Abstract
Climate change has led to an alarming increase in the frequency and severity of wildfires worldwide. While it is known that amphibians have physiological characteristics that make them highly susceptible to fire, the specific impacts of wildfires on their symbiotic skin bacterial communities (i.e., bacteriomes) and infection by the deadly chytrid fungus, Batrachochytrium dendrobatidis, remain poorly understood. Here, we address this research gap by evaluating the effects of fire on the amphibian skin bacteriome and the subsequent risk of chytridiomycosis. We sampled the skin bacteriome of the Neotropical species Scinax squalirostris and Boana leptolineata in fire and control plots before and after experimental burnings. Fire was linked with a marked increase in bacteriome beta dispersion, a proxy for skin microbial dysbiosis, alongside a trend of increased pathogen loads. By shedding light on the effects of fire on amphibian skin bacteriomes, this study contributes to our broader understanding of the impacts of wildfires on vulnerable vertebrate species.
Similar content being viewed by others
Introduction
Current climate change scenarios predict increased temperatures, more variable rainfall patterns, and more intense extreme weather events like wildfires1. In fire-adapted habitats, wildfires can occasionally contribute to an increase in biodiversity2. However, when they take place in fire-naive habitats, wildfires become a matter of critical conservation concern3. Controlled fire has been utilized as a preventive measure to effectively burn surplus organic matter (i.e., fuel) in natural ecosystems. The primary objective of this approach is to decrease the frequency of fire occurrences, thereby minimizing the risk of devastating wildfires4. Additionally, prescribed fire has been employed to manage seasonal weed growth in agricultural environments, although this particular practice is considered illegal in numerous countries5. Fire can dramatically impact vertebrate physiology, making animals more susceptible to diseases. Habitat loss and degradation caused by fires likely impact the immune defenses of animals. Change in host behavior and population demographics due to fires can also influence pathogen exposure and spread6. Nonetheless, little research has incorporated the cascading impacts of fire on wildlife health and disease risk. Considering progressing climate change scenarios and increasing agricultural expansion, fires are predicted to more frequently impact wildlife communities that are not adapted to this type of disturbance7.
Amphibians have received relatively little research attention in the field of fire ecology compared to other vertebrates8. Most amphibian species depend on permanent and temporary water bodies to complete their life cycle, are ectothermic, and have a permeable skin for osmoregulation; all of which compound their sensitivity to environmental disturbances 9. Fires can cause major disturbance to microhabitats by reducing humidity and increasing temperature variability10, and are thus likely to dramatically impact the amphibian immune system. Changes in the environmental pool of microorganisms due to fire11,12 could also affect the recruitment of skin-associated microbial taxa able to shield hosts from invading pathogens13. Fire also induces substantial alterations in arthropod communities within the environment14, potentially leading to modified dietary intake patterns that subsequently diminish the ability of amphibians to combat diseases15.
The amphibian skin microbiome is composed of the community of symbiotic microorganisms and their metabolic products16 and can help regulate metabolism, influence development, and mediate immune and stress responses17. The microbiome also plays an important role in amphibian health through its role in defense against chytridiomycosis, a disease caused by the chytrid fungus Batrachochytrium dendrobatidis (Bd)17. This pathogen has led to widespread amphibian population declines and extinctions, including in the Neotropics18, but relatively little work has examined the impact of fire on amphibian host–pathogen interactions. One field study found that boreal toads from areas that have experienced natural fires have lower Bd loads, indicating that natural fires may hamper Bd’s optimal microclimates and thus could decrease the likelihood of infection19. Conversely, a recent observational study found that wildfires could instead increase disease risk in salamanders by altering their protective skin microbiomes20. Thus, controlled experimental studies are sorely needed to disentangle the links between fires, microbiome, and disease risk in wildlife.
In this study, we experimentally tested whether controlled burning alters Bd infection, and symbiotic skin bacterial composition, diversity and beta dispersion (a proxy for bacterial community stochasticity) in Neotropical amphibians sampled at a highland grassland environment over more than one year. One of our hypotheses was that controlled burning, which is commonly employed in our focal study landscape, could directly hamper the presence of Bd in the environment, consequently reducing the risk of infection in amphibian hosts. However, in the event that burning lowers the protective function of the amphibian skin bacteriome, disrupting their immune capacity, we would expect a rise in Bd infections associated with controlled burning. To examine these divergent hypotheses, we collected samples from two species of endemic treefrogs at experimental fire plots and control plots, both before and after fire treatment. Our study illuminates the consequences of controlled burning and “escaped” controlled fires on the biodiversity of fragmented agricultural landscapes, highlighting implications for the conservation of tropical amphibians.
