Abstract
Majority of previous studies on alveolar ridge preservation (ARP) used collagen membranes as barrier membranes, and further evidence for ARP in dehiscent extraction sockets with a deproteinized bovine bone mineral (DBBM) and matrix is needed. The aim of this study is to assess the impact of non-cross linked collagen membranes (membrane) and crosslinked collagen matrices (matrix) on ARP using DBBM in extraction sockets with buccal dehiscence. In six mongrel dogs, the mesial roots of three mandibular premolars (P2, P3, and P4) were extracted 1 month after dehiscence defect induction. Two experimental groups were randomly assigned: (1) DBBM with a membrane (DBBM/membrane group) and (2) DBBM with a matrix (DBBM/matrix group). Three-dimensional (3D) volumetric, microcomputed tomography (μCT), and histologic analyses were performed to assess the ridge preservation. Both groups were effective to maintain the ridge width (p > 0.05), and the DBBM/matrix group showed more favorable soft tissue regeneration and bone quality in the histological analysis (p = 0.05). Based on these results, DBBM/matrix could be better choice for ARP in cases of buccal dehiscence defects.
Similar content being viewed by others
Introduction
Tooth extraction can cause horizontal and vertical bone losses. A systematic review found that tooth extraction can cause horizontal bone loss of 29–63% or 3.79 mm and vertical bone loss of 11–22% or 1.24 mm after 6 months1. In cases of severely inflamed tooth extraction, bone loss may be more delayed, incomplete, and extensive2,3.
Alveolar ridge preservation (ARP) refers surgical interventions designed to maintain the initial shape of the alveolar ridge to the greatest extent possible, thereby mitigating the process of alveolar bone resorption4 Typically, ARP denotes a procedure involving the placement of bone graft material within the extraction socket, with or without the application of a covering barrier membrane4. The objective of ARP is to mitigate alterations in the alveolar ridge following tooth extraction, facilitate the generation of new bone within the extraction socket, and foster soft tissue healing at the entrance of the extraction socket4.
The ARP procedure can help compromise the extraction sockets and reduce bone loss5. A recent systematic review found that alveolar ridge preservation (ARP) with a compromised buccal wall reduced horizontal bone loss by 2.37 mm and vertical bone loos by 1.10 mm6. Typically, a collagen membrane is utilized to induce bone regeneration by covering the extraction socket and the top of the defect in these scenarios as part of the socket seal technique7,8,9. Both animal studies and clinical trials have demonstrated that socket seal surgery can increase bone regeneration, decrease loss of soft tissue, and stabilize the grafted biomaterial10,11,12.
According to a recent meta-analysis, both primary closure and ARP techniques, such as flap advancement and open healing with a barrier, can effectively reduce bone remodeling after tooth extraction13. Various types of barrier membranes, including dense-polytetrafluoroethylene (d-PTFE)14,15, collagen plug16, and collagen membrane17,18,19, can be used successfully in open healing with barrier technique for ARP procedures. Clinicians may choose a particular graft material based on their preference, funding, or cultural background. Absorbable collagen membranes are commonly used for ARP among the available barrier membranes. Additionally, previous studies have shown that the collagen barrier plays an osteoconductive role in supporting the growth of bone tissue20,21.
The collagen matrix has been used in clinical practice based on research indicating that maintaining soft tissue integrity over the area of ARP has a significant positive effect on bone healing. Collagen matrix is widely used as an alternative in gingival graft procedures for soft tissue augmentation22,23. collagen matrix with its two layers is thicker than the collagen membranes used for ARP24,25. Collagen matrix has shown that it facilitates blood clot stabilization, promotes tissue integration with one porous layer, and accommodates soft tissue healing with the other layers26,27,28. Depending on these characteristics, the collagen matrix could be considered as an alternative to barrier membranes for ARP28,29,30,31.
Collagen barrier membrane can be categorized into two types based on their crosslinking status: crosslinked and non-crosslinked. Studies indicate that cross-linking the collagen barrier membrane can decelerate absorption32, preserving the transplanted bone volume over an extended period33. However, there are reports suggesting that early exposure of the membrane may elevate complications related to soft tissue34.
Most of the aforementioned studies examining the use of the collagen matrices have focused on non-crosslinked collagen matrices. However, there are still insufficient data on the behavior and integration of the cross-linked collagen matrix (matrix). To address this gap, this study assessed the volumetric, radiographic, and histologic outcomes of the matrix and compared them to those of non-cross linked collagen membranes (membrane).
The underlying hypothesis of this study posits that the performance of ARP on teeth with buccal dehiscence defect will result in better maintenance of alveolar ridge width when a collagen membrane is utilized compared to cases where a collagen matrix is employed. The present study focuses specifically on the alveolar ridge of the extraction socket, with a primary emphasis on the buccolingual dimension of the ridge in areas exhibiting buccal dehiscence. The primary outcome measure involves the three-dimensional (3D) volumetric changes within the soft and hard tissues, along with the buccolingual width of the alveolar ridge.
