Abstract
Adsorbents synthesized by activation and nanoparticle surface modifications are expensive and might pose health and ecological risks. Therefore, the interest in raw waste biomass materials as adsorbents is growing. In batch studies, an inexpensive and effective adsorbent is developed from raw olive stone (OS) to remove methylene blue (MB) from an aqueous solution. The OS adsorbent is characterized using scanning electron microscopy (SEM), Fourier Transform Infra-Red (FTIR), and Brunauer–Emmett–Teller (BET) surface area. Four isotherms are used to fit equilibrium adsorption data, and four kinetic models are used to simulate kinetic adsorption behavior. The obtained BET surface area is 0.9 m2 g−1, and the SEM analysis reveals significant pores in the OS sample that might facilitate the uptake of heavy compounds. The Langmuir and Temkin isotherm models best represent the adsorbtion of MB on the OS, with a maximum monolayer adsorption capacity of 44.5 mg g−1. The best dye color removal efficiency by the OS is 93.65% from an aqueous solution of 20 ppm at the OS doses of 0.2 g for 90 min contact time. The OS adsorbent serves in five successive adsorption cycles after a simple filtration-washing-drying process, maintaining MB removal efficiency of 91, 85, 80, and 78% in cycles 2, 3, 4, and 5, respectively. The pseudo second-order model is the best model to represent the adsorption process dynamics. Indeed, the pseudo second-order and the Elovich models are the most appropriate kinetic models, according to the correlation coefficient (R2) values (1.0 and 0.935, respectively) derived from the four kinetic models. The parameters of the surface adsorption are also predicted based on the mass transfer models of intra-particle diffusion and Bangham and Burt. According to the thermodynamic analysis, dye adsorption by the OS is endothermic and spontaneous. As a result, the OS material offers an efficient adsorbent for MB removal from wastewater that is less expensive, more ecologically friendly, and economically viable.
Similar content being viewed by others
Introduction
A growing worldwide interest has focused on developing wastewater treatment technologies to address the ever-increasing pollutants released into water, including industrial dyes1,2,3,4,5. The world uses about 7 million tonnes of dyes annually6. Moreover, dyes are used in the textile, food, paper, cosmetic, and pharmaceutical industries. During the dyeing process, 10 to 15% of these dyes reach the aquatic ecosystem7. Even though they may not enter aquatic habitats in most cases, their constant movement, because of the cumulative effect, poses a risk to aquatic ecosystems and the microorganisms that live there8,9,10. Existing conventional water treatment systems face a significant challenge due to the increasing rise in hazardous dye wastewater produced by various industrial/commercial sectors, which continues to be a serious public health problem and a top environmental protection priority11. Basic blue 9 is another name for methylene blue (MB), a cationic soluble dye or stain that can be found as a crystalline solid or a green/blue powder. MB is used in various fields, such as biology, medicine, and chemistry12. Wastewater is treated using different methods, such as membrane separation13,14, electrocoagulation15,16, flocculation17, precipitation18, ozonation19, and adsorption20,21. These well-established methods frequently fail to lower dye concentrations to desirable levels and are not economical for dye removal. Therefore, it is crucial to find treatment methods that are more efficient and economically feasible12. Adsorption is a technique capable of removing several types of pollutants from wastewater, including industrial dyes22. Nowadays, many adsorbents, including carbon-based nano-adsorbents23,24, transition metal-based oxides25, MOFs26, polymer-based adsorbents27, and low-cost adsorbents are used to treat dye-containing wastewater. It is possible to use inexpensive adsorbents from various sources, such as agricultural waste biomass, to remove contaminants from wastewater28,29. For wastewater treatment, agricultural wastes and cellulosic biomass hold tremendous promise as alternatives to activated carbon in adsorption30,31. Examples of low-cost adsorbents include pine leaves, pine cones, rice hull, tectona grandis sawdust, prickly pear seed cake, apricot seed, and sugarcane bagasse. These adsorbents showed promising results in removing several dyes such as Methylene Blue, Reactive Orange 16, Crystal Violet, Methyl Orange, Astrazone Black and Rhodamine B21,22,23,24,25,26,27. Table 1 presents the results of various reported studies of low-cost adsorbents on removal efficiency. Low-cost adsorbents demonstrated high removal efficiency (> 90%) for most of the dyes reported in Table 1.
The olive stone is a lignocellulosic biomass with lipids, polysaccharides, phenols, hemicelluloses, and celluloses as the main constituents32. There are many innovative uses for olive stones. An fascinating and expanding utilization of olive stones is as heavy metal and dye adsorbents in wastewater treatment facilities33.
This work focuses on using olive stone as a low cost bio-waste material, and efficient adsorbent for removal of methylene blue (MB) dye from wastewater in a batch adsorption system under various experimental settings/conditions, due to its availability as a waste resource. The olive stones are produced from locally available material that can be reused for removing the environmental pollution by using the low price, and environmentally friendly bio-adsorbent material. The determination analytic methods of XRD, SEM, BET, and FTIR are utilized to analyze the adsorption data. In addition, Freundlich and Langmuir adsorption isotherms are examined for finding the most suitable isotherm model. The adsorption kinetics is also studied. Furthermore, the mass transfer and thermodynamic studies are investigated.
Materials and methods
Sample collection and preparation
Olive stones (OS) are collected from local markets in Baghdad. Initially, the OS sample is washed several times with distilled water to remove any impurities on the OS surface. Then, the OS sample is dried in an oven at 110 °C for 24 h. After drying, the OS sample is ground (pulverized) using a mechanical mill, sieved into a fine powder, and kept in a moisture-proof container for further assessment.
Characterization of OS
The phases of crystalline materials are investigated using X-ray Diffraction (XRD) (type: Shimadzu-6000, origin: Japan) from 0° to 90° 2 theta degree with scan rate 2 (deg/min) and Cu–K (as a radiation source). FTIR spectroscopy (FTIR, Bruker Tensor 27, origin: Germany) is used to determine the functional groups on the surface of the adsorbent. The surface morphology for the OS is observed under a scanning electron microscope (SEM) (type: Tescan VEGA 3 SB, origin: Germany). The sample surface area is tested via BET instrument (Q-surf 9600, made in the USA).
