Abstract
The structuring of plant assemblages along environmental gradients is typically explained by shifts from competition (limiting similarity) to environmental filtering as the environment becomes more stressful. However, facilitation, weaker-competitor exclusion, environmental heterogeneity, and the colonization-competition tradeoff can also structure plant assemblages along gradients. These assembly processes act on different plant traits and organs, and their prevalence varies with respect to the spatial scale. Using patterns of functional diversity, coupled with patterns of species association at two spatial scales, here we discern the assembly processes that structure shrub communities in four localities along an aridity gradient of the Atacama Desert. At each site, we calculated functional dispersion indexes for above- and below-ground traits, and patterns of species association at a patch and neighborhood scale. Our results revealed that at the patch scale in intermediate levels of aridity, the dominant assembly process was within-site environmental heterogeneity. At the neighborhood scale, communities are assembled mainly through random processes. Nonetheless, in some communities, the dominant assembly process was competition via limiting similarity or exclusion of the weaker competitor, and these did not change along the gradient. Together, these results reveal that environmental heterogeneity and competition are the main drivers of plant community assembly in a hyper-arid environment.
Similar content being viewed by others
Introduction
Unravelling the processes that influence plant assemblages along environmental gradients is key to understanding patterns of plant diversity along such gradients1. Accordingly, the stress-dominance hypothesis (SDH)2,3 proposes that competition is the primary force structuring plant assemblages in the less stressful end of a gradient, but as environmental stress increases, so does the relative importance of abiotic filters. This shift is expected to lead to a change from a pattern of trait divergence to one of trait convergence2,4. Although some studies support the predictions of the SDH2, others do not3,5,6,7,8. The lack of consistent support to the SDH is partly explained because (1) other processes, such as facilitation, exclusion of the weaker competitor, and microenvironmental heterogeneity, can also structure plant assemblages along environmental gradients7,9,10,11,12,13, and (2) because the prevalence of these processes is scale-dependent; that is, the importance of the different assembly processes changes across different scales4,14,15.
Patterns of trait convergence and divergence change along environmental gradients; however, a simple trend of increasing or decreasing functional divergence is not an obvious indicator of the underlying assembly processes that structure plant assemblages11. For example, the widespread expectation of the SDH is that trait convergence should occur in the most stressful abiotic conditions, whereas trait divergence should be more prevalent in less stressful environments2,3; however, competition can also lead to trait convergence via weaker-competitor exclusion or hierarchical competition13,16. Alternatively, the stress gradient hypothesis (SGH) predicts that facilitation and competition vary inversely along environmental gradients, with facilitation becoming more common with increasing abiotic stress because it ameliorates micro-environmental conditions for less stress-tolerant species9. Consequently, facilitation can increase the functional diversity of plant assemblages, which should result in high trait divergence6,7,17,18,19. Furthermore, some studies suggest that the effect of facilitation can decrease when the environment becomes highly stressful20,21, leading to competition being the dominant driver of plant assemblages at both ends of the gradient22. Therefore, to better understand the processes that shape plant communities, patterns of functional diversity should be combined with patterns of spatial association11,23.
In the context of non-random patterns of species association24, segregation is typically associated with competition, while aggregation is associated with facilitation24,25. However, various factors can contribute to these spatial patterns10,11,14,23. For example, species may segregate due to either competition or environmental heterogeneity, where different species with varying abiotic tolerances can occupy distinct microenvironments within a community11,12. Conversely, aggregation may result from facilitation, seed trapping, dispersal limitation, and environmental heterogeneity, where plant species tend to cluster together in favorable habitats10,14. Segregation can also arise from differences in species dispersal abilities, resulting in a trade-off between their colonization and competitive abilities26,27,28,29.
Several assembly processes can result in trait convergence or divergence and species aggregation or segregation along an environmental gradient. However, the relative importance of these processes varies depending on the spatial scale under consideration4,14,15. Accordingly, competition and facilitation can change along environmental gradients at small (i.e., neighborhood) spatial scales11,12,13,23, while abiotic filtering tends to drive trait convergence at larger (i.e., patch) spatial scales. Conversely, environmental heterogeneity can lead to either trait convergence or divergence4,10,23,30. Additionally, at larger spatial scales, differences in the dispersal and competitive abilities of plants can also shape plant assemblages (via a colonization-competition trade-off) and drive patterns of trait divergence26,27,28,29. Therefore, it is crucial to examine the assembly processes operating at different spatial scales to untangle the factors that shape plant assemblages along environmental gradients.