Methods
Field methods
This study was carried out in an area within the lower extent of the Brazilian Atlantic Forest. The area, located in the municipality of São Francisco de Paula (29° 27′–29° 35′ S, 50° 08′–50° 15′ W), state of Rio Grande do Sul, is characterized by a mosaic of natural grasslands and subtemperate forests. The study sites are located within the Center for Research and Conservation of Nature Pró-Mata (CPCN Pró-Mata), a preserved area covering approximately 4500 ha. The local climate is classified as super-humid temperate, with rainfall well distributed throughout the year reaching 1700–2000 mm and an annual average temperature between 14 and 17 °C21. Although controlled burning is a regulated field management practice in the region through Law No. 11,498 of July 4, 2000, illegal burning practices have been employed frequently when carried out without authorization issued by the competent environmental agency, outside the regulations, or without limitations22.
We collected skin samples from two treefrog species, the fine-lined treefrog, Boana leptolineata and the long-snouted treefrog, Scinax squalirostris (both from the Hylidae family), using rayon swabs (Medical Wire), after rinsing animals with sterile water to minimize sampling of transient microorganisms, following standard methods23. After sampling, swabs were stored at − 20 °C in the field, then transported to the lab on ice and stored at − 80 °C. Frogs were sampled within 14 quadrats (70 × 70 m) located in grassland areas (Fig. 1). We took advantage of an ongoing controlled burning activity that exposed six plots to a controlled burn (after our baseline sampling), while eight plots were maintained as unburned controls over the entire sampling period. Burned plots were outlined with firebreaks to keep fires contained. We carried out field campaigns September through December 2021, and January through March 2022. Burned plots 1 and 4 were burned in December 2021 and plots 5A, 5B, 6A, and 6B in February 2022 (Fig. 1). Fire plots were sampled one month after burning.
Molecular methods
After fieldwork was completed, we extracted DNA from all skin swabs at Universidade do Vale do Rio dos Sinos, São Leopoldo, RS, Brazil using spin-column IBI extraction kits, following the standard protocol. For qPCR analysis we diluted DNA 1:10 and quantified Bd loads using Taqman qPCR assays on ITS and 5.8S genes24 and gBlock synthetic Bd standards diluted from 106 to 102 gene copies (gc). We ran plates in duplicate, with mismatching samples run on a triplicate plate. Only samples that were positive on 2 plates were recorded as positive. We averaged Bd loads across the duplicate plates and log10 transformed load values to account for non-normal residual distributions characteristic in models including pathogen load data as the response variable.
For metabarcoding bacterial communities from skin swabs, we followed the Earth Microbiome Project 16S Illumina Amplicon Protocol25,26, targeting the V4 region of the bacterial 16S rRNA gene using a dual-index approach with 515F and 806R barcoded primers. We PCR-amplified DNA extracted from skin swabs in duplicate plates using the following recipe per sample: 12.2 µL of UltraPure water, 4 µL of 5X Phire reaction buffer (Thermo Scientific), 0.4 µL of 2.5 mM dNTPs (Invitrogen), 0.4 µL of Phire Hot Start II DNA Polymerase (Thermo Scientific), 0.5 µL each of 10 µM barcoded forward and reverse primers (Integrated DNA Technologies), and 2 µL of sample DNA. We ran duplicate PCR plates on SimpliAmp thermal cyclers (Thermo Scientific) according to the following protocol: 98 °C for 3 min, 38 cycles of 98 °C for 5 s, 50 °C for 5 s, and 72 °C for 15 s, then 72 °C for 3 min before holding at 12 °C. We included a negative control (water without template DNA) in each plate to monitor any potential contamination of PCR reagents. We combined duplicate plates and visualized amplicons in 1% agarose gel to confirm DNA amplification, which revealed highly variable amplification among samples. We re-ran all poorly amplifying samples with doubled DNA concentration (4 µL instead of 2 µL) and halved DNA concentration (1:2 dilution) to account for low DNA concentrations and PCR inhibition. We then quantified DNA concentration for each sample using the Qubit 2.0 fluorometer with a dsDNA High-Sensitivity Assay Kit (Invitrogen) and pooled equimolar amounts of each sample (~ 10 nM) into a single amplicon library. We purified the library using a QIAquick Gel Extraction Kit (Qiagen), then measured amplicon library concentration using the Qubit 2.0 fluorometer with a dsDNA Broad-Range Assay Kit (Invitrogen). Concentrations of the purified library was 45.7 nM (11.1 ng/µL). The 16S library was sequenced using Illumina MiSeq platform using standard manufacturer protocols.
