Abstract
Accumulated evidence showed that thyroid diseases induced cognitive decline. However, the relationship between thyroid hormones (THs) and cognition in older euthyroid people is still unclear. Our study aimed to estimate the association between THs within the euthyroid range and cognition in community-dwelling older adults in China. Data were extracted from a cohort study on the health status of rural older adults from the Guizhou province in China (HSRO). Serum thyroid-stimulating hormone (TSH), free thyroxine (FT4), and free triiodothyronine (FT3) were measured using the electrochemiluminescence immunoassay. Cognitive function was evaluated by the Mini-Mental State Examination (MMSE). Linear regression and a binary logistic regression model were used to explore the relationship between THs and cognition in euthyroidism (TSH level of 0.27 ~ 4.20mIU/L). A total of 957 euthyroidism individuals were included in this study, with a mean (SD) age of 71.34 (6.35) years. In individuals with euthyroidism, serum TSH and FT3 levels were positively associated with cognition (TSH:β = 0.06, 95% CI 0.01 ~ 0.11, P = 0.03; FT3:β = 0.07, 95% CI 0.01 ~ 0.12, P = 0.01); and serum FT3 and TSH levels were significantly associated with cognitive domains (P < 0.05). Further, euthyroid individuals in the lowest serum FT3(OR = 1.96; 95% CI 1.27 ~ 3.03) quartile had a twofold increased risk of cognitive impairment compared to those in the highest quartile after adjusting for potential confounding factors. These findings suggested that low levels of FT3 could be an independent risk factor for cognitive impairment in older euthyroid adults. Additionally, a positive linear association exists between serum FT3 levels and cognitive domains (such as immediate memory, language, and attention). Further studies are needed to determine the underlying mechanisms and the community significance of these findings.
Similar content being viewed by others
Introduction
Due to the aging population, the incidence of Alzheimer’s Disease (AD) will increase to over 100 million people worldwide by 2050, which implies that 1 in every 85 individuals in the world may suffer from the disease1. No treatment is available to slow or stop the deterioration of brain cells in AD. The increasing prevalence of AD will lead to a huge burden for society and families1. Early detection of risk factors and incidence is a critical strategy in the prevention of AD. Thyroid hormones (THs) are essential for the development of the central nervous system (CNS) and for regulating metabolism, neurogenesis, myelination, and cellular repair in the brain throughout the lifespan of an individual2,3,4. Both in vitro and in vivo thyroid function studies have indicated that abnormal THs levels could lead to impaired cognitive function5,6. Studies derived from clinical institutions and patients also showed an association between thyroid function and cognitive function7,8,9,10.
Nevertheless, some observational studies on individuals in the community have yielded controversial results in older adults. A prospective investigation showed no significant association between subclinical thyroid d ysfunction and cognitive decline11. Similarly, a collaborative project in 25 cohorts did not support the association between thyroid dysfunction and impairment in cognitive function12. A cohort study found that thyroid disorders were associated with AD development13. Observational studies have yielded inconsistent results, which might be attributed to the unstable manifestations of that thyroid disorders among older adults. Some of these studies were limited by heterogeneity in definitions of thyroid dysfunction, while in others, subclinical thyroid dysfunction in older adults might return to normal over time14. Meanwhile, researchers have also studied the impact of changes in the levels of THs within the normal reference range on cognitive function, particularly in older adults. Such data could provide new insights into the association of levels of THs with cognitive decline15.
Some studies have found an association between cognition and thyroid indicators (thyroid-stimulating hormone (TSH) or free thyroxine (FT4)) within the normal reference range16,17,18. However, a cohort study by Samuels et al.19 discovered that minor thyroid hormone variations did not affect the cognition of community-dwelling older adults. Notably, these investigations primarily focused on examining the influence of a single thyroid indicator on cognitive function. Owing to the way the THs affect each other via the hypothalamus-pituitary-thyroid (HPT) axis, there is a lack of comprehensive exploration of the relationship between THs and cognitive function in community-dwelling older adults. Hence, it is necessary to observe the association between thyroid hormones in the euthyroid range and cognitive function in older populations. In this study, we used data from cross-sectional studies to investigate the aging population in a community in Guizhou province.