Preparation of MB stock solution
A 1 g of MB (C16H18ClN3S; MW = 319.85 g gmol−1) is dissolved in 1 L of distilled water to create a stock solution. Several initial MB concentrations (blank, 20, 40, 60, 80, 100, 120, and 140 mg L−1) are prepared. Under 663 nm, the MB calibration curve is performed by measuring the absorbance for each concentration (model JASCO V-630 Japan). From Eqs. (1) and (2), the dye removal from the solution and the adsorption capacity (qe) can be estimated34:
where Co and Ce are the initial and equilibrium concentrations of adsorbate (mg L−1), respectively, \(V\) denotes the volume (L) of the solution, \(M\) is the mass (g) of the adsorbent, and \({q}_{e}\) introduces the amount of the contaminant adsorbed (mg g−1).
Batch adsorption experiments
The batch adsorption experiments can evaluate several operational parameters, including the initial concentration, pH (2, 3, 5, 6, 7, 8, 9, and 12), temperature (25, 35, and 45 °C), and initial dye concentration (20–140 mg L−1). A 0.1 M of sodium hydroxide (NaOH) and/or hydrochloric acid (HCl) are used to adjust the pH. A shaker with a temperature control (Shaking Incubator, MODEL: SSI10R2) is utilized to shake 0.14 g of the OS adsorbent and 50 mL of MB solution at various starting concentrations for 24 h before centrifuging the mixture at 40,000 rpm. After each sample is vacuum-filtered through a 0.22 polycarbonate membrane, no dye tint is observed in the filter membrane. The MB concentration is measured using an ultraviolet–visible (UV) spectrophotometer.
Isotherm and kinetic adsorption of methylene blue
Three isotherm and kinetic adsorption models are used to represent the adsorbent's capacity, as given in Table 2, and to better comprehend the kinetics of the adsorption behaviors35.
Mass transfer
Adsorption, a mass transfer process, is the phenomenon of gases or solutes sticking to solid or liquid surfaces. Due to unequal forces, the molecules or atoms on the solid surface adsorb because they have excess surface energy36,37. The adsorption of contaminants on the adsorbent surface occurs in three steps40: (1) contaminants move from the liquid film's boundary layer to the adsorbent surface as explained by the extra-particle diffusion model, (2) molecules diffuse either within the pores, below the surface, or both as explained by the intra-particle diffusion theory, and (3) contaminants adsorb through electrostatic interaction and hydrogen bonding in a surface chemical reaction. Because the third step is the fastest one, the first and second steps of the surface adsorption process can influence the adsorption amount and rate. It should be noted that all three phases, occurring separately or simultaneously, contribute to the adsorption process.
The Weber and Morris (WM) model, also known as the intra-particle diffusion model, uses the intra-particle diffusion coefficient to determine the mass transfer rate of contaminant particles to the interior of the synthetic adsorbent38. The mechanism of surface mass transfer to the adsorbent pores is investigated using the WM model. The theoretical fundamental is Fick's mass transfer law. In practice, the pore-based diffusion of the particle may control the adsorption process. Normally, the adsorption process can be regulated by the diffusion of the particles inside the pores. The Bangham and Burt (BB) model can be effective in these cases39. Table 2 tabulates the mass transfer models.
Thermodynamics investigation
Another crucial aspect of adsorption studies is thermodynamics. The spontaneity and thermodynamic feasibility of the process are evaluated using thermodynamic parameters such entropy change (ΔSº), enthalpy change (ΔHº), and Gibbs free energy (ΔGº), as given below40:
where Kc represents the apparent equilibrium constant of the adsorption, expressed as follows41:
The standard thermodynamic equilibrium constant (Kc) of the adsorption system should be calculated using the activity rather than the concentration.
Equation (6) describes the relation between the entropy and enthalpy, as given below42:
Results and discussion
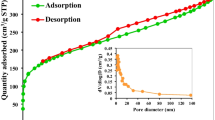
BET and scanning electron microscopy (SEM) analysis
The area of the olive stone (OS) sample is an irregular porous structure with average well-developed deep cavities and pores of diverse sizes and forms; the OS area is 0.9 m2 g−1. The SEM image of the OS powder is shown before MB adsorption in Fig. 1A, and after MB adsorption in Fig. 1B. The OS has a disorder structure, which favors the bio sorption of MB on different locations of the bio sorbent. This image confirms the previous results obtained by the analysis of the pore space/area of the OS samples48. Based on Fig. 1B, the particles of MB accumulation after adsorption can be seen, because of the adsorption of MB in the vacancies of the OS adsorbent material. It also demonstrates that the OS material is porous, giving MB molecules a considerable surface area to interact with and facilitate adsorption.
X-ray diffraction (XRD)
X-ray diffraction (XRD) is used to assess and validate the phase structures of the OS adsorbent. Figure 2 depicts the crystalline structure of OS powder, which is consistent with the findings of Tezcan et al.43, stating that the OS contains cellulose. Due to hydrogen bonding or van der Waals forces between nearby molecules, the final sample has a crystalline structure44. The similar results reveal the presence of a peak at 2θ = 19.85°, which is attributed to traces of lignin or hemicellulose45.