Bioinformatics and data processing
We received demultiplexed bacterial sequences from the sequencing facility, then imported forward and reverse reads for each sample into Quantitative Insights into Microbial Ecology II (QIIME2 version 2021.11). We used QIIME2 to generate amplicon sequence variant (ASV) tables and extract metrics of alpha and beta diversity for bacterial microbiomes. Prior to analyzing sequence data, we used the DADA2 paired-end pipeline to trim sequences to 250 bp based on quality scores and cluster sequences into ASVs. We used the SILVA 138 classifier to assign taxonomy to ASVs at 99% sequence similarity. We filtered out chloroplast and mitochondrial sequences then rarefied the ASV table to 4000 reads based on rarefaction curves (Figure S1), resulting in 26 of 262 samples excluded, including all PCR controls. For analyses of alpha diversity, we calculated the ASV richness for each sample. For analyses of beta diversity, we calculated Unweighted UniFrac dissimilarity (UU) and Bray–Curtis dissimilarity (BC) between samples, and used the first principal coordinate axis (PCo1) in analysis. We calculated beta dispersion, a community stochasticity metric, that has gained recognition as a proxy for microbiome dysbiosis27,28 using UU and BC distance matrices partitioned by treatment, which measures the relative distance from each individual bacteriome community to the centroid of each treatment in multidimensional space (betadisper function from vegan package in program R, version 4.2.229,30.
Statistical analyses
We used Zero-inflated Negative Binomial Models (ZINB) to test the effect of experimental fire on Bd infection loads (Bd ITS counts). ZINB models provide a distinct advantage as they eliminate the need for running separate models for proportion of infected individuals and infection loads (only including Bd-positive individuals and thus reducing sample size). This consolidated approach enhances our statistical power, allowing for a more robust analysis compared to having to run two distinct models. In our ZINB model, we considered the individual and interactive effects of treatment (control or burned) and timepoint (pre- and post-fire) as fixed effects, and sampling plot as a random effect. A generalized linear mixed model (GLMM) with binomial probability distribution and logit link is also available as supplementary information.
We used generalized linear mixed models (GLMMs) with normal probability distribution and identity link function to test for the effect of experimental fire on host skin bacteriome diversity (ASV richness) and beta dispersion (UU and BC beta dispersion). In these models, we considered the individual and interactive effects of treatment (control or burned) and time point (pre- and post-fire) as fixed effects, Bd loads (log10-transformed) as a fixed effect, and sampling plot as a random effect.
Using permutational analysis of variance (PERMANOVA; adonis2 function in vegan package29) on UU and BC dissimilarity matrices, we tested for differences in the skin bacteriome community between treatments, timepoints, and their interaction. We conducted PERMANOVAs separately for each host species. We ran models in R version 4.2.230.
Using the linear discriminant analysis effect size (LEfSe) method on the galaxy platform31 we tested for differentially abundant bacterial ASVs between treatments and timepoints for both host species. We ran analyses maintaining default parameters.
Ethics declaration
All experiments were performed in accordance with relevant Federal sampling permits (SISBio #78625-1; SISGen #A7080A8). Experimental protocols were approved by Universidade do Vale do Rio dos Sinos (Unisinos) and the local animal care committee (Comissão de Ética no Uso de Animal—PPECEUA0 #02.2021). The study adhered to the ARRIVE guidelines, providing a comprehensive account of the ten essential elements necessary for describing animal research that enables reviewers to effectively evaluate the credibility our study. All animals were released at the capture location following non-invasive swabbing.
Results
Overall Bd prevalence was 12.2% for Scinax squalirostris and 9.2% for Boana leptolineata. Average infection loads for the entire sampling population were 6,670 ± 46,579 for S. squalirostris (N = 114) and 6,970 ± 52,610 for B. leptolineata (N = 72). Average loads for Bd-positive individuals were 58,489 ± 130,948 for S. squalirostris (N = 13) and 82,489 ± 176,847 for B. leptolineata (N = 6). Our ZINB model indicated a significant increase in Bd infection loads in individuals of S. squalirostris after fire plots were experimentally burned, whereas in the unburned control plots, Bd loads showed a decline trend over time (fire treatment x timepoint: Z = 3.269, P = 0.001; Fig. 2; Table S1; Table S2; Fig. S1). Despite the statistical significance of these findings, we consider these results as indicative trends due to the limited number of Bd-positive frogs in both treatments. Fire treatment had no effect on Bd loads of B. leptolineata (Table S1; Fig. S2), and we found no significant effect of fire treatment, timepoint, and their one-level interaction on Bd prevalence of both focal amphibian species using GLMMs (Table S3).