Results
A total of 1235 subjects were included in this study for thyroid function assessment. Of these, 957 (77.49%) individuals had euthyroidism. The demographic characteristics and MMSE scores of the euthyroid participants are shown in Table 1. The mean (SD) age of the euthyroid participants was 71.34 (6.35) years. Women had lower cognitive scores than men (P < 0.001). Further, we observed that MMSE scores significantly declined with increasing age (P < 0.001). Participants with hypertension, depression, or anxiety, and those who were single or widowed/divorced had significantly lower MMSE scores (P < 0.001). Moreover, individuals from currently smoking and currently drinking groups got significantly higher MMSE scores (P < 0.001). Likewise, serum levels of TSH and THs differed by age, gender, and education level, as shown in Table S1. The fundamental demographic details of PHQ-2 and GAD-2 in Table S2.
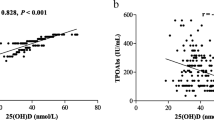
As shown in Fig. 1, we observed statistically significant differences in MMSE scores with varying FT3 concentrations. Individuals with lower FT3 concentrations had lower MMSE scores. The quartiles for the TSH concentration group showed statistically significant differences in MMSE scores for concentrations in Q3 and below. Further, we assessed the correlations between various thyroid markers and cognitive performance in different thyroid statuses, revealing that serum FT3 concentration was independently associated with cognitive function in euthyroidism (Table 2).
As shown in Table 3, the serum levels of FT3 (β = 0.07; 95% CI 0.01 ~ 0.12) and TSH (β = 0.06; 95% CI 0.01 ~ 0.11) were associated with the MMSE score. Further subgroup analysis revealed that in the TSH levels (Q4) group, FT3 was associated with MMSE scores, while in the high FT3 levels (Q4) group, TSH was not statistically associated with MMSE scores. Moreover, as shown in Fig. 2, the lowest level of FT3(OR = 1.96; 95% CI 1.27 ~ 3.03) was an independent risk factor for cognitive impairment after adjusting for age, gender, anxiety, depression, education level, and other confounding variables. The distribution of characteristics of all subjects assessed for thyroid function and the proportion of cognitive impairment in different groups are shown in Supplementary Tables S3, S4, and S5. Further, we found that serum FT3 levels were associated with the cognitive domains of immediate memory and language, while serum TSH levels were found to have a weak positive association with immediate memory and attention (Table S6).
Cognitive impairment and quartiles of thyroid indicators. TSH thyroid-stimulating hormone, FT3 free triiodothyronine, Q quartile, CI confidence interval. Q4 as reference. Model 1 is not adjusted; Model 2 is adjusted for age, gender, education, marital status, hypertension, smoking, drinking, anxiety, and depression.
Discussion
In this observational study of Chinese community-dwelling older euthyroid adults, we found a positive and a weak positive correlation of serum FT3 and TSH levels with cognition, respectively. Further, low FT3 levels were found to double the risk for cognitive impairment. Our results suggested that minor changes in thyroid indicators could impact cognition function even in older euthyroid adults. To the best of our knowledge, this was the first study that focused on the relationship between THs and cognition in older euthyroid adults in a Chinese community.