Fourier-transform infrared spectroscopy
Figure 3 provides the FTIR spectrum for the OS sample. The first broad peak spanning at 3371 cm−1 is identified as the O–H hydroxyl with a single H bond. The band at 2927 cm−1 shows symmetric and asymmetric stretching of the C–H alkane. Furthermore, the existence of hemicellulose is suggested by the C=O carbonyl stretching at 1743 cm−1. The bands can be attributed to C–O stretching and C=C aromatic groups at 1224 cm−1 and 1028 cm−1, respectively46. The presence of carboxyl groups in the composite components causes the peaks at 1743 cm−1 noticed in the OP spectra47. P-OH stretching vibrations are detected by the absorption maxima at 1159 cm−148. Figure 3 illustrates how the peaks at approximately 1591 cm−1 in the composites' spectra demonstrate the availability of OP within the composite49. The peaks at 2800–2900 cm−1 are noticeably amplified in accordance with symmetric vibrations of –CH. These peaks reveal the hydrogen bonds between the –CH and water or other hydroxyl groups in glycerol50. O–H stretching at 3371 cm−1 and O–H in plane bending at 1325 cm−1 are also observed, and N–H bending is noticed at 1500 cm−151. Inorganic groups or C–C stretching vibrations are responsible for the other bands seen at 820 and 889 cm−152.
Effects of adsorption parameters
Effect of pH
It is obvious that MB adsorption is pH-dependent. Figure 4A demonstrates that when the pH is increased from 2 to 12, the MB adsorption on the OS increases from 29.5% to around 93%. This shows that the adsorption process favors an alkaline environment. The surface deprotonation might cause this phenomenon/behavior due to the presence of hydroxyl ions on the adsorbent's surface, which has a strong negative charge46. The weak attraction of the MB-positive ions to the protonated hydroxyl and/or carbonyl groups on the adsorbent surface may lead to the low removal efficiency. In other words, because hydrogen ions compete with the adsorbent for active sites, the electrostatic attraction between the cation dye and adsorbent surface is not strong enough53. According to Mulugeta and Lelisa54, more active sites for adsorption become available by the negative charges on the adsorbent surface. Numerous investigations have also revealed that this advantageous adsorption occurs around pH of 8–1055,56,57,58.
The point of zero charge (pHpzc) can be employed to evaluate the surface charge characteristics of the OS particles and the interaction between the functional groups of MB and the adsorbent. The isoelectric point (IEP) of the adsorbent is determined to be 5.5, at which point the net surface charge becomes neutral (Fig. 4B). When the pH falls below 5.5, the adsorbent acquires a positive charge due to the hydrogen bond formation, potentially impeding the adsorption of MB by causing repulsive forces. Conversely, when the pH increases above 5.5 (e.g., 9), the OS surface becomes negatively charged, promoting the adsorption of cationic MB through electrostatic interactions59. This favorable basic environment can be attributed to the presence of OH and COO− groups on the adsorbent's surface.
Effect of adsorbent dosage
For a given initial concentration of adsorbate, the amount of adsorbent plays a significant role in determining the sorption capacity60. Figure 5A depicts the adsorption of MB dye (20 mg L−1) as a function of OS dosage. Increasing the adsorbent dose increases the percentage of dye removal. It is clear that increasing the adsorbent dose increases the number of accessible adsorption sites, which increases the extent of adsorbed dye. Adsorption density decreases upon an increase in the adsorbent dose, because adsorption does not completely saturate adsorption sites61. Another possibility is that the high adsorbent dose causes inter-particle interactions, such as aggregation, which would reduce the adsorbent's overall surface area and lengthen the diffusional path62.
Impact of initial MB concentration
Figure 5B shows how the initial MB concentration affects the adsorption of MB onto OS. The highest MB removal percentage is found to be at 20 mg L−1 equal to 91.02% for the OS adsorbent. The adsorption of MB decreases somewhat, reaching 89.31% and 86.83% at 60 and 80 mg L−1 of the MB dye, respectively. At higher concentrations, the amount of dye removal drops accordingly. This is as a result of the adsorbent surface's unoccupied binding sites being available63. The removal effectiveness for the OS declines to 72.03% at 140 mg L−1, as seen in Fig. 5B. The inhibitory effects of the high dye concentration may be responsible for the low decolorization percentage and gradual drop in the MB removal at high concentrations64, due to a reduction in adsorption's required active sites and an increase in mass transfer's driving force65. Low MB dye concentrations can speed up and improve the adsorption process, but high initial concentrations hold the dyes molecules in the solution by solubilizing them. Additionally, it is found that adsorption equilibrium is reached more quickly and with a higher extent of MB adsorption at lower initial dye concentrations. This result could be explained by the lower initial concentration of MB molecules becoming spontaneously accessible to the open active sites on the surface of the OS adsorbent66.
Effect of contact time
Over time intervals ranging from 15 to 180 min, the impact of contact time on the adsorption of MB dye on OS adsorbent is examined. The adsorption extent as a function of contact time is shown in Fig. 5C; the removal efficiency of MB dye by the OS increases with increasing contact time, demonstrating how time affects the adsorption process67. According to Fig. 5C, it peaks at around 90 min and then is nearly stabilized for the remaining 180 min. Thus, it can be concluded that 90 min would be a sufficient time to achieve equilibrium in subsequent trials. Hence, this time period is adopted for all subsequent observations.
Studying the key process variables demonstrates that OS is an effective material in removing the MB dye, with a good removal efficiency equal to 93.65% compared to other low-cost adsorbents such as pine leaves21 and pine cone22 with the removal efficiencies equal to ~ 80% and 63.83–94.82%, respectively, (Table 1).
Regeneration of adsorbent
During the regeneration phase, the OS used for adsorption is separated by filtration, washed with deionized water to remove residual MB, and finally dried at room temperature. Then, 200 mg of the spent adsorbent is added to 50 mL of 0.1 M HCL and stirred for 6 h. Later, the adsorbent is separated, washed, and oven-dried at 110 C for 3 h prior to being utilized in the regeneration cycles. The regeneration results are reported in Fig. 6. It is clear that the regeneration process of the adsorbent is successful, noting that the adsorption effectiveness decreases by a moderate level in the four cycles. Therefore, it can be concluded that the substance is effective even if it is used more than once.
