mater. Chem. Phys. 287, 126266 (2022).
Lopez-Ramon, M. V., Stoeckli, F., Moreno-Castilla, C. & Carrasco-Marin, F. On the characterization of acidic and basic surface sites on carbons by various techniques. Carbon N. Y. 37, 1215–1221 (1999).
Asfaram, A., Ghaedi, M., Hajati, S., Goudarzi, A. & Dil, E. A. Screening and optimization of highly effective ultrasound-assisted simultaneous adsorption of cationic dyes onto Mn-doped Fe3O4—Nanoparticle-loaded activated carbon. Ultrason. Sonochem. 34, 1–12 (2017).
Kumar, B. et al. Phytosynthesis and photocatalytic activity of magnetite (Fe3O4) nanoparticles using the Andean blackberry leaf. Mater. Chem. Phys. 179, 310–315 (2016).
Pan, S., Zhang, Y., Shen, H. & Hu, M. An intensive study on the magnetic effect of mercapto-functionalized nano-magnetic Fe3O4 polymers and their adsorption mechanism for the removal of Hg(II) from aqueous solution. Chem. Eng. J. 210, 564–574 (2012).
Arokiyaraj, S. et al. Enhanced antibacterial activity of iron oxide magnetic nanoparticles treated with Argemone mexicana L. leaf extract: An in vitro study. Mater. Res. Bull. 48, 3323–3327 (2013).
Gomez-Caballero, L. F. et al. Facile synthesis of Fe3O4 nanoparticles at room temperature coated with meso-2,3-dimercaptosuccinic acid for improved biocompatibility. J. Nanoparticle Res. 25, 66 (2023).
Sebeia, N., Jabli, M. & Ghith, A. Biological synthesis of copper nanoparticles, using Nerium oleander leaves extract: Characterization and study of their interaction with organic dyes. Inorg. Chem. Commun. 105, 36–46 (2019).
Prasad, C., Sreenivasulu, K., Gangadhara, S. & Venkateswarlu, P. Bio inspired green synthesis of Ni/Fe3O4 magnetic nanoparticles using Moringa oleifera leaves extract: A magnetically recoverable catalyst for organic dye degradation in aqueous solution. J. Alloys Compd. 700, 252–258 (2017).
Malek, N. N. A., Jawad, A. H., Ismail, K., Razuan, R. & ALOthman, Z. A. Fly ash modified magnetic chitosan-polyvinyl alcohol blend for reactive orange 16 dye removal: Adsorption parametric optimization. Int. J. Biol. Macromol. 189, 464–476 (2021).
Nasrollahzadeh, M., Mohammad Sajadi, S., Rostami-Vartooni, A. & Khalaj, M. Green synthesis of Pd/Fe3O4 nanoparticles using Euphorbia condylocarpa M. bieb root extract and their catalytic applications as magnetically recoverable and stable recyclable catalysts for the phosphine-free Sonogashira and Suzuki coupling reactions. J. Mol. Catal. A Chem. 396, 31–39 (2015).
Mashjoor, S., Yousefzadi, M., Zolgharnain, H., Kamrani, E. & Alishahi, M. Organic and inorganic nano-Fe3O4: Alga Ulva flexuosa-based synthesis, antimicrobial effects and acute toxicity to briny water rotifer Brachionus rotundiformis. Environ. Pollut. 237, 50–64 (2018).
Tran, H. N., Wang, Y.-F., You, S.-J. & Chao, H.-P. Insights into the mechanism of cationic dye adsorption on activated charcoal: The importance of π–π interactions. Process. Saf. Environ. Prot. 107, 168–180 (2017).
Patra, J. K. & Baek, K.-H. Green biosynthesis of magnetic iron oxide (Fe3O4) nanoparticles using the aqueous extracts of food processing wastes under photo-catalyzed condition and investigation of their antimicrobial and antioxidant activity. J. Photochem. Photobiol. B Biol. 173, 291–300 (2017).
Harifi, T. & Montazer, M. A novel magnetic reusable nanocomposite with enhanced photocatalytic activities for dye degradation. Sep. Purif. Technol. 134, 210–219 (2014).
Long, Z. et al. Hydrothermal synthesis of graphene oxide/multiwalled carbon nanotube/Fe3O4 ternary nanocomposite for removal of Cu(II) and methylene blue. J. Nanoparticle Res. 19, 318 (2017).
Uddin, M. T., Islam, M. A., Mahmud, S. & Rukanuzzaman, M. Adsorptive removal of methylene blue by tea waste. J. Hazard. Mater. 164, 53–60 (2009).
Liu, Y., Zheng, Y. & Wang, A. Enhanced adsorption of Methylene Blue from aqueous solution by chitosan-g-poly (acrylic acid)/vermiculite hydrogel composites. J. Environ. Sci. 22, 486–493 (2010).
Deng, H., Yang, L., Tao, G. & Dai, J. Preparation and characterization of activated carbon from cotton stalk by microwave assisted chemical activation—Application in methylene blue adsorption from aqueous solution. J. Hazard. Mater. 166, 1514–1521 (2009).
Es’haghi, Z., Vafaeinezhad, F. & Hooshmand, S. Green synthesis of magnetic iron nanoparticles coated by olive oil and verifying its efficiency in extraction of nickel from environmental samples via UV–Vis spectrophotometry. Process. Saf. Environ. Prot. 102, 403–409 (2016).