A further complexity in understanding how plant assemblages are organized along environmental gradients is that different assembly processes can operate within a single locality but on different traits or plant organs2,3,5,6,7,8. Consequently, if all plant traits are examined jointly, a dominant assembly process in a locality may not emerge. For example, Spasojevic and Suding6 found that when leaf traits and plant height were analyzed jointly, the pattern of trait dispersion of subalpine grassland communities along an environmental gradient did not change. However, when they examined each trait separately, divergence in plant height and leaf area increased at both ends of the gradient, suggesting that competition and facilitation, determined the structuring of these grassland assemblages in the least and most stressful ends. Similarly, in semi-arid scrubland communities along an aridity gradient, Gross et al.7 found that the signal of plant height shifted from competition in the less arid end of the gradient to facilitation towards the more arid end; conversely, the intensity of competition increased as the environment became more arid for the specific leaf area (SLA). These and other studies have focused on above-ground traits based on the assumption that aboveground competition is high due to light limitation (e.g., Chalmandrier et al.15; de Belo et al.23); however, when soil resources (e.g., water availability and nutrients) are limiting, below-ground traits play critical roles in response to environmental filtering (e.g., León et al.31) and concerning competitive and facilitative interactions10,32,33,34,35,36. For example, Butterfield, et al.35 studying perennial grassland communities along an aridity gradient in Arizona found that as aridity increased, variation in specific root length (SRL) also increased, promoting species coexistence via niche partitioning of soil resources. Consequently, incorporating below-ground traits into assembly studies can improve our understanding of the processes that structure plant assemblages, particularly in arid ecosystems.
The Atacama Desert provides an ideal model for studying how different assembly processes structure shrub communities due to its steep aridity gradient36. Additionally, it has been proposed that the main processes structuring shrub assemblages at the neighborhood scale along this gradient shift from competition to facilitation (mediated by below-ground traits) as the environment becomes increasingly arid10. Furthermore, evidence indicates that along the aridity gradient, shifts in plant resource use strategies of the above-ground traits are influenced by aridity. In contrast, those mediated by below-ground traits respond to aridity and biotic interactions36. In light of this information, two expectations emerge concerning the neighborhood scale: (1) below-ground traits should align with the predictions of the SGH9, and (2) above-ground traits should align with the SDH2,3. On the other hand, localities along this gradient exhibit high soil resource heterogeneity, especially at intermediate levels of aridity. Consequently, at the patch scale, the effect of soil heterogeneity should be greater at intermediate levels of aridity than at either end of the gradient.
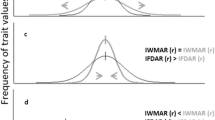
In this study, we employ a trait-based approach in conjunction with analyses of spatial patterns of species aggregation at two spatial scales (neighborhood and patch scale; Fig. 1) to examine if the assembly processes shaping shrub communities in the Atacama Desert change along the aridity gradient and across spatial scales. Specifically, we test two hypotheses. First, if the environmental heterogeneity at the patch scale changes along the gradient. We predict that shrub communities in sites with higher soil resource heterogeneity will exhibit either trait divergence and spatial aggregation or trait convergence and spatial segregation (Fig. 1). Second, we test if, at the neighborhood scale, increasing aridity leads to below-ground traits shifting from competition to facilitation (in accordance to the SGH), and to above-ground traits shifting from competition to habitat filtering (in accordance to the SDH). We predict that with increasing aridity, there will be a clinal change in below-ground traits, from segregation to aggregation, coupled with a pattern of trait divergence regardless of the level of aridity (Fig. 1). For above-ground traits; however, we predict a clinal change from segregation and divergence to aggregation and convergence (Fig. 1). At this scale, pooling of all traits should result in a shift from a pattern of trait divergence to a random pattern with increasing aridity. This is because the patterns of divergence and convergence for above- and below-ground traits, should cancel each other in the more arid end of the gradient.