Isotherm and kinetic investigation
Isotherm and equilibrium models
The adsorption isotherm model can explain the equilibrium uptake at a specific temperature for a certain adsorbent–adsorbate pair 68. The Langmuir, Freundlich, Dubinin–Radushkevich, and Temkin isotherms are examined in this study. The best-fit model is chosen based on the correlation coefficient (R2) value. Table 3 and Fig. 7 provide the results of various models considered in this study.
Among the models that fit the equilibrium data, the best ones are Langmuir and Temkin models. The RL value indicates the shape of the isotherm. Mckay et al.69 stated that RL values between 0 and 1 indicate favorable adsorption. In the present study, RL value for the MB dye adsorption equals 0.316. Therefore, the adsorption process is favorable. The maximum monolayer coverage capacity qmax = 44.5 mg g−1 with a correlation coefficient R2 = 0.9968. Temkin isotherm assumes that all molecules' adsorption heat will drop linearly as the adsorbent surface is covered in more molecules due to the adsorbent-adsorbate interactions. This model assumes that, up to a particular maximum binding energy, the adsorption is described by a regular distribution of binding energies in the layer70.
Adsorption kinetics
Table 4 and Fig. 8 provide the calculated parameters for various kinetics models as well as the validation results. Based on the outcomes, the Elovich and pseudo-second order models can accurately forecast the kinetics of MB adsorption onto the OS sample. The rate of solute adsorption is assumed proportional to the accessible sites on the adsorbent, according to the pseudo second-order model. The solute amount on the adsorbent surface determines the reaction rate, and the driving force is proportional to the total number of active sites present on the adsorbent surface71. Elovich model helps in predicting a system's activation and deactivation energy as well as mass and surface diffusion. The model assumes that as the amount of deposited solute increases, the rate of solute adsorption reduces exponentially72.
Mass transfer models
This study presents the primary stage in the analysis of the mass transfer of surface adsorption process to forecast the properties of the OS adsorbent and the surface adsorption behavior. The two mass transfer models (WM and BB) for the surface adsorption of dye molecules on the adsorbent are shown in Table 5 which implies that the experimental results suite the model well. The model constant and R2 values are displayed in Table 5. These values indicate that a rate-controlling step is necessary for the diffusion of MB molecules into the pores of the adsorbent.
Adsorption thermodynamics
It is important to calculate thermodynamic properties such as enthalpy, entropy, and Gibbs free energy in an adsorption process. Thermodynamic calculations are conducted for the investigated adsorbent at 25, 35, and 45 °C with a fixed MB concentration. At 25, 35, and 45 °C, the ∆G° values for the OS adsorbent case are determined to be −16, −20, and −23 kJ mol−1, respectively. In addition, the values of ∆H° and ∆S° are 70 kJ mol−1 K and 290 J mol−1 K−1 , respectively. Based on the negative values found for Gibbs free energy, it can be concluded that the MB adsorption on the OS is spontaneous at all temperatures73. Positive entropy values further support the spontaneity and highly irregular behavior of the adsorption process. The endothermic nature of the MB adsorption process is explained by the positive values of the enthalpy while dealing with the OS sample74. This is also confirmed by the findings related to the impact of temperature on the adsorption of MB onto OS. Table 6 provides the thermodynamic parameters.
Mechanisms of adsorption
Four steps are often involved in the mechanism of MB reduction by adsorption on OS69:(1) bulk diffusion, or the movement of the MB component from the solution's bulk to the adsorbent surface; (2) MB dye molucules transfer to the adsorbent surface across the boundary layer (film diffusion); (3) transport of the MB dye molucules from the particle's surface inside its interior pores (also known as intra-particle or pore diffusion); and (4) MB adsorption through physical adsorption using van der Waals forces at an active location on the material's surface70. To better understand the MB adsorption mechanisms, the kinetic and isotherms results are obtained. It makes the following assumptions in light of the Langmuir correlation coefficient (R2 = 0.996): (1) adsorption occurs in a monolayer; (2) a finite (fixed) number of equal and equivalent definite localized sites cannot host adsorption; and (3) even on nearby sites, steric hindrance and lateral contact between the adsorbed molecules can develop. The n value determines the Freundlich isotherm adsorption intensity; n = 2–10, n = 1–2, and n < 1 correspond to good features, moderate adsorption, and bad adsorption, respectively71. Consequently, the intra-particle diffusion and heterogeneous surface are confirmed by the Freundlich isotherm with 0.4 < n < 1, which is in line with the Temkin isotherm. The adsorption of the MB chemical by OS occurs in two phases, as shown by the kinetics of the intra-particle diffusion model and the impact of contact time: (1) the adsorption of MB compounds occurs on the OS external surface, which is the instantaneous adsorption stage (film diffusion); and (2) the movement of MB compound within the OS pores is confined (particle diffusion). According to Table 5, in addition to the surface adsorption characteristics, mass transfer mechanisms, such as intra-particle diffusion and external diffusion, and adsorption thermodynamics can contribute to the interactions between the MB compound and OS particles. The creation of an intra-bond between the OS and MB chemical controls the adsorption process on the OS in addition to surface interaction, particularly with low specific area. This explains the high adsorption capacity achieved based on the Temkin isotherm. The adsorption mechanisms are generally complicated and may include additional elements including temperature, ion exchange interactions, and dispersive force71,72.
Conclusions
Olive stone, a waste product, is an inexpensive adsorbent capable of removing methylene blue (MB) dye from aqueous solutions. This study assesses the removal of MB dye through batch tests. The MB dye adsorption on the OS follows the pseudo-second-order and the Elovich models based on the results and statistical analysis of various models. Modeling the adsorption isotherms with Langmuir and Temkin models offers a better fit. Additionally, the mass transfer mechanism of the surface adsorption for dye molecules on the OS adsorbent is examined using two mass transfer models. In the experimental phase of MB dye wastewater treatment, the tests are conducted to evaluate the effects of the solution pH, adsorbent dose, dye initial concentration, adsorption time, and temperature on the adsorption amount. According to the thermodynamic analysis, dye adsorption by the OS is endothermic and spontaneous. Overall, olive stone (as an adsorbent) effectively removes MB dye from aqueous solutions. Thus, it is a good choice for this purpose in wastewater treatment. This is because chemically-processed adsorbents entail pollution risks. As a result, OS offers an adsorbent for MB removal from wastewater that is low-cost, more ecologically friendly, and economically viable. Systematic tests might be implemented successfully to increase the use of OS and sustainably treat MB-contaminated wastewater at small to large scales. For future work, it is recommended to conduct the adsorption tests at broad ranges of temperatures, pressures, concentrations/compositions, and types of contaminants in various water and wastewater streams.