Prasad, C. et al. Bio-inspired green synthesis of RGO/Fe3O4 magnetic nanoparticles using Murrayakoenigii leaves extract and its application for removal of Pb(II) from aqueous solution. J. Environ. Chem. Eng. 5, 4374–4380 (2017).
Bhatti, A. A., Oguz, M. & Yilmaz, M. Magnetizing calixarene: Azo dye removal from aqueous media by Fe3O4 nanoparticles fabricated with carboxylic-substituted calix [4] arene. J. Chem. Eng. Data 62, 2819–2825 (2017).
Lagergren, S. About the theory of so-called adsorption of soluble substances. K. Sven. Vetenskapsakademiens Handl. 24, 1–39 (1898).
Zhao, Y.-G., Shen, H.-Y., Pan, S.-D. & Hu, M.-Q. Synthesis, characterization and properties of ethylenediamine-functionalized Fe3O4 magnetic polymers for removal of Cr(VI) in wastewater. J. Hazard. Mater. 182, 295–302 (2010).
Ho, Y. & McKay, G. Pseudo-second order model for sorption processes. Process. Biochem. 34, 451–465 (1999).
Shen, H. et al. New Insights into the sorption and detoxification of chromium(VI) by tetraethylenepentamine functionalized nanosized magnetic polymer adsorbents: Mechanism and pH effect. Ind. Eng. Chem. Res. 52, 12723–12732 (2013).
Pan, S., Shen, H., Xu, Q., Luo, J. & Hu, M. Surface mercapto engineered magnetic Fe3O4 nanoadsorbent for the removal of mercury from aqueous solutions. J. Colloid Interface Sci. 365, 204–212 (2012).
Sebastian, A., Nangia, A. & Prasad, M. N. V. A green synthetic route to phenolics fabricated magnetite nanoparticles from coconut husk extract: Implications to treat metal contaminated water and heavy metal stress in Oryza sativa L.. J. Clean. Prod. 174, 355–366 (2018).
Zhao, Y.-G., Shen, H.-Y., Pan, S.-D., Hu, M.-Q. & Xia, Q.-H. Preparation and characterization of amino-functionalized nano-Fe3O4 magnetic polymer adsorbents for removal of chromium(VI) ions. J. Mater. Sci. 45, 5291–5301 (2010).
Nyankson, E. et al. Characterization and evaluation of zeolite A/Fe3O4 Nanocomposite as a potential adsorbent for removal of organic molecules from wastewater. J. Chem. 2019, 1–13 (2019).
Pandian, C. J., Palanivel, R. & Dhananasekaran, S. Green synthesis of nickel nanoparticles using Ocimum sanctum and their application in dye and pollutant adsorption. Chin. J. Chem. Eng. 23, 1307–1315 (2015).
Saruchi, M., Thakur, P. & Kumar, V. Kinetics and thermodynamic studies for removal of methylene blue dye by biosynthesize copper oxide nanoparticles and its antibacterial activity. J. Environ. Heal. Sci. Eng. 17, 367–376 (2019).
Ramesh, A. V., Rama Devi, D., Mohan Botsa, S. & Basavaiah, K. Facile green synthesis of Fe3O4 nanoparticles using aqueous leaf extract of Zanthoxylum armatum DC. For efficient adsorption of methylene blue. J. Asian Ceram. Soc. 6, 145–155 (2018).
Ramesh, A. V. et al. A facile plant mediated synthesis of magnetite nanoparticles using aqueous leaf extract of Ficus Hispida L. for adsorption of organic dye. IOSR J. Appl. Chem. 10, 35–43 (2017).
Allafchian, A., Mousavi, Z. S. & Hosseini, S. S. Application of cress seed musilage magnetic nanocomposites for removal of methylene blue dye from water. Int. J. Biol. Macromol. 136, 199–208 (2019).
Singh, K. K., Senapati, K. K. & Sarma, K. C. Synthesis of superparamagnetic Fe3O4 nanoparticles coated with green tea polyphenols and their use for removal of dye pollutant from aqueous solution. J. Environ. Chem. Eng. 5, 2214–2221 (2017).
Acknowledgements
The authors thank the Hormozgan University Research Council for support of this work.
Author information
Authors and Affiliations
Contributions
F.S. was the supervisor of work in all steps such as design of study, data collection and management, and manuscript preparation. Experimental was mainly done by E.G. and some contribution was also done by A.H. Inclusion/exclusion of articles was done by all authors’ contribution. The preparation of the main draft of manuscript and figures/Tables was done by F.S and E.G. and was edited by S. Yousefinejad. All author had contribution in revision and correction of the final version.
Corresponding author
Ethics declarations
Competing interests
The authors confirm that there are no conflicts of interest associated with this publication.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ghoohestani, E., Samari, F., Homaei, A. et al. A facile strategy for preparation of Fe3O4 magnetic nanoparticles using Cordia myxa leaf extract and investigating its adsorption activity in dye removal. Sci Rep 14, 84 (2024). https://doi.org/10.1038/s41598-023-50550-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-50550-1
- Springer Nature Limited
This article is cited by
-
Fabrication of a Fe3O4/CS/AgNPs Composite from Indigenous Iron Sand for Enhanced Methylene Blue Adsorption
Journal of Cluster Science (2024)