Results
Clinical findings
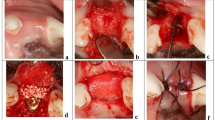
After induction of the dehiscent defect, clinical signs of inflammation, namely gingival swelling, redness, and calculus deposition, were observed around the mesial root. Healing after the surgical procedure occurred without primary closure, but there were no signs of inflammation or other complications. A total of 24 sections were considered for 3D volumetric, radiographic, and histological analyses, with 12 sections in each group (Fig. 1).
Analyses of the 3D digital images
The widths is shown in Fig. 2 and Table S1. The change over time was significant within both groups as a result of repeated-measures ANOVA at 1, 3, and 5 mm below the crest (p < 0.001), and was used as a linear model (p < 0.001).
Results of repeated measures ANOVA of the linear and volumetric measurements (a–d) in both groups (DBBM/membrane and DBBM/matrix). (a–c) Buccolingual alveolar ridge width (mm) at 1 (B-level), 3 (C-level), and 5 (D-level) mm below the reference line over time. (d) Volume changes (%) in the volume of interest (VOI) between 0 and 4 weeks and between 0 and 20 weeks. DBBM: bovine-derived xenograft (Bio-Oss®), membrane: type I non-crosslinked collagen derived from pigs (Bio-Gide®), and matrix: type I crosslinked collagen derived from pigs (Collagen Graft 2®).
Linear changes in the membrane and matrix were not statistically significant at 1, 3, and 5 mm below the crest (Table S1). Volumetric measurements of the volume of interest (VOI) are shown in Fig. 2 and Table S1. The volume change ratios of DBBM/membrane and DBBM/matrix were statistically insignificant.
Analyses of micro-CT radiographic images
The results of the qualitative and quantitative analyses of the micro-CT images are presented in Table 1. The BV/TV values were 32.53 ± 3.93 and 32.42 ± 3.24% in the DBBM/membrane and DBBM/matrix group, respectively. The BS/BV values were 6.727 ± 2.10 and 6.296 ± 1.94, in the DBBM/membrane and DBBM/matrix groups, respectively. The TbPf values, which indicate the level of interconnectivity of the bone, were − 6.31 ± 3.19 and − 6.08 ± 2.96 in the DBBM/membrane and DBBM/matrix groups, respectively. SMI, similar to TbPf, displayed no significant differences; thus, no significant qualitative differences were observed between the two groups.
The dimensional proportions at the coronal area were 27.47 ± 17.15 and 29.53 ± 20.60% in the DBBM/membrane and DBBM/matrix groups, respectively; there were no statistically significant differences between the two groups at the 20-week observation. The dimensions in the middle and apical areas were 78.65 ± 14.64 and 79.86 ± 5.65%, along with 92.08 ± 4.49 and 92.42 ± 4.37% in the DBBM/membrane and DBBM/matrix groups, respectively; there were no significant intergroup differences at the middle and apical areas.
Analyses of histologic samples
Following a 20-week period of socket healing, both groups exhibited robust healing of the extraction sockets characterized by the presence of a solid osseous structure interlaced with connective tissue (Fig. 3). In regions where DBBM was employed, there was a mixture of newly generated bone, vestiges of pre-existing bone particles, and connective tissue.
Buccolingual section obtained at 20 weeks after staining with Goldner's trichrome (a–j). (a, b) Low-magnification images of the membrane and matrix groups (scale bar = 1 mm). High-magnification images of (c, d) crestal, (e, f) apical, (g, h) soft tissue areas (scale bar = 100 um) are shown in panels (a, b), and high-magnification images of (i, j) pseudo-periosteum (scale bar = 50 um) are shown in panels (g–h), respectively. *: pseudo-periosteum. DBBM: bovine-derived xenograft (Bio-Oss®), membrane: type I non-crosslinked collagen derived from pigs (Bio-Gide®), and matrix: type I crosslinked collagen derived from pigs (Collagen Graft 2®).
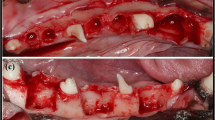
In the DBBM/membrane group, the augmented area remained well-preserved (Fig. 3B), and newly mineralized bone was observed in the coronal part of the sockets below the newly formed collagen matrix. In the coronal and apical areas of the extraction sockets, DBBM particles were dispersed in the connective tissue, and osteoclasts were present in their lacunae at the boundary (Fig. 3C). A pseudo-periosteum was observed along the outer surface of the alveolar ridge (Fig. 3G). The membrane was almost completely absorbed and integrated into the newly formed soft connective tissue. Clusters of red-stained fibers were embedded in and transversed by prominent newly formed collagen fiber bundles with interspersed slender fibroblasts. Blood vessels are also present in the collagen matrix.