Data availability
All relevant data and material are presented in the main paper.
References
Ali, N. S. et al. Applying MCM-48 mesoporous material, equilibrium, isotherm, and mechanism for the effective adsorption of 4-nitroaniline from wastewater. Sci. Rep. 13, 9837. https://doi.org/10.1038/s41598-023-37090-4 (2023).
Humadi, J. I. et al. Recovery of fuel from real waste oily sludge via a new eco-friendly surfactant material used in a digital baffle batch extraction unit. Sci. Rep. 13, 9931. https://doi.org/10.1038/s41598-023-37188-9 (2023).
Ali, N. S. et al. Utilization of loaded cobalt onto MCM-48 mesoporous catalyst as a heterogeneous reaction in a fixed bed membrane reactor to produce isomerization product from n-heptane. Catalysts 13, 1138. https://doi.org/10.3390/catal13071138 (2023).
Mahdi, A. E. et al. Effective adsorption of 2-nitroaniline from wastewater applying mesoporous material MCM-48: Equilibrium, isotherm, and mechanism investigation. Desalin. Water Treat. 300, 120–129. https://doi.org/10.5004/dwt.2023.29741 (2023).
Khudhur, R. H. et al. Adsorption of anionic azo dye from aqeous wastewater using zeolite NaX as an efficient adsorbent. Desalin. Water Treat. 306, 245–252. https://doi.org/10.5004/dwt.2023.29861 (2023).
Bhagat, C., Kumar, M., Tyagi, V. K. & Mohapatra, P. K. Proclivities for prevalence and treatment of antibiotics in the ambient water: A review. npj Clean Water 3(1), 42. https://doi.org/10.1038/s41545-020-00087-x (2020).
Ali, S. S. et al. Construction of a novel cold-adapted oleaginous yeast consortium valued for textile azo dye wastewater processing and biorefinery. Fuel 285, 119050. https://doi.org/10.1016/j.fuel.2020.119050 (2021).
Maged, A., Iqbal, J., Kharbish, S., Ismael, I. S. & Bhatnagar, A. Tuning tetracycline removal from aqueous solution onto activated 2:1 layered clay mineral: Characterization, sorption and mechanistic studies. J. Hazard. Mater. 384, 121320. https://doi.org/10.1016/j.jhazmat.2019.121320 (2020).
Ali, N. S., Majdi, HSh., Albayati, T. M. & Jasim, D. J. Adsorption of aniline from aqueous solutions onto a nanoporous material adsorbent: Isotherms, kinetics, and mass transfer mechanisms. Water Pract. Technol. 18(9), 2136. https://doi.org/10.2166/wpt.2023.132 (2021).
Mahdi, A. E. et al. Investigation of equilibrium, isotherm, and mechanism for the efficient removal of 3-nitroaniline dye from wastewater using mesoporous material MCM-48. Prog. Color Colorants Coat. 16, 387–398. https://doi.org/10.30509/pccc.2023.167111.1205 (2023).
Ali, N. S., Kalash, K. R., Ahmed, A. N. & Albayati, T. M. Performance of a solar photocatalysis reactor as pretreatment for wastewater via UV, UV/TiO2, and UV/H2O2 to control membrane fouling. Sci. Rep. 12, 16782. https://doi.org/10.1038/s41598-022-20984-0 (2022).
Jabbar, N. M., Alardhi, S. M., Mohammed, A. K., Salih, I. K. & Albayati, T. M. Challenges in the implementation of bioremediation processes in petroleum-contaminated soils: A review. Environ. Nanotechnol. Monit. Manag. 18, 100694. https://doi.org/10.1016/j.enmm.2022.100694 (2022).
Khadim, A. T., Albayati, T. M. & Cata Saady, N. M. Removal of sulfur compounds from real diesel fuel employing the encapsulated mesoporous material adsorbent Co/MCM-41 in a fixed-bed column. Microporous Mesoporous Mater. 341, 112020. https://doi.org/10.1016/j.micromeso.2022.112020 (2022).
Hamedi, H., Ehteshami, M., Mirbagheri, S. A., Rasouli, S. A. & Zendehboudi, S. Current status and future prospects of membrane bioreactors (MBRs) and fouling phenomena: A systematic review. Can. J. Chem. Eng. 97(1), 32–58. https://doi.org/10.1002/cjce.23345 (2019).
Ali, N. S. et al. Modification of SBA-15 mesoporous silica as an active heterogeneous catalyst for the hydroisomerization and hydrocracking of n-heptane. Heliyon 8, e09737. https://doi.org/10.1016/j.heliyon.2022.e09737 (2022).
Al-Jaaf, H. J., Ali, N. S., Alardhi, S. M. & Albayati, T. M. Implementing eggplant peels as an efficient bio-adsorbent for treatment of oily domestic wastewater. Desalin. Water Treat. 245, 226–237. https://doi.org/10.5004/dwt.2022.27986 (2022).
Abbood, N. S. et al. Photocatalytic degradation of cefotaxime pharmaceutical compounds onto a modified nanocatalyst. Res. Chem. Intermediat. 49, 43–56. https://doi.org/10.1007/s11164-022-04879-3 (2023).