After filtering and rarefaction, we detected a total of 2,406 unique ASVs, with an average of 155 ± 106 from S. squalirostris and 198 ± 135 from B. leptolineata. These ASVs were primarily in the phyla Proteobacteria, Actinobacteriota, Bacteroidota, Acidobacteriota, Planctomycetota, and Firmicutes (Fig. 3). We found no significant effect of treatment, timepoint, and their one-level interaction on ASV richness for either host species (Table S5).
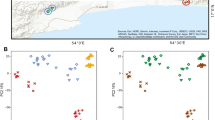
We found significant differences in skin bacteriome composition using permutational analysis of variance (PERMANOVA) on UU distance matrices between treatments, and timepoints, but not their interaction for S. squalirostris and B. leptolineata (Table S6; Fig. 4). We found similar differences in skin bacteriome composition when using BC matrices (Table S6; Fig. S3). We did not detect any differentially abundant bacteria between treatments or timepoints for either host species using LEfSe analysis.
Plots showing differences in bacteriome composition between treatments. Spider plots show unweighted UniFrac skin bacteriome similarity for Scinax squalirostris (A) and Boana leptolineata (B). Centroids indicate average bacteriome composition for each timepoint within each treatment. Average dispersion between treatments over time indicate an increase in bacteriome beta dispersion, a proxy for microbiome dysbiosis, for both B. leptolineata (C) and S. squalirostris (D). Connecting lines visually highlight changes in microbiome dispersion and do not represent regression fit.
We found a significant influence of the interaction between treatment and timepoint on UU bacteriome beta dispersion for S. squalirostris (Table 1; Fig. 4C). We found a similar, but non-significant trend, for B. leptolineata (Table S7; Fig. 4D). Finally, we found no associations between bacteriome beta dispersion and treatment, timepoint, or their interaction for S. squalirostris and B. leptolineata when using BC dispersion as response variable; (Table S8; Fig. S4 C, D).
Discussion
Throughout history, fire has exerted its influence on ecological communities and plays a pivotal role in shaping biodiversity. However, the impact of fire has expanded beyond its traditional boundaries due to human activities such as land use change and global warming. These factors have heightened the frequency and severity of fires, resulting in their unprecedented effects on biodiversity and ecosystem function8. In this field experiment, we found links between controlled burning, higher pathogen loads, and skin microbiome disruption in an endemic Neotropical treefrog. Specifically, we detected a marked increase in bacteriome beta dispersion and higher Bd loads in individuals of Scinax squalirostris after individual plots were burned.
Fire is a driver of biodiversity turnover globally, shaping communities and ecosystems 10. Areas that experience regular fire exhibit high levels of endemism, making fire, in conjunction with factor such as climate, resource availability, and environmental variation, a significant catalyst behind the richness of species in these regions32,33. Natural fire regimes creates conditions for plant species to thrive and reproduce34, create unique habitats where specialization can emerge35 and maintain a diversity of ecosystems36. Out of context, however, fire can have devastating impacts on ecosystems. Climate change and human-induced fires are altering fire regimes and bringing fire to places that are not fire-adapted, such as forests in South America, West-central Africa, Southeast Asia and the Tundra at the Arctic Circle37,38,39. Regions that have a long history of recurrent fire have also witnessed the occurrence of larger and more severe fires, as seen in the boreal forests of Canada and Russia40,41, as well as mixed forests, shrublands, and grasslands in places like Australia, southern Europe, and the western United States42,43,44. In contrast, fire-dependent ecosystems like grasslands and savannas in Brazil and the United States have experienced a irregularity or exclusion of fire activity 45,46. These emerging changes pose global challenges in effectively preserving biodiversity.
Fire can lead to significant changes in ecosystems, altering vegetation structure, microclimates, and microbial communities in soil and plants11,12. One recent review study indicated that amphibian abundance, species richness, and individual behavior are also strongly influenced by fire47. Specifically, 26% of the reviewed studies showed negative effects of fire, including decreased species richness48, 20% showed positive effects such as increased abundance 49, and 47% showed no significant effects50. Most studies focused on North American taxa were conducted in fire-dependent landscapes. In a recent study by Mulla & Hernández-Gómez20, microbiome diversity of salamanders was higher in areas prone to recurrent wildfires, which could suggest colonization of opportunistic microbial taxa due to wildfires.
The combustion of vegetation and soil organic matter results in the production of ash, which can pose risks to amphibians due to its content of inorganic metals and organic polycyclic aromatic hydrocarbons. These substances are well-known for their significant toxicity, long-lasting nature, and ability to accumulate in biological systems51. Some studies have found that ashes can affect growth of bacteria in the skin microbiome of amphibians52,53. Fire-driven shifts in the skin microbiome of amphibians may in turn compromise immune system function and lead to an increased susceptibility to pathogenic infections or diseases.