In the DBBM/matrix group, the augmented area was well maintained and dome-shaped (Fig. 3B), with most DBBM particles tightly attached to the newly formed woven bone. Newly formed bone had pores, and osteoblast-like cells were observed on its surface. The DBBM particles were in contact with the surrounding mineralized bone rather than with the fibrovascular tissue (Fig. 3F). Blood vessels and a pseudo-periosteum were observed and the matrix was almost fully integrated into the newly formed soft connective tissue above the pseudo-periosteum (Fig. 3H). The matrix was mostly absorbed; however, there were areas where the matrix was occasionally observed. Clusters of red-stained fibers were embedded in and transversed by prominent newly formed collagen fiber bundles with interspersed slender fibroblasts. Blood vessels that sprouted into the matrix-stained light pink were also present in the new collagen matrix and pseudo-periosteum.
Histomorphometric analyses
The width of the ridge at different points was not significantly different between the two groups (Table 2). Similarly, there were no significant differences in the amounts of mineralized bone, or fibrovascular connective tissue between the two groups, as determined by histomorphometry (Table 3). However, the number of residual bone particles was two times higher in the DBBM/membrane group, which was statistically significant.
Discussion
In this study, we compared the effects of two ARP procedures, using DBBM with a membrane and DBBM with a matrix, in extraction sockets with buccal dehiscence in dogs. Crest height and width were markedly reduced following extraction and defect induction. Although no significant differences in the 3D volumetric and radiographic analyses were found, a significant difference was observed in the residual bone particles in the DBBM/membrane.
In this study, the upper portion of the socket underwent coverage with either a collagen membrane or collagen matrix, with open healing being deliberately induced. While open healing comes with the drawback of exposing the bone graft material to the oral environment during the initial stages of healing, it offers the benefit of reducing trauma35 and minimizing alveolar ridge absorption19.
The different properties and preparations of the membrane and matrix explain the different behaviors observed in this study. In this study, membranes (derived from porcine dermis and consisting of type I and III collagen) were non-crosslinked36, whereas the matrix (derived from porcine tendon and consisting of type I collagen) was crosslinked with 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) to improve resistance to enzymatic degradation. The amount of water that collagen can bind to depends on its crosslinking37. Non-crosslinked collagen binds more water than crosslinked collagen. The membrane swelled more initially, but degraded faster over time than the matrix38. The matrix exhibited more stable swelling and showed good results in a previous study39.
Collagen membrane resorption generally takes 4–12 weeks40, but the rate of absorption is influenced by the composition of the collagen membrane and local environmental factors such as pH and temperature41. Previous studies have shown that the structural properties of non-crosslinked collagen matrices are designed to maintain barrier function for at least 30 days42,43. However, in this study, a cross-linked collagen matrix was used, which was expected to maintain its barrier function for an even longer period, thereby holding the potential to enhance outcomes through the mitigation of grafting material collapse. The results of this study are similar to those of previous studies31,44 that used DBBM and a matrix for ARP, suggesting that the matrix could be useful for soft tissue reconstruction during ARP.
However, the lack of significant differences in the residual bone particles between the two groups requires cautious interpretation. Histomorphometric analysis of the DBBM/membrane and DBBM/matrix groups showed that the residual bone particles in the region of interest (ROI) is almost double, which indicates the need for further discussion concerning the large standard deviation of the DBBM/membrane group. The slightly higher proportion of residual bone particles in the DBBM/membrane group is consistent with previous studies that reported incomplete integration and reabsorption even after 24 months of DBBM45. Therefore, it is possible that a few residual bone materials may still exist even after sufficient time for delayed implant placement following ARP with DBBM/membrane.
While the utilization of various bone graft materials during ARP procedures often does not significantly enhance new bone formation compared to natural healing, employing DBBM with a collagen barrier in teeth with dehiscence demonstrates a noteworthy reduction in the future need for guided bone regeneration (GBR)46. Additionally, the execution of ARP results in diminished horizontal and vertical bone loss compared to cases where ARP is not performed6.
This study has some limitations. First, the absence of multiple time points for radiographic and histological analyses may have limited the interpretation of the results. Short-term observational periods could provide further insights into the healing process of extraction sockets with buccal dehiscence and the behavior of the membrane or matrix. Secondly, the relatively small dimensions of the defects could promote regeneration and limit the applicability of the data in clinical settings. Third, the lack of a negative control group without ARP after the induction of dehiscence should be considered.
Within the limitations of this study, the effects of volume change and bone quality and quantity in radiographic analysis were not statistically significant between the two groups; however, the DBBM/matrix showed slightly favorable histologic results. DBBM/matrix could be a candidate therapy for successful ridge regeneration in extraction sockets with buccal dehiscence defects.