Mohammed, M. Y., Ali, A. M. & Albayati, T. M. Choline chloride-based deep eutectic solvents for ultrasonic-assisted oxidative desulfurization of actual heavy crude oil. Chem. Eng. Res. Des. 182, 659–666. https://doi.org/10.1016/j.cherd.2022.03.047 (2022).
Abd Al-Khodor, Y. A. & Albayati, T. M. Real heavy crude oil desulfurization onto nanoporous activated carbon implementing batch adsorption process: Equilibrium, kinetics, and thermodynamic studies. Chem. Afr. 6, 747–756. https://doi.org/10.1007/s42250-022-00482-6 (2023).
Muslim, W. A., Albayati, T. M. & Al Nasri, S. K. Decontamination of actual radioactive wastewater containing 137Cs using bentonite as a natural adsorbent: Equilibrium, kinetics, and thermodynamic studies. Sci. Rep. 12, 13837. https://doi.org/10.1038/s41598-022-18202-y (2022).
Hamedi, H., Zendehboudi, S., Rezaei, N., Azizi, A. & Shahhoseini, F. Application of functionalized Fe(3)O(4) magnetic nanoparticles using CTAB and SDS for oil separation from oil-in-water nanoemulsions. Langmuir 39(23), 7995–8007. https://doi.org/10.1021/acs.langmuir.2c03266 (2023).
Gutiérrez, M., Grillini, V., Mutavdžić Pavlović, D. & Verlicchi, P. Activated carbon coupled with advanced biological wastewater treatment: A review of the enhancement in micropollutant removal. Sci. Total Environ. 790, 148050. https://doi.org/10.1016/j.scitotenv.2021.148050 (2021).
Bezerra de Araujo, C. M. et al. Adsorptive removal of dye from real textile wastewater using graphene oxide produced via modifications of hummers method. Chem. Eng. Commun. 206(11), 1375–1387. https://doi.org/10.1080/00986445.2018.1534232 (2019).
Al-Jadir, T. et al. Modeling of lead (II) ion adsorption on multiwall carbon nanotubes using artificial neural network and Monte Carlo technique. Chem. Eng. Commun. 210(10), 1642–1658. https://doi.org/10.1080/00986445.2022.2129622 (2023).
Dubey, K. et al. Defects and oxygen vacancies modified properties of transition metal doped Ce0.95X0.05O2 (X = Fe Co, Ni) nanoparticles. Mater. Sci. Eng. B 288, 116154. https://doi.org/10.1016/j.mseb.2022.116154 (2023).
Uddin, M. J., Ampiaw, R. E. & Lee, W. Adsorptive removal of dyes from wastewater using a metal-organic framework: A review. Chemosphere 284, 131314. https://doi.org/10.1016/j.chemosphere.2021.131314 (2021).
Moradi, O. & Sharma, G. Emerging novel polymeric adsorbents for removing dyes from wastewater: A comprehensive review and comparison with other adsorbents. Environ. Res. 201, 111534. https://doi.org/10.1016/j.envres.2021.111534 (2021).
Karić, N. et al. Bio-waste valorisation: Agricultural wastes as biosorbents for removal of (in)organic pollutants in wastewater treatment. Chem. Eng. J. Adv. 9, 100239. https://doi.org/10.1016/j.ceja.2021.100239 (2022).
dos Santos, K. J. L. et al. Syagrus oleracea-activated carbon prepared by vacuum pyrolysis for methylene blue adsorption. Environ. Sci. Pollut. Res. 26(16), 16470–16481. https://doi.org/10.1007/s11356-019-05083-4 (2019).
Pang, X. et al. Adsorption of crystal violet on biomasses from pecan nutshell, para chestnut husk, araucaria bark and palm cactus: Experimental study and theoretical modeling via monolayer and double layer statistical physics models. Chem. Eng. J. 378, 122101. https://doi.org/10.1016/j.cej.2019.122101 (2019).
dos Santos, K. J. L. et al. Wodyetia bifurcata biochar for methylene blue removal from aqueous matrix. Bioresour. Technol. 293, 122093. https://doi.org/10.1016/j.biortech.2019.122093 (2019).
Espadas-Aldana, G., Vialle, C., Belaud, J.-P., Vaca-Garcia, C. & Sablayrolles, C. Analysis and trends for Life Cycle Assessment of olive oil production. Sustain. Prod. Consumpt. 19, 216–230. https://doi.org/10.1016/j.spc.2019.04.003 (2019).
Boubakri, S. et al. Removal of two anionic reactive textile dyes by adsorption into MgAl-layered double hydroxide in aqueous solutions. Environ. Sci. Pollut. Res. 25, 23817–23832 (2018).
Alardhi, S. M., Fiyadh, S. S., Salman, A. D. & Adelikhah, M. Prediction of methyl orange dye (MO) adsorption using activated carbon with an artificial neural network optimization modeling. Heliyon https://doi.org/10.1016/j.heliyon.2023.e12888 (2023).
Mahdia, A. H., Jaida, G. M. & Alardhib, S. M. Artificial neural network modelling for the removal of lead from wastewater by using adsorption process. Desalin. Water Treat. 244, 110–119 (2021).
Hu, H. & Xu, K. Chapter 8—Physicochemical technologies for HRPs and risk control. In High-Risk Pollutants in Wastewater (Ren, H., Zhang, X. Eds.). 169–207. (Elsevier, 2020). https://doi.org/10.1016/B978-0-12-816448-8.00008-3.
Nouri, N., Tasviri, M. & Zendehboudi, S. Effect of poly(vinyl alcohol) on catalytic performance of Al-pillared clay in alkylation of aromatic hydrocarbons with olefins. Ind. Eng. Chem. Res. 62(17), 6612–6625. https://doi.org/10.1021/acs.iecr.2c02295 (2023).
Cabooter, D. et al. Measurement and modelling of the intra-particle diffusion and b-term in reversed-phase liquid chromatography. J. Chromatogr. A 1637, 461852. https://doi.org/10.1016/j.chroma.2020.461852 (2021).