Conceptual diagram illustrating the predictions derived from patterns of spatial association and functional diversity at different scales. At finer spatial scales (neighborhood), the combination of spatial association patterns and functional diversity provides four possible scenarios regarding the dominant assembly processes: (1) Facilitation is inferred when species are spatially aggregated and traits exhibit divergence; (2) limiting similarity is expected to prevail when species are spatially segregated and traits show a pattern of divergence; (3) abiotic constraints are assumed to be at play when species are spatially aggregated and traits exhibit convergence and; (4) weaker-competitor exclusion is inferred if species are spatially segregated, and traits show convergence. At a broader scale (among patches), we anticipate the following scenarios: (1) Habitat filtering is expected to result in trait convergence and spatial aggregation (abiotic constraint), (2) micro-Environmental Filtering (Environmental Heterogeneity) is indicated by trait convergence and spatial segregation, because species with varying ecological tolerances or resource-use strategies occupy different habitats or patches within a site. The effects of environmental heterogeneity can also be detected if plants are spatially aggerated and their traits diverge because plant species are occupying the more favorable patches within a site (environmental heterogeneity). Finally, (3) a competition-colonization tradeoff will lead to trait divergence and spatial segregation of species.
Results
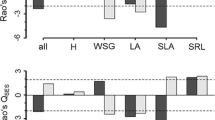
The mean standardized effect size (SES) of the functional dispersion index (FDis) was only significantly divergent at the patch scale and not at the neighborhood scale (Fig. 2). Specifically, the SES of FDis at the patch scale was significantly divergent in the two sites with intermediate levels of aridity (LLACHA and CHA). This was true when analyzing all traits together, as well as separately for below-ground traits at LLCHA (Fig. 2a,c) and above-ground traits at CHA (Fig. 2b). C-Scores revealed that shrub species were aggregated only in LLCHA, the second most arid location (Fig. 2a–c). In contrast, they were segregated in the wettest site (ROM; Fig. 2a–c) and were not different from random in the other sites (CHA and QL; Fig. 2a–c). At the neighborhood scale, the FDis did not differ significantly from the null distribution along the aridity gradient when all traits were examined jointly nor when above- and below-ground traits were examined separately (Fig. 2d–f). Nevertheless, some plots in all sites showed significant differences from the null distribution, indicating divergence or convergence (Fig. S1d–f). Additionally, at the neighborhood scale and irrespective of the aridity level, the SES of C-scores that significantly deviated from the null distribution indicated species segregation (Fig. 2d–f, Fig. S1d–f).
Mean standardized effect size (SES) of the functional diversity index (FDis) and SES of C-Scores (triangles) along the aridity gradient in the Atacama Desert as indicated by DEMAI (De Martonne Aridity Index) at the patch and neighborhood scale for: (a,d) all traits, (b,e) aboveground traits, (c,f) belowground traits. *Indicates significant statistical differences from the null expectation based on two tailed t-tests for FDis and, filled orange triangles represent SES C-scores values significantly different from the null expectation. Lower DEMAI index values indicate greater aridity. QL Quebrada El León, LLCHA Norte Llanos de Challe, CHA Chañaral de Aceituno, ROM Romeral.
Discussion
Our findings suggest that, at the patch scale, in the site with intermediate aridity levels (LLCHA), environmental heterogeneity was the main driver in structuring shrub assemblages; no distinct assembly process was evident in the other localities (Fig. 1a–c). These results provided partial support for our initial hypothesis, indicating that environmental heterogeneity exerts a more pronounced influence in locations with greater overall heterogeneity. Environmental heterogeneity in soil resources can shape plant communities by either enabling species with differing abiotic tolerances or resource utilization strategies to occupy different habitats within sites11,12 or by facilitating the coexistence of multiple species within more favorable patches10,14. In our study, the observed pattern in LLCHA, where plants were spatially aggregated and exhibited divergent traits (Fig. 2a,c), aligns with the latter explanation. Our findings also suggest that below-ground traits may have significantly contributed to the level of divergence observed when examining all plant traits collectively. In LLCHA, plants are likely using the most favorable habitats, and the reduction in niche overlap is achieved through greater trait divergence among species within a patch. This differentiation is largely influenced by below-ground traits, which may reduce interspecific niche overlap12,13. For example, in this same locality, Carvajal et al.36 observed a high level of diversity in root dry matter content (RDMC). This trait is associated with root lifespan, hence the diversity of RDMC may facilitate temporal partitioning of soil resources, thereby reducing competition among species13.