Materials and methods
Animals
The study was conducted between August 2021 and February 2022 at the CRONEX Animal Facility in Seoul, Korea. Six male mongrel dogs, weighing 31–35 kg and aged 10–12 months, were housed in a cage with a constant temperature (22 ± 2 °C) and humidity (50 ± 10%). All experimental protocols were approved by the Institutional Animal Care and Use Committee of CRONEX (IACUC; approval no. 202108010) and carried out according to the guideline and regulations of IACUC of CRONEX, which adhered to the ARRIVE guidelines for pre-clinical studies47.
Based on the previous study48,49, the sample size was determined to minimize the number of animals in each group and to distribute the samples equally across each experimental site.
Study design
The overall study flow was presented in Fig. 1. A buccal dehiscence defect model50 was constructed in order to simulate a damaged extraction socket (the experimental site). After inducing the dehiscence defect, the mesial roots of the mandibular second, third, and fourth premolars (P2, P3, and P4, respectively) were extracted 4 weeks later. Each distal root was subjected to root canal treatment after hemisection and was kept as a reference pristine site for the corresponding mesial root. The following two extraction sockets on the unilateral alveolar ridge were rotated into the following groups to produce an equal distribution of three other premolar sites:
DBBM/membrane group: ARP with DBBM (Bio-Oss®; Geistlich Pharma AG, Wolhusen, Switzerland) and a membrane (Bio-Gide®, Geistlich Pharma AG, Wolhusen, Switzerland)
DBBM/matrix group: ARP with DBBM (Bio-Oss®; Geistlich Pharma AG, Wolhusen, Switzerland) and a matrix (Collagen Graft 2®, Genoss, Suwon, Korea)
Surgical procedures
All surgical procedures were performed under general anesthesia induced by a 1:1 mixture of tiletamine hydrochloride, zolazepam hydrochloride (2 mL/10 kg intravenously, Zoletil® 50; Virbac S.A., France), and xylazine hydrochloride (Rompun®; Bayer, Leverkusen, Germany). Inhalation anesthesia was induced by the inhalation of a 2:1 mixture of isoflurane and oxygen. Local infiltration anesthesia (Lidocaine HCL 2% with epinephrine 1:100,000; Huons, Gyeonggi-do, Korea) was administered via infiltration at the buccal and lingual sides of teeth P2, P3, and P4. Scaling was performed prior to surgery. To manage postoperative infection and pain, enrofloxacine (0.2 mL/kg intramuscular, Komibiotril 100 Injection®; Komipharm Co. Ltd., Siheung-si, Korea) and meloxicam (0.4 mg/kg analgesics intramuscular, Metacam®; Labiana Life Science, S.A., Spain) were administered for 3 days. The oral mucosa and wound were disinfected seven times a week using a 0.12% chlorhexidine solution (Hexamedin®; Bukwang Pharm Co., Seoul, Korea).
Induction of dehiscence defect
In this study, the surgical techniques were similar to those of a previous study50 except for the bone graft materials used and healing periods. This study involved hemisection of three premolars on one side of the mandible of six male mongrel dogs after flap elevation with an intracrevicular incision. The remaining distal root was subjected to root canal treatment and filled with gutta-perch and a sealer (AH Plus; Dentsply DeTrey, Konstanz, Germany), and the coronal access to the pulp chamber was sealed with intermediate restorative material (IRM; Dentsply Sirona, Milford, DE, USA). A dehiscent defect was created by performing osteotomy of the buccal wall at the mesial root and creating a groove on the buccal area to expose the dental pulp. The mesiodistal (MD) width of dehiscence was standardized to 2/3 of the MD width of the mesial root. Additionally, the height (H) of the dehiscence was established at half the H of the mesial root.
Extractions and ridge preservation procedures
Following 4 weeks, the corresponding mesial roots were extracted and group-specific interventions were conducted. The DBBM was filled into the extraction socket up to an imaginary line between the adjacent bone walls. The membrane or matrix was trimmed into a rectangular shape to cover the top of the extraction socket, which is grafted area48. Open healing was induced by exposing the collagen membrane or collagen matrix without primary closure of the flap.
Sacrifice
The animals were sacrificed 5 months51 after surgery using suxamethonium chloride hydrate (50 mg/mL intraveneously, Succipharm®; Komipharm Co. Ltd, Siheung-si, Korea) for radiographic and histologic analyses. The acquired specimens were fixed in 10% neutral buffered formalin for 10 days.
Analyses of the 3D digital images
The mandibles of the dogs were scanned using a dental scanner (Medit, Seoul, Korea), and the obtained images were superimposed at 0, 4, and 20 weeks after surgery for 3D digital evaluation using software (Geomagic Design X and Control X, 3DSYSTEMS, SC, USA). Buccolingual alveolar ridge width was measured on cross-sectional images according to previous studies52,53,54. A reference line and curve were created, and three parallel lines (B, C, and D-line) were drawn to connect the buccal and lingual contours 1, 3, and 5 mm below the top of the alveolar crest at three time points. A rectangular area of 5 × 4 mm was selected as the region of interest (ROI) to measure volume changes during the three overlapping observational periods55.