Prajapati, A. K. & Mondal, M. K. Comprehensive kinetic and mass transfer modeling for methylene blue dye adsorption onto CuO nanoparticles loaded on nanoporous activated carbon prepared from waste coconut shell. J. Mol. Liq. 307, 112949 (2020).
De Castro, M. L. F. A. et al. Adsorption of methylene blue dye and Cu(II) ions on EDTA-modified bentonite: Isotherm, kinetic and thermodynamic studies. Sustain. Environ. Res. 28(5), 197–205. https://doi.org/10.1016/j.serj.2018.04.001 (2018).
Jua, L. Y. et al. Modeling of methylene blue adsorption using functionalized buckypaper/polyvinyl alcohol membrane via ant colony optimization. Environ. Pollut. 259, 113940. https://doi.org/10.1016/j.envpol.2020.113940 (2020).
Awad, A. M. et al. Adsorption of organic pollutants by nanomaterial-based adsorbents: An overview. J. Mol. Liq. 301, 112335. https://doi.org/10.1016/j.molliq.2019.112335 (2020).
Sounni, F., Aissam, H., Ghomari, O., Merzouki, M. & Benlemlih, M. Electrocoagulation of olive mill wastewaters to enhance biogas production. Biotechnol. Lett. 40(2), 297–301. https://doi.org/10.1007/s10529-017-2464-5 (2018).
Zhang, Y. H. & Lynd, L. R. Toward an aggregated understanding of enzymatic hydrolysis of cellulose: Noncomplexed cellulase systems. Biotechnol. Bioeng. 88(7), 797–824. https://doi.org/10.1002/bit.20282 (2004).
Allaoui, S. et al. Valorization of crude olive stone in the removing of polyphenols from crude olive mill wastewater: Kinetic, isotherm and mechanism study. Heliyon 7(7), e07525. https://doi.org/10.1016/j.heliyon.2021.e07525 (2021).
Malekbala, M. R., Hosseini, S., Kazemi Yazdi, S., Masoudi Soltani, S. & Malekbala, M. R. The study of the potential capability of sugar beet pulp on the removal efficiency of two cationic dyes. Chem. Eng. Res. Des. 90(5), 704–712. https://doi.org/10.1016/j.cherd.2011.09.010 (2012).
Akar, T. et al. An attractive agro-industrial by-product in environmental cleanup: Dye biosorption potential of untreated olive pomace. J. Hazard. Mater. 166(2), 1217–1225. https://doi.org/10.1016/j.jhazmat.2008.12.029 (2009).
Han, M. H. & Yun, Y.-S. Mechanistic understanding and performance enhancement of biosorption of reactive dyestuffs by the waste biomass generated from amino acid fermentation process. Biochem. Eng. J. 36(1), 2–7. https://doi.org/10.1016/j.bej.2006.06.010 (2007).
Kaya, N. et al. Fabrication and characterization of olive pomace filled PP composites. Compos. Part B Eng. 150, 277–283. https://doi.org/10.1016/j.compositesb.2017.08.017 (2018).
Jabeen, B. & Rafique, U. Synthesis and application of metal doped silica particles for adsorptive desulphurization of fuels. Environ. Eng. Res. 19(3), 205–214. https://doi.org/10.4491/eer.2014.017 (2014).
Gülel, Ş. & Güvenilir, Y. Using olive stone powder for biodegradation of bio-based polyamide 5.6. Proceedings 69(1), 2 (2021).
Amar, M. B., Walha, K. & Salvadó, V. Evaluation of olive stones for Cd(II), Cu(II), Pb(II) and Cr(VI) biosorption from aqueous solution: Equilibrium and kinetics. Int. J. Environ. Res. 14(2), 193–204. https://doi.org/10.1007/s41742-020-00246-5 (2020).
Manna, S., Roy, D., Saha, P., Gopakumar, D. A. & Thomas, S. Rapid methylene blue adsorption using modified lignocellulosic materials. Process Saf. Environ. Protect. 107, 346–356 (2017).
Mulugeta, M. & Lelisa, B. Removal of methylene blue (Mb) dye from aqueous solution by bioadsorption onto untreated parthenium hystrophorous weed. Mod. Chem. Appl. 2(4), 146 (2014).
Ovando-Medina, V. M. et al. Composite of cellulosic agricultural waste coated with semiconducting polypyrrole as potential dye remover. Polymer Compos. 35(1), 186–193. https://doi.org/10.1002/pc.22649 (2014).
Hassaan, M. A. et al. Isotherm and kinetic investigations of sawdust-based biochar modified by ammonia to remove methylene blue from water. Sci. Rep. 13(1), 12724. https://doi.org/10.1038/s41598-023-39971-0 (2023).
Egbosiuba, T. C. et al. Ultrasonic enhanced adsorption of methylene blue onto the optimized surface area of activated carbon: Adsorption isotherm, kinetics and thermodynamics. Chem. Eng. Res. Des. 153, 315–336. https://doi.org/10.1016/j.cherd.2019.10.016 (2020).
Babaei, A. A. et al. Experimental and modeling study on adsorption of cationic methylene blue dye onto mesoporous biochars prepared from agrowaste. Desalin. Water Treat. 57(56), 27199–27212. https://doi.org/10.1080/19443994.2016.1163736 (2016).
Ahmad, R. & Ansari, K. Comparative study for adsorption of congo red and methylene blue dye on chitosan modified hybrid nanocomposite. Process. Biochem. 108, 90–102. https://doi.org/10.1016/j.procbio.2021.05.013 (2021).
Hojati, S. & Landi, A. Removal of zinc from a metal plating wastewater using an Iranian sepiolite: Determination of optimum conditions. Desalin. Water Treat. 53(8), 2117–2124. https://doi.org/10.1080/19443994.2013.861771 (2015).
Yu, L. J., Shukla, S. S., Dorris, K. L., Shukla, A. & Margrave, J. L. Adsorption of chromium from aqueous solutions by maple sawdust. J. Hazard. Mater. 100(1), 53–63. https://doi.org/10.1016/S0304-3894(03)00008-6 (2003).