In CHA, plants exhibited divergent above-ground traits, but the spatial distribution of species was random. The higher functional diversity in CHA can be attributed to the greater variation in leaf traits associated with resource acquisition strategies, including leaf area, leaf dry matter content, and long-term water use efficiency36. However, because shrub species were randomly distributed in space, we cannot conclusively determine that the trade-off between colonization and competition is the primary mechanism structuring these shrub communities. This randomness in spatial distribution might contribute to species coexistence at large spatial scales26,27,28,29,32.
At the neighborhood scale, while c-scores indicated spatial segregation of shrubs, we did not observe a shift in the dominant assembly process along the aridity gradient. This remained true whether we analyzed all traits collectively or separately for above- and below-ground traits, as the FDis did not significantly differ from the null distribution across the aridity gradient (Fig. 2d–f). Consequently, we found no support to the hypothesis that as aridity increases, below-ground traits should reveal a shift from competition to facilitation (as predicted by the SGH), while above-ground traits should indicate a shift from competition to habitat filtering (as predicted by the SDH). At the neighborhood scale, it appears that shrub species exhibit a random assembly pattern across all sites along the gradient. There are several possible explanations for these results. First, it is worth noting that we did not account for intraspecific trait variation (ITV) in our analysis. Because ITV reduces the similarity among neighboring species in response to biotic interactions, it can increase the probability of detecting non-random assembly processes in plant communities (e.g., Jung et al.37; Siefert38). However, even if we had considered ITV, it is unlikely that it would have contributed to detecting assembly processes in our system. This is because among-patch ITV in our system is lower than 17% (Carvajal et al. unpublished data), and a substantial effect in detecting assembly processes typically requires ITV to exceed 30%39. Second, disparities in dispersal limitation can obscure the effects of the assembly process and result in random patterns. However, because dispersal limitation is believed to be more pronounced at larger spatial scales23, it is less likely to generate random patterns at the neighborhood scale. Third, intransitive competition (i.e., when there is no unique best competitor; sensu Soliveres et al.40) could also lead to a random pattern. This occurs because intransitive competition produces a spectrum of trait values, that may not necessarily result in trait convergence or divergence41. Fourth, neutrality and ecological drift can contribute to random trait patterns. This happens because (a) individual fitness becomes independent of species identity and community composition (i.e., there is functional equivalence) and (b) the stochastic natures of births and deaths can lead to poor regulation of plant abundance42,43. Fifth, a random pattern can also emerge when multiple assembly processes simultaneously act on plant assemblages, concealing the underlying processes that underlie divergence or convergence30,44. In the Atacama Desert, at least three of these factors appear to be contributing to the random assembly of shrub communities: intransitive competition, neutrality and ecological drift, and multiple assembly processes acting simultaneously. Hence, further studies are required to untangle the complex processes influencing plant communities in this desert. However, despite these explanations, it is noteworthy that a few plots deviated significantly from the null distribution at this scale, both in terms of the SES of C-Scores and the SES of the FDis. These deviations suggest that regardless of the aridity level in the Atacama Desert, plant competition may play a central role in shaping plant assemblages, either through limiting similarity or by excluding the weaker competitors. This observation holds true not only for above- and below-ground traits but also when considering all traits together. These findings contrast theoretical and empirical studies that show a shift from competition to facilitation (e.g., Bertness and Callaway9; López et al.10) or a shift from competition to environmental filtering (e.g., Coyle et al.3) towards the most stressful end of the gradient.
In conclusion, our study revealed that at the patch scale, environmental heterogeneity emerged as the dominant assembly processes in only one site. At neighborhood scale, we observed a weak signal of competition as the dominant assembly process. Specifically, signs of competition were present in all sites in all sites but were only evident in a few plots. Our results underscore the need to apply both trait-based metrics and spatial association analyses at different spatial scales to understand community assembly. For example, at the neighborhood scale, divergence detected by the functional dispersion index could indicate either competition or facilitation, while convergence might stem from abiotic constraints or weaker-competitor exclusion. Conversely, segregation patterns identified by C-scores suggest competition, however they do not clarify whether it is due to limiting similarity or weaker-competitor exclusion. Employing both methods allows us to infer not only the predominant process shaping plant communities but also the specific drivers behind these processes.