Analyses of micro-computed tomography radiographic images
Micro-CT (Skyscan 1173, Konitch, Belgium) was used to obtain the scan data at a resolution of 35 μm, and DataViewer (Skyscan, Konitch, Belgium) was used for visualization. Bone morphometric parameters were measured for each volume of interest (VOI), according to the American Society for Bone and Mineral Research Histomorphometry Nomenclature Committee56,57: bone volume (BV), tissue volume (TV), volume density (BV/TV), bone surface (BS), surface-to-volume ratio (BS/BV), trabecular bone pattern factor (TbPf), structure model index (SMI). Buccolingual sectioning was performed, and four horizontal lines were drawn to calculate the dimensional changes between the experimental and reference areas, expressed as percentages9,58.
Analyses of histologic samples
The sectioned specimens were embedded in acrylic resin (Technovit 7200 VLC; Heraeus Kulzer, Wehrheim, Germany) and cut into 40 μm slices, then stained with Goldner’s trichrome stain and analyzed by an experienced investigator using a software (CaseViewer; 3DHISTECH Ltd., Budapest, Hungary, ImageJ; Bethesda, Maryland, USA). The landmarks were identified, and the buccolingual alveolar bone width was measured at four points: A, the crest of the buccolingual bone wall of the ridge; V, the vertical line drawn in the center of the ridge through the most apical point of the osseous basal body that separated the buccolingual compartments of the section; T, the line perpendicular to V drawn through the apical point of the basal bone; and B, C, and D, the lines perpendicular to V at 1, 3, and 5 mm below peak A51. An imaginary line was drawn from the alveolar bone crest to set the ROI. Surface area of the mineralized bone, residual bone particles, and fibrovascular connective tissue on the ROI were measured and calculated9,59. The investigator performed histomorphometry on 36 sections twice over 2 weeks to adjust for errors.
Statistical analyses
Statistical analyses were performed using SPSS version 26.0. The data are presented as mean ± standard deviation, and normality and sphericity assumptions were tested using the Shapiro–Wilk and Mauchly’s tests, respectively. Changes between the two groups were compared using repeated-measures analysis of variance (ANOVA) with the Bonferroni correction. For intergroup comparisons, the Student's t-test or Mann–Whitney U test was used. The significance threshold was set at p < 0.05 The intraclass correlation coefficient (ICC) was used to assess examiner reliability, which was high for both radiographic and histomorphometric evaluations. The concordance coefficients were 0.984 and 0.93, respectively, indicating a high reliability.
Ethical approval
This study was approved by the Institutional Animal Care and Use Committee of CRONEX, Seoul, Korea (approval No. 202108010) according to the ARRIVE guidelines for preclinical studies.
Data availability
All data generated by this study are included in this manuscript.
References
Tan, W. L., Wong, T. L., Wong, M. C. & Lang, N. P. A systematic review of post-extractional alveolar hard and soft tissue dimensional changes in humans. Clin. Oral Implants Res. 23(Suppl 5), 1–21. https://doi.org/10.1111/j.1600-0501.2011.02375.x (2012).
Marcaccini, A. M., Novaes, A. B. Jr., Souza, S. L., Taba, M. Jr. & Grisi, M. F. Immediate placement of implants into periodontally infected sites in dogs. Part 2: A fluorescence microscopy study. Int. J. Oral Maxillofac. Implants 18, 812–819 (2003).
Ahn, J. J. & Shin, H. I. Bone tissue formation in extraction sockets from sites with advanced periodontal disease: A histomorphometric study in humans. Int. J. Oral Maxillofac. Implants 23, 1133–1138 (2008).
Mardas, N., Macbeth, N., Donos, N., Jung, R. E. & Zuercher, A. N. Is alveolar ridge preservation an overtreatment?. Periodontology 2000 https://doi.org/10.1111/prd.12508 (2023).
Kim, J. J. et al. Is ridge preservation/augmentation at periodontally compromised extraction sockets safe? A retrospective study. J. Clin. Periodontol. 44, 1051–1058. https://doi.org/10.1111/jcpe.12764 (2017).
Garcia-Gonzalez, S. et al. Volumetric changes in alveolar ridge preservation with a compromised buccal wall: A systematic review and meta-analysis. Med. Oral Patol. Oral Cir. Bucal 25, e565–e575. https://doi.org/10.4317/medoral.23451 (2020).
Tien, H. K. et al. Alveolar ridge regeneration in two-wall-damaged extraction sockets of an in vivo experimental model. Clin. Oral Implants Res. 32, 971–979. https://doi.org/10.1111/clr.13791 (2021).
BenAmara, H. et al. Is ridge preservation effective in the extraction sockets of periodontally compromised teeth? A randomized controlled trial. J. Clin. Periodontol. 48, 464–477. https://doi.org/10.1111/jcpe.13412 (2021).