Previous studies have primarily focused on exploring the relationship between thyroid dysfunction and cognition. However, thyroid dysfunction has been found to be less prevalent in older communities, which might be attributed to the heterogeneity in definitions of thyroid dysfunction20. This might have led to subclinical hypothyroidism and hyperthyroidism not being found to be associated with cognitive function12. While investigating the association between cognition and thyroid function, we used continuous measurements to address the above-mentioned limitations and received the same results. Our study, in line with a longitudinal investigation in Brazil21, found no significant correlation between FT4 and cognitive function. The nuanced actions of thyroid hormones are likely mediated through interactions with specific thyroid nuclear receptors, as FT4 and FT3 are secreted by the thyroid gland and transported to target cells via distinct serum proteins. Given that FT3 is the active form of the hormone, the existing literature supports the notion that FT3, rather than FT4, is more likely to instigate changes in cognitive function22. In contrast, a clinical study involving individuals with normal thyroid function reported an association between serum FT4 levels and Alzheimer’s disease pathology23, contradicting our findings. This discrepancy may arise from the limited subject pool in the clinical study, leading to a narrow distribution of FT4 levels. Additionally, an alternative mechanism underlying the association between thyroid hormones and cognitive decline could be the dynamic changes in the expression of these hormones throughout an individual’s lifespan in response to the evolving needs of various organs and the aging process24,25. These factors deserve further exploration in future studies. Additionally, our findings revealed that when TSH levels are high (Q4), there is an observed association between FT3 and MMSE scores. Conversely, when FT3 levels are high (Q4), TSH is not found to be statistically associated with MMSE scores. The relationship between FT3 and TSH in older adults with euthyroidism involves a dynamic interplay. Low FT3 concentrations may lead to increased TSH secretion, and changes in TSH levels can also influence FT3 concentrations. The findings suggested that the influence of thyroid hormones on cognitive function may vary based on hormone concentrations. Future research should explore the temporal dynamics and bidirectional influences within the hypothalamus–pituitary–thyroid axis and their specific impact on cognitive function.
In our study, we observed no significant association between thyroid-stimulating hormone levels and cognitive impairment, and the lowest concentration of FT3 was linked to a twofold risk of cognitive impairment. These findings were in line with a single-center study that evaluated patients with dementia10, which may be attributed to the altered activity of deiodinase type 2 (D2), expressed in various tissues, which regulates the conversion of T4 to T3 in the central nervous system. D2 activity could be considered as another possible mechanism contributing to the association between thyroid hormones and cognitive decline. Further investigations are warranted to delve into this mechanism and establish a more comprehensive understanding. Similarly, our results align with cohort studies that found no correlation between normal range or mild changes in TSH and cognitive dysfunction26. This concordance may be attributed to our study population, consisting of elderly individuals with normal thyroid function. In post-mortem and mono-center studies, AD patients have been found to exhibit low serum levels of FT3, which was consistent with our findings10,25. Similarly, in older euthyroid adults, a previous cross-sectional study found that the risk factors for cognitive impairment were positively associated with FT3 levels27. Our study contributes to the growing evidence associating FT3 levels with cognition in older euthyroid adults, suggesting a nuanced interplay between TSH and FT3 within the normal thyroid function range. Furthermore, we also found that TSH and FT3 levels were associated with the cognitive domain of immediate memory. Previous observable research found an association between TSH levels in the normal range and memory performance28,29. The prefrontal cortex is a brain region of great importance for memory30. A study on older mice with significantly impaired learning and memory showed lower serum levels of FT3 and higher levels of SNAP-25 and MUNC18-1 in the frontal lobe of the mice31. This finding held potential significance in enhancing our understanding of the mechanisms linking TSH and FT3 levels with cognitive domains in euthyroidism.
The current study had several limitations. Firstly, this is a cross-sectional epidemiological survey in rural Guizhou province, with no evidence of age-related cognitive changes in the selected cohort. Secondly, this study lacks the exploration of inflammatory markers. All the associations might have arisen due to the modulation of inflammatory factors produced during the various non-thyroidal diseases. Thirdly, the lack of data regarding serum total T3, total T4, thyroid-specific antibodies, and enzymes involved in the conversion of T4 to T3 might have limited the interpretation of our findings. There is a need for a more comprehensive assessment to elaborate on the mechanisms underlying the impact of thyroid hormones on the central nervous system. Fourthly, the MMSE is a brief assessment of overall cognitive function and may not be sensitive to the more subtle changes across our lifespan.
In conclusion, euthyroidism is mostly neglected by researchers. However, this state is also essential in the community, particularly among older adults with cognitive decline. Our findings from older adults in the Chinese community suggested an independent relationship between serum FT3 levels and cognitive function within normal thyroid function, with low levels of FT3 associated with the risk of cognitive impairment. Additionally, TSH and FT3 levels also impacted the cognitive domain of immediate memory. As these findings are derived from cross-sectional data, further confirmation is needed from a longitudinal exploration of impact of FT3 supplementation on the cognitive function of euthyroid older adults.