Methods
Study site
We conducted this study in the Atacama Desert (Chile) along an aridity gradient that expands approximately 440 km (from 26° 57ʹ S to 29° 43ʹ S). Rainfall across this area is limited to the winter months45, and is more variable towards the north46. The mean annual precipitation along the gradient ranges between 14 and 80 mm, while the mean annual temperature is relatively constant, fluctuating between 15 and 17 °C (Table 1). We selected four sites along the gradient that differ in their degree of aridity, which was estimated using De Martonne’s aridity index: DEMAI = MAP/(MAT + 10)47, where MAP and MAT are the mean annual precipitation and temperature, respectively. From the most to the least arid site, these are: Quebrada El León (QL), Norte de Llanos de Challe (LLCHA), Chañaral de Aceituno (CHA) and Romeral (ROM) (Table 1). Of these, LLCHA exhibited the highest variability in soil physico-chemical properties (Table 1).
Data collection
At each site, vegetation was sampled in stabilized dunes (sandy soils) of west-facing, gentle slopes (< 5%)36. The sampling procedure was carried out at two scales: the patch scale (100 m2 plots) and the neighborhood scale (5 m2 plots). For the patch scale, we established 20 plots of 50 × 2 m separated by at least 100 m in each of the four sites (N = 20/site, except for QL that had 22 plots). We recorded all plant species present in each plot, as well as the number of individuals per species, which was used to estimate the relative abundance of species per plot. For the neighborhood scale, we selected 10 of the 20 plots established per site (except for QL that hat 11 plots), divided the area of each selected plot into five subplots and recorded the number of individuals per species in three 1 m2 quadrants within each subplot.
Trait measurements
We measured plant height and a set of leaf, stem and root traits following standardized protocols48. At each site, we randomly selected 50 individuals to measure all leaf traits, except leaf chemistry traits for which we sampled five individuals per species per site. Similarly, only five individuals per species per site were used to measure stem traits. We selected only three individuals per species per site to measure coarse-root traits and five individuals to measure fine-root traits because the methodology used to quantify roots traits is destructive36. In total, we measured 14 traits: Plant height (cm), leaf area (LA—cm2), specific leaf area (SLA; the ratio of leaf area to leaf mass (cm g−2)), leaf dry matter content (LDMC; the ratio of leaf dry mass to fresh mass (mg g−1)), leaf nitrogen concentration (LNC (%)), foliar carbon isotope ratio (δ13C (‰)), carbon to nitrogen ratio (CNleaf (mg·mg−1)), stem wood density (WDs; the ratio of oven dry mass to green volume (g·cm-3)), root dry matter content (RDMC; the ratio of root dry mass to fresh mass (mg·g−1)), specific root length (SRL; the ratio of root dry mass to length (g·cm−1)), root nitrogen concentration (RNC (%)), carbon to nitrogen ratio (CNroot (mg·mg−1)), root depth distribution (β index) and root wood density (WDr; the ratio of oven-dried mass to green volume (g·cm−3)). For a detailed description of trait measurements and their functional significance see36.
Data analysis
Trait-based assembly processes were estimated using the functional dispersion index (FDis; Laliberté and Legengre49); this index can accommodate multiple traits, considers species abundance and is independent of species richness49. Calculations of the FDis at both scales involved computing the trait means for each site using the dbFd function in the FD package50. To test for non-random assembly processes along the aridity gradient at each spatial scale, we conducted analyses for all traits combined, as well as above- and below-ground traits separately. The species pool was restricted according to the scale51: at the patch scale, we used the whole set of species (i.e., all of the species found in all of the plots within a site), while at the neighborhood scale, we used all of the species found within a single plot. To calculate null distributions, we generated 9999 randomly assembled communities using constrained randomizations, which keep fixed the total number of species and abundance in the samples (i.e., plots or subplots) and reshuffle species abundances while keeping the same number of species (species richness) within samples24,52. Subsequently, we calculated the standardized effect size (SES) as: SES = (Tobs − Tnull)/SDnull, where Tobs is the observed FDis, Tnull is the mean FDis of the null distribution, and SDnull is the standard deviation of the null distribution. To test whether SES values of the FDis were significantly lower (SES < 0 = convergence) or greater (SES > 0 = divergence) than expected, we estimated p-values using two-tailed tests of the quantile scores, which test whether an observed FDis value falls outside the null distribution using the randomizeMatrix function in Picante package53. The final number of randomizations at the patch and neighborhood scale varied (Table S1) because some samples contained less than three species, which rendered it impossible to perform null models.