Shukla, A., Zhang, Y.-H., Dubey, P., Margrave, J. L. & Shukla, S. S. The role of sawdust in the removal of unwanted materials from water. J. Hazard. Mater. 95(1), 137–152. https://doi.org/10.1016/S0304-3894(02)00089-4 (2002).
Vijayaraghavan, K. & Yun, Y.-S. Biosorption of C.I. reactive black 5 from aqueous solution using acid-treated biomass of brown seaweed Laminaria sp. Dyes Pigments 76(3), 726–732. https://doi.org/10.1016/j.dyepig.2007.01.013 (2008).
Anjaneya, O., Souche, S. Y., Santoshkumar, M. & Karegoudar, T. B. Decolorization of sulfonated azo dye Metanil Yellow by newly isolated bacterial strains: Bacillus sp. strain AK1 and Lysinibacillus sp. strain AK2. J. Hazard Mater. 190(1–3), 351–358. https://doi.org/10.1016/j.jhazmat.2011.03.044 (2011).
Pathania, D., Sharma, S. & Singh, P. Removal of methylene blue by adsorption onto activated carbon developed from Ficus carica bast. Arab. J. Chem. 10, S1445–S1451 (2017).
Marrakchi, F., Auta, M., Khanday, W. A. & Hameed, B. H. High-surface-area and nitrogen-rich mesoporous carbon material from fishery waste for effective adsorption of methylene blue. Powder Technol. 321, 428–434. https://doi.org/10.1016/j.powtec.2017.08.023 (2017).
Potgieter, J. H., Potgieter-Vermaak, S. S. & Kalibantonga, P. D. Heavy metals removal from solution by palygorskite clay. Miner. Eng. 19(5), 463–470. https://doi.org/10.1016/j.mineng.2005.07.004 (2006).
Muttakin, M., Mitra, S., Thu, K., Ito, K. & Saha, B. B. Theoretical framework to evaluate minimum desorption temperature for IUPAC classified adsorption isotherms. Int. J. Heat Mass Transf. 122, 795–805. https://doi.org/10.1016/j.ijheatmasstransfer.2018.01.107 (2018).
Azaman, S. H., Afandi, A., Hameed, B. & Din, A. M. Removal of Malachite green from aqueous phase using coconut shell activated carbon: Adsorption. Desorpt. Reusabil. Stud. J. Appl. Sci. Eng. 21(3), 317–330 (2018).
Iqbal, J. et al. Adsorption of acid yellow dye on flakes of chitosan prepared from fishery wastes. Arab. J. Chem. 4(4), 389–395. https://doi.org/10.1016/j.arabjc.2010.07.007 (2011).
Tan, K. L. & Hameed, B. H. Insight into the adsorption kinetics models for the removal of contaminants from aqueous solutions. J. Taiwan Inst. Chem. Eng. 74, 25–48. https://doi.org/10.1016/j.jtice.2017.01.024 (2017).
George William, K., Serkan, E., Atakan, Ö., Özcan, H.K. & Serdar, A. Modelling of adsorption kinetic processes—Errors, theory and application. In Advanced Sorption Process Applications, IntechOpen, Rijeka. Chap. 10 (Serpil, E. Ed.) (2018). https://doi.org/10.5772/intechopen.80495.
Ebelegi, A. N., Ayawei, N. & Wankasi, D. Interpretation of adsorption thermodynamics and kinetics. Open J. Phys. Chem. 10(03), 17. https://doi.org/10.4236/ojpc.2020.103010 (2020).
Hasanpour, M. & Hatami, M. Application of three dimensional porous aerogels as adsorbent for removal of heavy metal ions from water/wastewater: A review study. Adv. Colloid Interface Sci. 284, 102247. https://doi.org/10.1016/j.cis.2020.102247 (2020).
Acknowledgements
The authors are grateful to the Department of Chemical Engineering at the University of Technology-Iraq, the Mustansiriyah University/College of Engineering/Materials Engineering Department, Baghdad-Iraq, the Department of Chemical and Petroleum Industries Engineering, Al-Mustaqbal University College, Babylon, Iraq, and the Memorial University, St. John’s, NL A1B 3X5, Canada. The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University of funding this work through large group Research Project under grant number RGP2/133/44.
Funding
This research was funded by the Dean of Scientific Research at King Khalid University under the grant number RGP2/133/44.
Author information
Authors and Affiliations
Contributions
S.M.A., H.G.S., and N.S.A.: Conceptualization, Methodology, Investigation, Data curation, Writing-original draft; A.H.K., I.K.S., and N.M.C.S.: Writing-review & editing, Supervision; S.Z., T.M.A., H.N.H.: Conceptualization, Supervision, Writing-review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alardhi, S.M., Salih, H.G., Ali, N.S. et al. Olive stone as an eco-friendly bio-adsorbent for elimination of methylene blue dye from industrial wastewater. Sci Rep 13, 21063 (2023). https://doi.org/10.1038/s41598-023-47319-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-47319-x
- Springer Nature Limited
This article is cited by
-
Banana leaves powder as an effective, low-cost adsorbent for methyl blue dye removal: kinetics, isothermal, thermodynamics, ANN and DFT analysis
International Journal of Environmental Science and Technology (2024)
-
Zeolite 13X incorporated with Zn-Ce oxide nanocatalyst for removal of Reactive Red 120 dye: RSM-based approach
Environmental Monitoring and Assessment (2024)
-
Utilizing olive leaves biomass as an efficient adsorbent for ciprofloxacin removal: characterization, isotherm, kinetic, and thermodynamic analysis
Environmental Monitoring and Assessment (2024)
-
Scalable production of efficient hybrid nanoadsorbent based on porous carbon nanoparticles and polyaniline nanofibers for removal of toxic methylene blue
Journal of Thermal Analysis and Calorimetry (2024)