Materials and methods
Study design and participants
This is a cross-sectional analysis of the baseline survey data obtained from the cohort study on the health status of rural older adults from the Guizhou province in China (HSRO). The data were obtained using multistage cluster sampling. A total of 12 villages in the province were selected, and the baseline survey was conducted from July to August 2019. The inclusion criteria included community-dwelling volunteers aged ≥ 60 years who were long-term residents of the area (at least > 6 months). A total of 1795 older adults were enrolled in this study. Among them, 560 individuals were excluded for the following reasons: (i) did not cooperate in the blood sample collection, (ii) did not have normal communication skills, (iii) had been diagnosed with severe mental illness by the medical center (including severe depression and anxiety), and (iv) had taken drugs that affected thyroid function, such as levothyroxine and thyroxine. Thus, 1235 community-dwelling older adults were included in the thyroid function assessment analysis, of whom 957 were euthyroid individuals. The study was approved by the Ethics Committee of Guizhou Medical University, and all the participants signed informed consent.
Analyses
Assessment of thyroid function
Blood samples were collected the morning after an overnight fast. Serum levels of TSH, free thyroxine (FT4), and free triiodothyronine (FT3) were determined using the Roche Cobase601 automated chemiluminescent immunoassays (Roche Group, Switzerland). The reference ranges provided by the manufacturer were as follows: FT3, 3.10 ~ 6.80 pmol/L; FT4, 12.00 ~ 22.00 pmol/L; and TSH, 0.27 ~ 4.20 mIU/L.
Participants were categorized based on TSH, FT3, and FT4 concentrations, the normal thyroid function also called euthyroidism (TSH = 0.27 ~ 4.20mUI/L); overt hypothyroidism (TSH > 4.20 mIU/L and FT4 < 12.00 pmol/L); subclinical hypothyroidism (TSH > 4.20mIU/L and FT4 = 12.00 ~ 22.00 pmol/L); subclinical hyperthyroidism (TSH < 0.27 mIU/L, FT4 = 12.00 ~ 22.00 pmol/L, and FT3 = 3.10 ~ 6.80 pmol/L) and overt hyperthyroidism (TSH < 0.27 mIU/L, FT4 > 22.00 pmol/L, and FT3 > 6.80 pmol/L)32.
Assessment of cognitive function
The mini-mental state examination (MMSE) score was used to assess the cognitive function of participants33. MMSE results can quickly reflect a global cognition in clinical, research, and community settings. The test comprises 11 items divided into five domains: orientation, immediate memory, attention, language, and delayed recall. Trained investigators recorded the participants’ responses while observing their behavior and then summed the scores assigned to each question. The 30-point questionnaire contains a total of 30 test questions. For each correct response, the participants would score 1 point; thus, each participant could obtain a total score of 0 to 30 points. Cognitive impairment was defined as MMSE score of ≤ 17 for illiterate participants, ≤ 20 for those with primary school education and below, and ≤ 24 for those with junior high school education and above34.
Covariates
In this study, we examined several demographic characteristics of the participants, including gender, marital status (single, married, and widowed/divorced), and education (illiteracy, primary, and high level), age (age groups of 60–69, 70–79, ≥ 80 years). Based on their smoking status, the participants were divided into two categories: currently smoking (defined as a total of > 100 cigarettes smoked in the past year) or not smoking (including quitting smoking (defined as quitting for > 6 months) and never smoking). The drinking category was divided into 2 categories: currently drinking (defined as drinking on an average of ≥ 1 days per week in the past year) or not drinking (including never/occasional drinking for > 6 months). Hypertension was assessed through self-report; participants needed to answer the question, “Have you ever been told by a doctor, nurse, or another health professional that you have hypertension?” Responses were grouped into two categories: Yes and no.
Depressive symptoms assessment
Utilizing the Patient Health Questionnaire-2 (PHQ-2), the evaluation of depressive symptoms was conducted. Comprising two items, this questionnaire probes into the frequency of depressive mood and feelings of loss over the preceding two weeks. Response options, including "not at all", "a few days", "more than half of the days", and "almost every day", are associated with scores of 0, 1, 2, and 3, respectively. Consequently, PHQ-2 scores span from 0 to 6, with a score of 3 or more indicating the potential presence of a depressive disorder35.