Patterns of interspecific spatial association were estimated using the checkerboard score (C-score; Stone and Roberts 1990). C-score calculations were performed using species occurrence data summarized into presence–absence matrices (species in rows, quadrats in columns) for each scale (patch and neighborhood). We then tested for non-random pairwise species associations by constructing null models with 10,000 randomly assembled communities for each scale using fixed-equiprobable and fixed-proportional algorithms. Specifically, we used the fixed-equiprobable algorithm at the patch scale, which keeps the total number of species in a site fixed and randomizes species occurrences among plots. Thus, this algorithm assumes that plots are homogeneous and can be occupied by any species within a site and thus, is appropriate to detect the signal of abiotic constraints. Conversely, at the neighborhood scale, we used the fixed-proportional algorithm, which also keeps the total number of species in a plot fixed but randomizes species within subplots proportional to the observed species richness within a plot. Because this algorithm maintains the differences in species occurrences among plots but randomizes occurrences within a plot, it is apt to detect the signal of biotic interactions24. We calculated the SES using the formula described above. To examine patterns of spatial association, we tested whether C-scores values were significantly lower (SES < 0 = aggregated) or greater (SES > 0 = segregated) than expected by comparing the observed C-score of each matrix to the 95% confidence interval of simulated C-scores generated by the null models. These analyses were performed using the cooc_null_model function in the EcoSimR package54. All statistical analyses were performed using the R statistical environment55.
Ethics declarations
Experimental research and field studies on plants (either cultivated or wild), including the collection of plant material, must comply with relevant institutional, national, and international guidelines and legislation.
Data availability
All data generated or analyzed during this study are included in this published article (and its Supplementary Information files).
References
Whittaker, R. H. Dominance and diversity in land plant communities: Numerical relations of species express the importance of competition in community function and evolution. Science 147, 250–260 (1965).
Swenson, N. G. & Enquist, B. J. Ecological and evolutionary determinants of a key plant functional trait: Wood density and its community-wide variation across latitude and elevation. Am. J. Bot. 94, 451–459. https://doi.org/10.3732/ajb.94.3.451 (2007).
Coyle, J. R. et al. Using trait and phylogenetic diversity to evaluate the generality of the stress-dominance hypothesis in eastern North American tree communities. Ecography 37, 814–826. https://doi.org/10.1111/ecog.00473 (2014).
Weiher, E. & Keddy, P. A. Assembly rules, null models, and trait dispersion—New questions front old patterns. Oikos 74, 159–164. https://doi.org/10.2307/3545686 (1995).
Cornwell, W. K. & Ackerly, D. D. Community assembly and shifts in plant trait distributions across an environmental gradient in coastal California. Ecol. Monogr. 79, 109–126. https://doi.org/10.1890/07-1134.1 (2009).
Spasojevic, M. J. & Suding, K. N. Inferring community assembly mechanisms from functional diversity patterns: The importance of multiple assembly processes. J. Ecol. 100, 652–661. https://doi.org/10.1111/j.1365-2745.2011.01945.x (2012).
Gross, N. et al. Uncovering multiscale effects of aridity and biotic interactions on the functional structure of Mediterranean shrublands. J. Ecol. 101, 637–649. https://doi.org/10.1111/1365-2745.12063 (2013).
Lhotsky, B. et al. Changes in assembly rules along a stress gradient from open dry grasslands to wetlands. J. Ecol. 104, 507–517. https://doi.org/10.1111/1365-2745.12532 (2016).