Our study location is characterized by a cool, humid climate at 1000 m elevation, ideal conditions for Bd growth and transmission54. Despite this, we found very low Bd prevalence in our samples. We expected, according to a previous study, that fires could reduce Bd infection through shifting microenvironmental conditions beyond Bd’s optimal temperature range19. However, our ZINB model taking into account both shifts in baseline infection probability and Bd infection intensity pointed to a statistically significant increase in Bd loads in amphibians sampled from areas following controlled burning. While interpreting these findings with caution, our results indicate that Bd loads could still spike in amphibians through mechanisms other than suboptimal environmental conditions for Bd in post-fire environments. Our findings highlight that reduction in host immune capacity including stress and microbiome-related responses deserve further mechanistic investigation. Although we did not observe a significant link between fire and a reduction in bacteriome diversity as observed recently in salamanders20, the observed increase in bacteriome stochasticity is an indication that fire could indeed reduce microbiome resilience and anti-pathogen function27. Changes in the composition of the host microbiome after this type of environmental disturbances could also lead to sub-lethal suppression of amphibian immunity, increasing susceptibility to diseases55.
We found higher bacteriome beta dispersion for S. squalirostris in burned plots after experimental fires. Thus, our results add fire as a potential disturbance that can drive microbiome variability and, consequently, could lead to microbiome dysbiosis. Studies characterize dysbiosis as a disruption of the relationships between microbiome and host that may negatively impact host health56. We did not evaluate functional changes in the microbiome or aspects of host health because bacterial culturing and challenge assays with Bd were beyond the scope of our study. Although further studies are needed to unravel all the mechanisms driving the observed pattern of high microbiome stochasticity post-fire (and the Anna Karenina theory of microbiome ecology), considering beta dispersion as a proxy for dysbiosis has gained growing recognition and acceptance27,57,58.
Indeed, diverging microbiome community composition among individuals occupying the same environment could reflect other biological processes. For instance, reduction in habitat complexity or host movement could significantly impact exposure and microbial recruitment from divergent environmental pools, not necessarily reflecting dysbiosis. Our focal study species are commonly found foraging and vocalizing on grasses and shrubs59,60, which suggests that they could potentially avoid contact with soil microbial reservoirs in typical grassland conditions. After fire disturbance, we observed that grasses were completely burned, driving amphibians using those habitats to move over soil to cross the remnant vegetation (woody shrubs that were not completely burned). This change in behavior may have led to a change in the dynamics of microbial recruitment of our focal amphibian species. Additionally, burning likely causes significant changes in environmental soil chemistry and microclimates10, and drive nutrient runoff into amphibian breeding sites61,62,63. Even if we disregard changes in amphibian behavior, fire can alter environmental microbiomes, especially in plants and soil11,12. All these factors would greatly alter microbial pools in the environment and subsequently shift composition of microbial communities that are filtered/recruited into the amphibian skin microbiome, considering that most of the bacterial ASVs of amphibian skin are shared with their perching environment13. Although we expect that fire should homogenize the environmental microbial pool, amphibians in the post-fire treatment still showed stochastic, unpredictable bacteriome assembly compared to the control group, further supporting the Anna Karenina principle and the observed high beta dispersion as proxy for microbiome disruption.
Chronic stress driven by environmental change could also suppress host pathogen defenses64. Burned sites were drier than control sites due to physical drying from the fire itself and the subsequent loss of plant cover65. Changes in water availability in burned areas could also be an added stressor for frogs. Amphibians experiencing the stress of water loss require high energy demands and have negative cardiovascular impacts66. Stress hormones like corticosterone mediates the physiological response to dehydration stress67 and are involved in water-seeking behavior68. Burned areas in our study may have reduced food quality and availability64, disrupting corticosterone balance69 to levels that are potentially immunosuppressive in amphibians70.
This study brings novel findings linking wildfires and amphibian bacteriome health. We show that intermittent burning could have hidden effects on biodiversity through disruption of host-associated symbionts. Over time, bacteriome disruption caused by fires could potentially impact amphibian population viability, especially when combined with additional stressors like habitat loss, disease and climate change, further threatening amphibians, one of the most vulnerable vertebrate taxa.
Data availability
Raw data used for analyses has been uploaded as a supplemental file. Any additional data is available upon request to Laura K Schuck (laurakauerschuck@gmail.com). Microbiome sequence reads generated in this study have been uploaded to the NCBI Sequence Read Archive (BioProject PRJNA999620).