Lee, J. S. et al. Ridge regeneration of damaged extraction sockets using rhBMP-2: An experimental study in canine. J. Clin. Periodontol. 42, 678–687. https://doi.org/10.1111/jcpe.12414 (2015).
Kim, S. Y. et al. Extraction socket sealing using palatal gingival grafts and resorbable collagen membranes. Maxillofac. Plast. Reconstr. Surg. 39, 39. https://doi.org/10.1186/s40902-017-0137-x (2017).
Barone, A. et al. Buccal bone deficiency in fresh extraction sockets: a prospective single cohort study. Clin. Oral Implants Res. 26, 823–830. https://doi.org/10.1111/clr.12369 (2015).
Guarnieri, R., Di Nardo, D., Di Giorgio, G., Miccoli, G. & Testarelli, L. Effectiveness of xenograft and porcine-derived resorbable membrane in augmentation of posterior extraction sockets with a severe wall defect. A radiographic/tomographic evaluation. J. Oral Maxillofac. Res. 10, 53. https://doi.org/10.5037/jomr.2019.10103 (2019).
Martins, J. R. et al. Comparison of the efficacy of different techniques to seal the alveolus during alveolar ridge preservation: Meta-regression and network meta-analysis. J. Clin. Periodontol. 49, 694–705. https://doi.org/10.1111/jcpe.13628 (2022).
Barber, H. D., Lignelli, J., Smith, B. M. & Bartee, B. K. Using a dense PTFE membrane without primary closure to achieve bone and tissue regeneration. J. Oral Maxillofac. Surg. 65, 748–752. https://doi.org/10.1016/j.joms.2006.10.042 (2007).
Sun, D. J., Lim, H. C. & Lee, D. W. Alveolar ridge preservation using an open membrane approach for sockets with bone deficiency: A randomized controlled clinical trial. Clin. Implant Dent. Relat. Res. 21, 175–182. https://doi.org/10.1111/cid.12668 (2019).
Kotsakis, G. A., Salama, M., Chrepa, V., Hinrichs, J. E. & Gaillard, P. A randomized, blinded, controlled clinical study of particulate anorganic bovine bone mineral and calcium phosphosilicate putty bone substitutes for socket preservation. Int. J. Oral Maxillofac. Implants 29, 141–151. https://doi.org/10.11607/jomi.3230 (2014).
Iorio-Siciliano, V. et al. Dimensional changes following alveolar ridge preservation in the posterior area using bovine-derived xenografts and collagen membrane compared to spontaneous healing: A 6-month randomized controlled clinical trial. Clin. Oral Investig. 24, 1013–1023. https://doi.org/10.1007/s00784-019-02979-w (2020).
Cavdar, F. H., Keceli, H. G., Hatipoglu, H., Demiralp, B. & Caglayan, F. Evaluation of extraction site dimensions and density using computed tomography treated with different graft materials: A preliminary study. Implant Dent. 26, 270–274. https://doi.org/10.1097/ID.0000000000000567 (2017).
Lim, H. C., Shin, H. S., Cho, I. W., Koo, K. T. & Park, J. C. Ridge preservation in molar extraction sites with an open-healing approach: A randomized controlled clinical trial. J. Clin. Periodontol. 46, 1144–1154. https://doi.org/10.1111/jcpe.13184 (2019).
Kuchler, U. et al. Bone-conditioned medium modulates the osteoconductive properties of collagen membranes in a rat Calvaria defect model. Clin. Oral Implants Res. 29, 381–388. https://doi.org/10.1111/clr.13133 (2018).
Feher, B. et al. Osteoconductive properties of upside-down bilayer collagen membranes in rat calvarial defects. Int. J. Implant Dent. 7, 50. https://doi.org/10.1186/s40729-021-00333-y (2021).
Song, Y. W. et al. Soft tissue substitutes to increase gingival thickness: Histologic and volumetric analyses in dogs. J. Clin. Periodontol. 46, 96–104. https://doi.org/10.1111/jcpe.13034 (2019).
Schmitt, C. M. et al. Soft tissue volume alterations after connective tissue grafting at teeth: The subepithelial autologous connective tissue graft versus a porcine collagen matrix—A pre-clinical volumetric analysis. J. Clin. Periodontol. 43, 609–617. https://doi.org/10.1111/jcpe.12547 (2016).
Thoma, D. S., Benic, G. I., Zwahlen, M., Hammerle, C. H. & Jung, R. E. A systematic review assessing soft tissue augmentation techniques. Clin. Oral Implants Res. 20(Suppl 4), 146–165. https://doi.org/10.1111/j.1600-0501.2009.01784.x (2009).
Cardaropoli, D., De Luca, N., Tamagnone, L. & Leonardi, R. Bone and soft tissue modifications in immediate implants versus delayed implants inserted following alveolar ridge preservation: A randomized controlled clinical trial. Part I: Esthetic outcomes. Int. J. Periodontics Restor. Dent. 42, 195–202. https://doi.org/10.11607/prd.5565 (2022).