Bertness, M. D. & Callaway, R. Positive interactions in communities. Trends Ecol. Evol. 9, 191–193. https://doi.org/10.1016/0169-5347(94)90088-4 (1994).
López, R. P., Squeo, F. A., Armas, C., Kelt, D. A. & Gutiérrez, J. R. Enhanced facilitation at the extreme end of the aridity gradient in the Atacama Desert: A community-level approach. Ecology 97, 1593–1604. https://doi.org/10.1890/15-1152.1 (2016).
Conti, L., de Bello, F., Lepš, J., Acosta, A. T. R. & Carboni, M. Environmental gradients and micro-heterogeneity shape fine-scale plant community assembly on coastal dunes. J. Veg. Sci. 28, 762–773. https://doi.org/10.1111/jvs.12533 (2017).
Adler, P. B., Fajardo, A., Kleinhesselink, A. R. & Kraft, N. J. B. Trait-based tests of coexistence mechanisms. Ecol. Lett. 16, 1294–1306. https://doi.org/10.1111/ele.12157 (2013).
Chesson, P. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 31, 343–366. https://doi.org/10.1146/annurev.ecolsys.31.1.343 (2000).
Dullinger, S. et al. Weak and variable relationships between environmental severity and small-scale co-occurrence in alpine plant communities. J. Ecol. 95, 1284–1295. https://doi.org/10.1111/j.1365-2745.2007.01288.x (2007).
Chalmandrier, L. et al. Spatial scale and intraspecific trait variability mediate assembly rules in alpine grasslands. J. Ecol. 105, 277–287. https://doi.org/10.1111/1365-2745.12658 (2017).
Mayfield, M. M. & Levine, J. M. Opposing effects of competitive exclusion on the phylogenetic structure of communities. Ecol. Lett. 13, 1085–1093. https://doi.org/10.1111/j.1461-0248.2010.01509.x (2010).
Vega-Álvarez, J., García-Rodríguez, J. A. & Cayuela, L. Facilitation beyond species richness. J. Ecol. 107, 722–734. https://doi.org/10.1111/1365-2745.13072 (2019).
Madrigal-González, J., Cano-Barbacil, C., Kigel, J., Ferrandis, P. & Luzuriaga, A. L. Nurse plants promote taxonomic and functional diversity in an arid Mediterranean annual plant community. J. Veg. Sci. 31, 658–666. https://doi.org/10.1111/jvs.12876 (2020).
Bashirzadeh, M., Soliveres, S., Farzam, M. & Ejtehadi, H. Plant–plant interactions determine taxonomic, functional and phylogenetic diversity in severe ecosystems. Glob. Ecol. Biogeogr. 31, 649–662. https://doi.org/10.1111/geb.13451 (2022).
Michalet, R. et al. Do biotic interactions shape both sides of the humped-back model of species richness in plant communities? Ecol. Lett. 9, 767–773. https://doi.org/10.1111/j.1561-0248.2006.00935.x (2006).
Maestre, F. T., Callaway, R. M., Valladares, F. & Lortie, C. J. Refining the stress-gradient hypothesis for competition and facilitation in plant communities. J. Ecol. 97, 199–205. https://doi.org/10.1111/j.1365-2745.2008.01476.x (2009).
Maestre, F. T. & Cortina, J. Do positive interactions increase with abiotic stress?—A test from a semi-arid steppe. Proc. R. Soc. Lond. Ser. B Biol. Sci. 271, S331–S333. https://doi.org/10.1098/rsbl.2004.0181 (2004).
de Bello, F. et al. Evidence for scale- and disturbance-dependent trait assembly patterns in dry semi-natural grasslands. J. Ecol. 101, 1237–1244. https://doi.org/10.1111/1365-2745.12139 (2013).
Gotelli, N. J. Null model analyisis of species co-occurrence patterns. Ecology 81, 2606–2621. https://doi.org/10.1890/0012-9658(2000)081(2606:nmaosc)2.0.co;2 (2000).
Gotelli, N. J. & McCabe, D. J. Species co-occurrence: A meta-analysis of J. M. Diamond’s assembly rules model. Ecology 83, 2091–2096. https://doi.org/10.1890/0012-9658(2002)083(2091:scoama)2.0.co;2 (2002).