Anxiety symptoms assessment
For the assessment of anxiety symptoms, the Generalized Anxiety Disorder 2-Item Scale (GAD-2) is employed as a concise and user-friendly tool designed for the initial screening of generalized anxiety disorder. This scale consists of two items that investigate the frequency of anxiety over the past two weeks. Response options, ranging from "not at all" to "almost every day", are correspondingly assigned scores of 0, 1, 2, and 3. Consequently, GAD-2 scores range from 0 to 6, and a score of 3 or higher suggests the potential presence of an underlying anxiety disorder36.
Statistical analysis
All statistical analyses were performed using SPSS for Windows version 22.0 (IBM Corp., Armonk, NY, USA). MMSE scores and, THs were analyzed by quartile grouping in order to observe cognitive differences. Spearman’s correlation coefficients were calculated to estimate the association of MMSE scores with thyroid hormones. Bootstrapping was used to obtain 95% confidence intervals (CIs). Multiple linear regression and binary logistic regression analysis were used to assess the relationship between THs and cognitive function (domains) after adjustment for age, gender, marriage, and other relevant variables (smoking, drinking, anxiety, and depression). The difference between all subjects and euthyroidism was assessed and shown in the appendix. All statistical tests were 2-sided and the difference were considered significant at P < 0.05.
Ethics approval and consent to participate
Written informed consent was obtained from each participant before any study procedure was initiated, and the collection of data on human subjects was approved by the medical ethics committee of Guizhou Medical University (approval No. 2018-092). All methods in this study were performed in accordance with the guidelines of the Declaration of Helsinki.
Data availability
The datasets that support the findings of this study are available on request from the corresponding author (Jingyuan Yang, e-mail: yang8880@sina.com. The data is not publicly available due to privacy or ethical restrictions.
Abbreviations
- AD:
-
Alzheimer’s disease
- THs:
-
Thyroid hormones
- TSH:
-
Thyroid-stimulating hormone
- FT4:
-
Free thyroxine
- FT3:
-
Free triiodothyronine
- MMSE:
-
The mini-mental state examination (MMSE)
References
Alzheimer’S, A. 2009 Alzheimer’s disease facts and figures. Alzheimers Dement. 5, 234–270. https://doi.org/10.1016/j.jalz.2009.03.001 (2009).
Boelaert, K. Thyroid dysfunction in the elderly. Nat. Rev. Endocrinol. 9, 194–204. https://doi.org/10.1038/nrendo.2013.30 (2013).
Calza, L., Fernandez, M. & Giardino, L. Role of the thyroid system in myelination and neural connectivity. Compr. Physiol. 5, 1405–1421. https://doi.org/10.1002/cphy.c140035 (2015).
Korevaar, T. I. et al. Association of maternal thyroid function during early pregnancy with offspring IQ and brain morphology in childhood: A population-based prospective cohort study. Lancet Diabetes Endocrinol. 4, 35–43. https://doi.org/10.1016/S2213-8587(15)00327-7 (2016).
Belakavadi, M., Dell, J., Grover, G. J. & Fondell, J. D. Thyroid hormone suppression of beta-amyloid precursor protein gene expression in the brain involves multiple epigenetic regulatory events. Mol. Cell. Endocrinol. 339, 72–80. https://doi.org/10.1016/j.mce.2011.03.016 (2011).
Chaalal, A., Poirier, R., Blum, D., Laroche, S. & Enderlin, V. Thyroid hormone supplementation restores spatial memory, hippocampal markers of neuroinflammation, plasticity-related signaling molecules, and beta-amyloid peptide load in hypothyroid rats. Mol. Neurobiol. 56, 722–735. https://doi.org/10.1007/s12035-018-1111-z (2019).
Chen, Z. et al. Correlation of thyroid dysfunction and cognitive impairments induced by subcortical ischemic vascular disease. Brain Behav. 6, e00452. https://doi.org/10.1002/brb3.452 (2016).