McGuire, M. K. & Scheyer, E. T. Xenogeneic collagen matrix with coronally advanced flap compared to connective tissue with coronally advanced flap for the treatment of dehiscence-type recession defects. J. Periodontol. 81, 1108–1117. https://doi.org/10.1902/jop.2010.090698 (2010).
McGuire, M. K., Scheyer, E. T. & Gwaltney, C. Commentary: Incorporating patient-reported outcomes in periodontal clinical trials. J. Periodontol. 85, 1313–1319. https://doi.org/10.1902/jop.2014.130693 (2014).
Fickl, S., Kauffmann, F., Stappert, C. F., Kauffmann, A. & Schlagenhauf, U. Scar tissue formation following alveolar ridge preservation: A case control study. Int. J. Periodontics Restor. Dent. 38, e1–e7. https://doi.org/10.11607/prd.3347 (2018).
Jung, R. E. et al. Radiographic evaluation of different techniques for ridge preservation after tooth extraction: A randomized controlled clinical trial. J. Clin. Periodontol. 40, 90–98. https://doi.org/10.1111/jcpe.12027 (2013).
Natto, Z. S. et al. Efficacy of collagen matrix seal and collagen sponge on ridge preservation in combination with bone allograft: A randomized controlled clinical trial. J. Clin. Periodontol. 44, 649–659. https://doi.org/10.1111/jcpe.12722 (2017).
Schneider, D. et al. Labial soft tissue volume evaluation of different techniques for ridge preservation after tooth extraction: A randomized controlled clinical trial. J. Clin. Periodontol. 41, 612–617. https://doi.org/10.1111/jcpe.12246 (2014).
Brunel, G. et al. Regeneration of rat calvarial defects using a bioabsorbable membrane technique: Influence of collagen cross-linking. J. Periodontol. 67, 1342–1348. https://doi.org/10.1902/jop.1996.67.12.1342 (1996).
Friedmann, A., Strietzel, F. P., Maretzki, B., Pitaru, S. & Bernimoulin, J. P. Observations on a new collagen barrier membrane in 16 consecutively treated patients. Clinical and histological findings. J. Periodontol. 72, 1616–1623. https://doi.org/10.1902/jop.2001.72.11.1616 (2001).
Bornstein, M. M., Bosshardt, D. & Buser, D. Effect of two different bioabsorbable collagen membranes on guided bone regeneration: A comparative histomorphometric study in the dog mandible. J. Periodontol. 78, 1943–1953. https://doi.org/10.1902/jop.2007.070102 (2007).
Lee, J., Lee, J. B., Koo, K. T., Seol, Y. J. & Lee, Y. M. Flap management in alveolar ridge preservation: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Implants 33, 613–621. https://doi.org/10.11607/jomi.6368 (2018).
Radenkovic, M. et al. Comparative in vivo analysis of the integration behavior and immune response of collagen-based dental barrier membranes for guided bone regeneration (GBR). Membranes (Basel) https://doi.org/10.3390/membranes11090712 (2021).
Kopp, J., Bonnet, M. & Renou, J. P. Effect of collagen crosslinking on collagen-water interactions (a DSC investigation). Matrix 9, 443–450. https://doi.org/10.1016/s0934-8832(11)80013-2 (1989).
Owens, K. W. & Yukna, R. A. Collagen membrane resorption in dogs: A comparative study. Implant Dent. 10, 49–58. https://doi.org/10.1097/00008505-200101000-00016 (2001).
Rothamel, D. et al. Biodegradation of differently cross-linked collagen membranes: An experimental study in the rat. Clin. Oral Implants Res. 16, 369–378. https://doi.org/10.1111/j.1600-0501.2005.01108.x (2005).
Bunyaratavej, P. & Wang, H. L. Collagen membranes: A review. J. Periodontol. 72, 215–229. https://doi.org/10.1902/jop.2001.72.2.215 (2001).
Hammerle, C. H. & Jung, R. E. Bone augmentation by means of barrier membranes. Periodontology 2000(33), 36–53. https://doi.org/10.1046/j.0906-6713.2003.03304.x (2003).
Parashis, A. O., Kalaitzakis, C. J., Tatakis, D. N. & Tosios, K. Alveolar ridge preservation using xenogeneic collagen matrix and bone allograft. Int. J. Dent. 2014, 172854. https://doi.org/10.1155/2014/172854 (2014).
Ghanaati, S. et al. Evaluation of the tissue reaction to a new bilayered collagen matrix in vivo and its translation to the clinic. Biomed. Mater. 6, 015010. https://doi.org/10.1088/1748-6041/6/1/015010 (2011).
Maiorana, C. et al. Alveolar socket preservation with demineralised bovine bone mineral and a collagen matrix. J. Periodontal Implant Sci. 47, 194–210. https://doi.org/10.5051/jpis.2017.47.4.194 (2017).