References
Nimmo, D. G., Carthey, A. J., Jolly, C. J. & Blumstein, D. T. Welcome to the Pyrocene: Animal survival in the age of megafire. Glob. Change Biol. 27, 5684–5693 (2021).
Archibald, S. et al. Biological and geophysical feedbacks with fire in the earth system. Environ. Res. Lett. 13, 033003 (2018).
Kelly, L. T. et al. Fire and biodiversity in the Anthropocene. Science 370, eabb0355 (2020).
Fernandes, P. M. Empirical support for the use of prescribed burning as a fuel treatment. Curr. For. Rep. 1, 118–127 (2015).
Valkó, O., Török, P., Deák, B. & Tóthmérész, B. Prospects and limitations of prescribed burning as a management tool in European grasslands. Basic Appl. Ecol. 15, 26–33 (2014).
Albery, G. F. et al. From flames to inflammation: How wildfires affect patterns of wildlife disease. Fire Ecol. 17, 1–17 (2021).
Bush, M. B., Silman, M. R., McMichael, C. & Saatchi, S. Fire, climate change and biodiversity in Amazonia: A Late-Holocene perspective. Philos. Trans. R. Soc. B Biol. Sci. 363, 1795–1802 (2008).
Pastro, L. A., Dickman, C. R. & Letnic, M. Burning for biodiversity or burning biodiversity? Prescribed burn vs. wildfire impacts on plants, lizards, and mammals. Ecol. Appl. 21, 3238–3253 (2011).
Wells, K. D. The ecology and behavior of Amphibians (University of Chicago Press, 2019).
He, T., Lamont, B. B. & Pausas, J. G. Fire as a key driver of Earth’s biodiversity. Biol. Rev. 94, 1983–2010 (2019).
Dove, N. C., Klingeman, D. M., Carrell, A. A., Cregger, M. A. & Schadt, C. W. Fire alters plant microbiome assembly patterns: Integrating the plant and soil microbial response to disturbance. New Phytol. 230, 2433–2446 (2021).
Nelson, A. R. et al. Wildfire-dependent changes in soil microbiome diversity and function. Nat. Microbiol. 7, 1419–1430 (2022).
Rebollar, E. A. et al. The Skin microbiome of the Neotropical frog Craugastor fitzingeri: Inferring potential bacterial-host-pathogen interactions from metagenomic data. Front. Microbiol. 9, 466 (2018).
Bieber, B. V. et al. Increasing prevalence of severe fires change the structure of arthropod communities: Evidence from a meta-analysis. Funct. Ecol. 37, 2096–2109 (2023).
Venesky, M. D. et al. Dietary protein restriction impairs growth, immunity, and disease resistance in southern leopard frog tadpoles. Oecologia 169, 23–31 (2012).
Thaiss, C. A., Levy, M., Suez, J. & Elinav, E. The interplay between the innate immune system and the microbiota. Curr. Opin. Immunol. 26, 41–48 (2014).
Rebollar, E. A., Martínez-Ugalde, E. & Orta, A. H. The amphibian skin microbiome and its protective role against chytridiomycosis. Herpetologica 76, 167–177 (2020).
Scheele, B. C. et al. Amphibian fungal panzootic causes catastrophic and ongoing loss of biodiversity. Science 363, 1459–1463 (2019).
Hossack, B. R., Lowe, W. H., Ware, J. L. & Corn, P. S. Disease in a dynamic landscape: Host behavior and wildfire reduce amphibian chytrid infection. Biol. Conserv. 157, 293–299 (2013).
Mulla, L. & Hernández-Gómez, O. Wildfires disturb the natural skin microbiota of terrestrial salamanders. Environ. Microbiol. (2023).
Rossato, M. S. Os climas do Rio Grande do Sul: uma proposta de classificação climática. ENTRE-LUGAR 11, 57–85 (2020).
Pillar, V. D. P. & Lange, O. Os Campos do Sul (UFRGS, 2015).
Boyle, A. D. H. D. G. et al. Diagnostic assays and sampling protocols for the detection of Batrachochytrium dendrobatidis. Dis. Aquat. Organ. 73, 175–192 (2007).
Boyle, D. G., Boyle, D. B., Olsen, V., Morgan, J. A. & Hyatt, A. D. Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real-time Taqman PCR assay. Dis. Aquat. Organ. 60, 141–148 (2004).
Caporaso, J. G. et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 6, 1621–1624 (2012).
Kozich, J. J., Westcott, S. L., Baxter, N. T., Highlander, S. K. & Schloss, P. D. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the miseq illumina sequencing platform. Appl. Environ. Microbiol. 79, 5112–5120 (2013).