Hu, Y., Wang, Z. C., Guo, Q. H., Cheng, W. & Chen, Y. W. Is thyroid status associated with cognitive impairment in elderly patients in China?. BMC Endocr. Disord. 16, 11. https://doi.org/10.1186/s12902-016-0092-z (2016).
Burkauskas, J. et al. Cognitive functioning in coronary artery disease patients: Associations with thyroid hormones, N-terminal Pro-B-type natriuretic peptide and high-sensitivity C-reactive protein. Arch. Clin. Neuropsychol. 32, 245–251. https://doi.org/10.1093/arclin/acx004 (2017).
Quinlan, P., Horvath, A., Wallin, A. & Svensson, J. Low serum concentration of free triiodothyronine (FT3) is associated with increased risk of Alzheimer’s disease. Psychoneuroendocrinology 99, 112–119. https://doi.org/10.1016/j.psyneuen.2018.09.002 (2019).
Wijsman, L. W. et al. Subclinical thyroid dysfunction and cognitive decline in old age. PLoS One 8, e59199. https://doi.org/10.1371/journal.pone.0059199 (2013).
van Vliet, N. A. et al. Association of thyroid dysfunction with cognitive function: An individual participant data analysis. JAMA Intern. Med. 181, 1440–1450. https://doi.org/10.1001/jamainternmed.2021.5078 (2021).
Kim, J. H. et al. The association between thyroid diseases and Alzheimer’s disease in a national health screening cohort in Korea. Front. Endocrinol. 13, 815063. https://doi.org/10.3389/fendo.2022.815063 (2022).
Jasim, S. & Gharib, H. Thyroid and aging. Endocr. Pract. 24, 369–374. https://doi.org/10.4158/EP171796.RA (2018).
Ritchie, M. & Yeap, B. B. Thyroid hormone: Influences on mood and cognition in adults. Maturitas 81, 266–275. https://doi.org/10.1016/j.maturitas.2015.03.016 (2015).
Chaker, L. et al. Thyroid function and the risk of dementia. Neurology 87, 1688. https://doi.org/10.1212/WNL.0000000000003227 (2016).
Moon, J. H. et al. Lower-but-normal serum TSH level is associated with the development or progression of cognitive impairment in elderly: Korean longitudinal study on health and aging (KLoSHA). J. Clin. Endocrinol. Metab. 99, 424–432. https://doi.org/10.1210/jc.2013-3385 (2014).
Yeap, B. B. et al. Higher free thyroxine levels predict increased incidence of dementia in older men: The health in men study. J. Clin. Endocrinol. Metab. 97, E2230–E2237. https://doi.org/10.1210/jc.2012-2108 (2012).
Samuels, M. H. et al. Thyroid function variations within the reference range do not affect quality of life, mood, or cognitive function in community-dwelling older men. Thyroid 26, 1185–1194. https://doi.org/10.1089/thy.2016.0104 (2016).
Yeap, B. B. et al. Reference ranges for thyroid-stimulating hormone and free thyroxine in older men: Results from the health in men study. J. Gerontol. A Biol. Sci. Med. Sci. 72, 444–449. https://doi.org/10.1093/gerona/glw132 (2017).
Szlejf, C. et al. Subtle thyroid dysfunction is not associated with cognitive decline: Results from the ELSA-Brasil. J. Alzheimers Dis. 81, 1529–1540. https://doi.org/10.3233/JAD-210018 (2021).
Grigorova, M. & Sherwin, B. B. Thyroid hormones and cognitive functioning in healthy, euthyroid women: A correlational study. Horm. Behav. 61, 617–622. https://doi.org/10.1016/j.yhbeh.2012.02.014 (2012).
Choi, H. J. et al. Associations of thyroid hormone serum levels with in-vivo Alzheimer’s disease pathologies. Alzheimers Res. Ther. 9, 64. https://doi.org/10.1186/s13195-017-0291-5 (2017).
Luongo, C., Dentice, M. & Salvatore, D. Deiodinases and their intricate role in thyroid hormone homeostasis. Nat. Rev. Endocrinol. 15, 479–488. https://doi.org/10.1038/s41574-019-0218-2 (2019).