Artzi, Z. et al. Biomaterial resorption rate and healing site morphology of inorganic bovine bone and beta-tricalcium phosphate in the canine: A 24-month longitudinal histologic study and morphometric analysis. Int. J. Oral Maxillofac. Implants 19, 357–368 (2004).
MacBeth, N. D., Donos, N. & Mardas, N. Alveolar ridge preservation with guided bone regeneration or socket seal technique. A randomised, single-blind controlled clinical trial. Clin. Oral Implants Res. 33, 681–699. https://doi.org/10.1111/clr.13933 (2022).
Kilkenny, C., Browne, W. J., Cuthill, I. C., Emerson, M. & Altman, D. G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. J. Pharmacol. Pharmacother. 1, 94–99. https://doi.org/10.4103/0976-500X.72351 (2010).
Araujo, M., Linder, E., Wennstrom, J. & Lindhe, J. The influence of Bio-Oss Collagen on healing of an extraction socket: An experimental study in the dog. Int. J. Periodontics Restor. Dent. 28, 123–135 (2008).
Netto, H. D. et al. Histometric analyses of cancellous and cortical interface in autogenous bone grafting. Int. J. Clin. Exp. Pathol. 6, 1532–1537 (2013).
Lee, J., Lee, Y. M., Lim, Y. J. & Kim, B. Ridge augmentation using beta-tricalcium phosphate and biphasic calcium phosphate sphere with collagen membrane in chronic pathologic extraction sockets with dehiscence defect: a pilot study in beagle dogs. Materials (Basel) https://doi.org/10.3390/ma13061452 (2020).
Roman, A. et al. Ridge preservation using a new 3D collagen matrix: a preclinical study. Clin. Oral Investig. 19, 1527–1536. https://doi.org/10.1007/s00784-014-1368-1 (2015).
Di Raimondo, R. et al. Alveolar crest contour changes after guided bone regeneration using different biomaterials: An experimental in vivo investigation. Clin. Oral Investig. 24, 2351–2361. https://doi.org/10.1007/s00784-019-03092-8 (2020).
Sapata, V. M. et al. Deproteinized bovine bone mineral is non-inferior to deproteinized bovine bone mineral with 10% collagen in maintaining the soft tissue contour post-extraction: A randomized trial. Clin. Oral Implants Res. 31, 294–301. https://doi.org/10.1111/clr.13570 (2020).
Lee, D., Lee, Y., Kim, S., Lee, J. T. & Ahn, J. S. Evaluation of regeneration after the application of 2 types of deproteinized bovine bone mineral to alveolar bone defects in adult dogs. J. Periodontal Implant Sci. 52, 370–382. https://doi.org/10.5051/jpis.2106080304 (2022).
Borges, T. et al. Correlation between alveolar bone morphology and volumetric dimensional changes in immediate maxillary implant placement: A 1-year prospective cohort study. J. Periodontol. 91, 1167–1176. https://doi.org/10.1002/JPER.19-0606 (2020).
Dempster, D. W. et al. Standardized nomenclature, symbols, and units for bone histomorphometry: A 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Min. Res. 28, 2–17. https://doi.org/10.1002/jbmr.1805 (2013).
Parfitt, A. M. Bone histomorphometry: Standardization of nomenclature, symbols and units. Summary of proposed system. Bone Min. 4, 1–5 (1988).
Kim, J. J. et al. Biomodification of compromised extraction sockets using hyaluronic acid and rhBMP-2: An experimental study in dogs. J. Periodontol. 90, 416–424. https://doi.org/10.1002/JPER.18-0348 (2019).
Ramaglia, L. et al. Histologic evaluation of soft and hard tissue healing following alveolar ridge preservation with deproteinized bovine bone mineral covered with xenogenic collagen matrix. Int. J. Periodontics Restor. Dent. 38, 737–745. https://doi.org/10.11607/prd.3565 (2018).
Funding
This Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (No. HI20C2114) and National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2020R1C1C1005830/2022M3A9F3082330).
Author information
Authors and Affiliations
Contributions
S.T.K. was mainly responsible for the conception, design, and methodology. Y.D.C. was mainly responsible for the interpretation and extraction of data. S.H.O. was mainly responsible for the methodology. J.T.L. was mainly responsible for the conception and data interpretation. H.S.H. was mainly responsible for the acquisition, analysis and interpretation of data. H.S.H. and J.T.L. drafted the manuscript. Y.D.C., S.H.O. and S.T.K. critically revised the manuscript. All authors including H.S.H., J.T.L., S.H.O., Y.D.C. and S.T.K. gave final approval of the version to be published. Each author agrees to be accountable for all aspects of the accuracy or integrity of the work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Han, Hs., Lee, JT., Oh, S. et al. Effectiveness of a collagen matrix seal and xenograft in alveolar ridge preservation: an experimental study in dogs. Sci Rep 14, 163 (2024). https://doi.org/10.1038/s41598-023-50370-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-50370-3
- Springer Nature Limited