Neely, W. J. et al. Habitat Disturbance linked with host microbiome dispersion and Bd dynamics in temperate amphibians. Microb. Ecol. 84, 901–910 (2022).
Zaneveld, J. R., McMinds, R. & Vega Thurber, R. Stress and stability: Applying the Anna Karenina principle to animal microbiomes. Nat. Microbiol. 2, 1–8 (2017).
Oksanen, J. et al. Vegan: Community ecology package. R package version 2.5-7. https://CRAN.R-project.org/package=vegan (2017).
R Core Team. R: A language and environment for statistical computing (2022).
Chang, F., He, S. & Dang, C. Assisted selection of biomarkers by linear discriminant analysis effect size (LEfSe) in microbiome data. J. Vis. Exp. https://doi.org/10.3791/61715 (2022).
Rundel, P. W. et al. Mediterranean biomes: Evolution of their vegetation, floras, and climate. Annu. Rev. Ecol. Evol. Syst. 47, 383–407 (2016).
Fernandes, G. W. et al. The deadly route to collapse and the uncertain fate of Brazilian rupestrian grasslands. Biodivers. Conserv. 27, 2587–2603 (2018).
Rundel, P. W. et al. Fire and plant diversification in Mediterranean-climate regions. Front. Plant Sci. 9, 851 (2018).
Kelly, L. T. & Brotons, L. Using fire to promote biodiversity. Science 355, 1264–1265 (2017).
Pausas, J. G. & Bond, W. J. Alternative biome states in terrestrial ecosystems. Trends Plant Sci. 25, 250–263 (2020).
Chisholm, R. A., Wijedasa, L. S. & Swinfield, T. The need for long-term remedies for Indonesia’s forest fires. Conserv. Biol. 30, 5–6 (2016).
Barlow, J., Berenguer, E., Carmenta, R. & França, F. Clarifying Amazonia’s burning crisis. Glob. Change Biol. 26, 319–321 (2020).
Hu, F. S. et al. Arctic tundra fires: Natural variability and responses to climate change. Front. Ecol. Environ. 13, 369–377 (2015).
Whitman, E., Parisien, M.-A., Thompson, D. K. & Flannigan, M. D. Short-interval wildfire and drought overwhelm boreal forest resilience. Sci. Rep. 9, 18796 (2019).
Barrett, K. et al. Postfire recruitment failure in Scots pine forests of southern Siberia. Remote Sens. Environ. 237, 111539 (2020).
Schoennagel, T. et al. Adapt to more wildfire in western North American forests as climate changes. Proc. Natl. Acad. Sci. 114, 4582–4590 (2017).
Boer, M. M., Resco de Dios, V. & Bradstock, R. A. Unprecedented burn area of Australian mega forest fires. Nat. Clim. Change 10, 171–172 (2020).
Moreira, F. et al. Wildfire management in Mediterranean-type regions: Paradigm change needed. Environ. Res. Lett. 15, 011001 (2020).
Twidwell, D., Bielski, C. H., Scholtz, R. & Fuhlendorf, S. D. Advancing fire ecology in 21st century rangelands. Rangel. Ecol. Manag. 78, 201–212 (2021).
Andela, N. et al. A human-driven decline in global burned area. Science 356, 1356–1362 (2017).
dos Anjos, A. G., Solé, M. & Benchimol, M. Fire effects on anurans: What we know so far?. For. Ecol. Manag. 495, 119338 (2021).
Matthews, C. E., Moorman, C. E., Greenberg, C. H. & Waldrop, T. A. Response of reptiles and amphibians to repeated fuel reduction treatments. J. Wildl. Manag. 74, 1301–1310 (2010).
Brown, D. J., Baccus, J. T., Means, D. B. & Forstner, M. R. J. Potential positive effects of fire on juvenile amphibians in a Southern USA pine forest. J. Fish Wildl. Manag. 2, 135–145 (2011).
Engbrecht, N. J. & Lannoo, M. J. Crawfish frog behavioral differences in postburned and vegetated grasslands. Fire Ecol. 8, 63–76 (2012).
Chen, H. et al. Wildfire burn intensity affects the quantity and speciation of polycyclic aromatic hydrocarbons in soils. ACS Earth Space Chem. 2, 1262–1270 (2018).
Afonso, M. et al. Effects of pine and eucalypt ashes on bacterial isolates from the skin microbiome of the fire salamander (Salamandra salamandra). Sci. Total Environ. 841, 156677 (2022).
Coelho, L. et al. Effects of eucalypt ashes from moderate and high severity wildfires on the skin microbiome of the Iberian frog (Rana iberica). Environ. Pollut. 313, 120065 (2022).