Davis, J. D. et al. Thyroid hormone levels in the prefrontal cortex of post-mortem brains of Alzheimer’s disease patients. Curr. Aging Sci. 1, 175–181. https://doi.org/10.2174/1874609810801030175 (2008).
Aubert, C. E. et al. The association between subclinical thyroid dysfunction and dementia: The health, aging and body composition (Health ABC) study. Clin. Endocrinol. (Oxf.) 87, 617–626. https://doi.org/10.1111/cen.13458 (2017).
Ceresini, G. et al. Physical performance across the thyroid function values within the normal range in adult and older persons. Aging Clin. Exp. Res. 31, 385–391. https://doi.org/10.1007/s40520-018-0975-0 (2019).
Livner, A., Wahlin, A. & Backman, L. Thyroid stimulating hormone and prospective memory functioning in old age. Psychoneuroendocrinology 34, 1554–1559. https://doi.org/10.1016/j.psyneuen.2009.05.016 (2009).
Wahlin, A., Bunce, D. & Wahlin, T. B. Longitudinal evidence of the impact of normal thyroid stimulating hormone variations on cognitive functioning in very old age. Psychoneuroendocrinology 30, 625–637. https://doi.org/10.1016/j.psyneuen.2005.01.010 (2005).
Volle, E., Gonen-Yaacovi, G., Costello Ade, L., Gilbert, S. J. & Burgess, P. W. The role of rostral prefrontal cortex in prospective memory: A voxel-based lesion study. Neuropsychologia 49, 2185–2198. https://doi.org/10.1016/j.neuropsychologia.2011.02.045 (2011).
Cao, L. et al. Reduced thyroid hormones with increased hippocampal SNAP-25 and Munc18-1 might involve cognitive impairment during aging. Behav. Brain Res. 229, 131–137. https://doi.org/10.1016/j.bbr.2012.01.014 (2012).
Liu, Y., Shan, Z., Endocrine Metabolic Diseases Group of the Chinese Geriatrics S, Thyroid Group of the Chinese Society of Endocrinology CMA. Expert consensus on diagnosis and treatment for elderly with thyroid diseases in China. Aging Med. (Milton) 4(70–92), 2021. https://doi.org/10.1002/agm2.12165 (2021).
Folstein, M. F., Folstein, S. E. & McHugh, P. R. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12, 189–198. https://doi.org/10.1016/0022-3956(75)90026-6 (1975).
Qi, S. et al. Undetected dementia in community-dwelling older people—6 Provincial- level administrative divisions, China, 2015–2016. China CDC Wkly. 2, 731–735. https://doi.org/10.46234/ccdcw2020.200 (2020).
Kroenke, K., Spitzer, R. L. & Williams, J. B. The patient health questionnaire-2: Validity of a two-item depression screener. Med. Care 41, 1284–1292. https://doi.org/10.1097/01.MLR.0000093487.78664.3C (2003).
Kroenke, K., Spitzer, R. L., Williams, J. B., Monahan, P. O. & Lowe, B. Anxiety disorders in primary care: Prevalence, impairment, comorbidity, and detection. Ann. Intern. Med. 146, 317–325. https://doi.org/10.7326/0003-4819-146-5-200703060-00004 (2007).
Acknowledgements
The authors would like to acknowledge the efforts of the participants who voluntarily gave their time to participate in the study.
Funding
The study was supported by the National Natural Science Foundation of China (Grant No. 81860598).
Author information
Authors and Affiliations
Contributions
J.Y. conceived and designed the study. H.C., J.H., X.Y., Q.Z., Y.H., X.T., J.T., and L.Z. took responsibility for data collection. H.C. conducted the statistical analysis and wrote the original draft. J.Y. revised the paper. All authors contributed to the final version of the paper and have read as well as approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, H., Hu, J., Yang, X. et al. Low levels of free triiodothyronine are associated with risk of cognitive impairment in older euthyroid adults. Sci Rep 13, 22133 (2023). https://doi.org/10.1038/s41598-023-49285-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-49285-w
- Springer Nature Limited