Stevenson, L. A. et al. Variation in thermal performance of a widespread pathogen, the amphibian chytrid fungus Batrachochytrium dendrobatidis. PLOS One 8, e73830 (2013).
Brand, A. B., Snodgrass, J. W., Gallagher, M. T., Casey, R. E. & Van Meter, R. Lethal and sublethal effects of embryonic and larval exposure of hyla versicolor to stormwater pond sediments. Arch. Environ. Contam. Toxicol. 58, 325–331 (2010).
Croswell, A., Amir, E., Teggatz, P., Barman, M. & Salzman, N. H. Prolonged impact of antibiotics on intestinal microbial ecology and susceptibility to enteric salmonella infection. Infect. Immun. 77, 2741–2753 (2009).
Jiménez, R. R., Alvarado, G., Sandoval, J. & Sommer, S. Habitat disturbance influences the skin microbiome of a rediscovered Neotropical-montane frog. BMC Microbiol. 20, 292 (2020).
Greenspan, S. E. et al. Warming drives ecological community changes linked to host-associated microbiome dysbiosis. Nat. Clim. Change 10, 1057–1061 (2020).
Maneyro, R., Loebman, D. & Tozetti, A. M. Anfíbios das planícies costeiras do extremo sul do Brasil e Uruguai (Anolis Book, 2017).
Kwet, A., Lignau, R. & Di-Bernardo, M. Pró-Mata: Anfíbios da Serra Gaúcha, sul do Brasil (EDIPUCRS, 2010).
Pulido-Chavez, M. F., Alvarado, E. C., DeLuca, T. H., Edmonds, R. L. & Glassman, S. I. High-severity wildfire reduces richness and alters composition of ectomycorrhizal fungi in low-severity adapted ponderosa pine forests. For. Ecol. Manag. 485, 118923 (2021).
Whitman, T. et al. Soil bacterial and fungal response to wildfires in the Canadian boreal forest across a burn severity gradient. Soil Biol. Biochem. 138, 107571 (2019).
Brown, S. P. et al. Context dependent fungal and bacterial soil community shifts in response to recent wildfires in the Southern Appalachian Mountains. For. Ecol. Manag. 451, 117520 (2019).
Becker, C. G. et al. Habitat split as a driver of disease in amphibians. Biol. Rev. 98, 727–746 (2023).
Pilliod, D. S., Bury, R. B., Hyde, E. J., Pearl, C. A. & Corn, P. S. Fire and amphibians in North America. For. Ecol. Manag. 178, 163–181 (2003).
Hillman, S. S., Withers, P. C., Drewes, R. C. & Hillyard, S. D. Ecological and Environmental Physiology of Amphibians (Oxford University Press, 2008). https://doi.org/10.1093/acprof:oso/9780198570325.001.0001.
Uchiyama, M. & Konno, N. Hormonal regulation of ion and water transport in anuran amphibians. Gen. Comp. Endocrinol. 147, 54–61 (2006).
Madelaire, C. B. et al. Challenges of dehydration result in a behavioral shift in invasive toads. Behav. Ecol. Sociobiol. 74, 83 (2020).
Crespi, E. J. & Denver, R. J. Roles of stress hormones in food intake regulation in anuran amphibians throughout the life cycle. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 141, 381–390 (2005).
Rollins-Smith, L. A. Neuroendocrine-immune system interactions in amphibians. Immunol. Res. 23, 273–280 (2001).
Acknowledgements
We thank Gabriela Taíza de Souza, Amanda da Silva Paim, Natália Mendonça and Michele Machado Gonçalvez for field assistance. We also thank Victor Hugo Valiatti and his lab technicians for the help with extractions. This research was funded by Conselho Nacional de Desenvolvimento Científco e Tecnológico – CNPq PELD Pró-Mata and The National Science Foundation (DEB #2003523; IOS #1947681).
Author information
Authors and Affiliations
Contributions
L.K.S., A.M.T. and C.G.B. conceived the idea of the experiment. W.J.N. and C.G.B. performed the statistical analyses. L.K.S. wrote the manuscript with important contributions from all other authors. L.K.S., C.A.C., C.F.M. and P.B. conducted the experiment and the field campaigns. L.K.S., W.J.N. and S.M.B. performed the PCRs and qPCRs. L.K.S. conducted the DNA extraction with support from V.H.V. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schuck, L.K., Neely, W.J., Buttimer, S.M. et al. Effects of grassland controlled burning on symbiotic skin microbes in Neotropical amphibians. Sci Rep 14, 959 (2024). https://doi.org/10.1038/s41598-023-50394-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-50394-9
- Springer Nature Limited