Turnbull, L. A., Crawley, M. J. & Rees, M. Are plant populations seed-limited? A review of seed sowing experiments. Oikos 88, 225–238. https://doi.org/10.1034/j.1600-0706.2000.880201.x (2000).
Leibold, M. A. et al. The metacommunity concept: A framework for multi-scale community ecology. Ecol. Lett. 7, 601–613. https://doi.org/10.1111/j.1461-0248.2004.00608.x (2004).
Kneitel, J. M. & Chase, J. M. Trade-offs in community ecology: Linking spatial scales and species coexistence. Ecol. Lett. 7, 69–80. https://doi.org/10.1046/j.1461-0248.2003.00551.x (2004).
Cadotte, M. W. et al. On testing the competition-colonization trade-off in a multispecies assemblage. Am. Nat. 168, 704–709. https://doi.org/10.1086/508296 (2006).
Scherrer, D. et al. Disentangling the processes driving plant assemblages in mountain grasslands across spatial scales and environmental gradients. J. Ecol. 107, 265–278. https://doi.org/10.1111/1365-2745.13037 (2019).
León, M. F., Squeo, F. A., Gutierrez, J. R. & Holmgren, M. Rapid root extension during water pulses enhances establishment of shrub seedlings in the Atacama Desert. J. Veg. Sci. 22, 120–129. https://doi.org/10.1111/j.1654-1103.2010.01224.x (2011).
Tilman, D. Competition and biodiversity in spatially structured habitats. Ecology 75, 2–16. https://doi.org/10.2307/1939377 (1994).
Goldberg, D. & Novoplansky, A. On the relative importance of competition in unproductive environments. J. Ecol. 85, 409–418. https://doi.org/10.2307/2960565 (1997).
Cahill, J. F. Fertilization effects on interactions between above- and belowground competition in an old field. Ecology 80, 466–480. https://doi.org/10.1890/0012-9658(1999)080(0466:feoiba)2.0.co;2 (1999).
Butterfield, B. J., Bradford, J. B., Munson, S. & Gremer, J. Aridity increases below-ground niche breadth in grass communities. Plant Ecol. 218, 385–394. https://doi.org/10.1007/s11258-016-0696-4 (2017).
Carvajal, D. E., Loayza, A. P., Rios, R. S., Delpiano, C. A. & Squeo, F. A. A hyper-arid environment shapes an inverse pattern of the fast–slow plant economics spectrum for above-, but not below-ground resource acquisition strategies. J. Ecol. 107, 1079–1092. https://doi.org/10.1111/1365-2745.13092 (2019).
Jung, V., Violle, C., Mondy, C., Hoffmann, L. & Muller, S. Intraspecific variability and trait-based community assembly. J. Ecol. 98, 1134–1140. https://doi.org/10.1111/j.1365-2745.2010.01687.x (2010).
Siefert, A. Incorporating intraspecific variation in tests of trait-based community assembly. Oecologia 170, 767–775. https://doi.org/10.1007/s00442-012-2351-7 (2012).
Garnier, E., Navas, M.-L. & Grigulis, K. Plant Functional Diversity: Organism Traits, Community Structure, and Ecosystem Properties 1st edn. (Oxford University Press, 2016).
Soliveres, S. et al. Intransitive competition is widespread in plant communities and maintains their species richness. Ecol. Lett. 18, 790–798. https://doi.org/10.1111/ele.12456 (2015).
Gallien, L. Intransitive competition and its effects on community functional diversity. Oikos 126, 615–623. https://doi.org/10.1111/oik.04033 (2017).
Vellend, B. M. Conceptual synthesis in community ecology. Q. Rev. Biol. 85, 183–206. https://doi.org/10.1086/652373 (2010).
Hubbell, S. P. The Unified Neutral Theory of Biodiversity and Biogeography Vol. 32 (Princeton University Press Princeton, 2001).
Swenson, N. G. & Enquist, B. J. Opposing assembly mechanisms in a Neotropical dry forest: Implications for phylogenetic and functional community ecology. Ecology 90, 2161–2170. https://doi.org/10.1890/08-1025.1 (2009).
Di Castri, F. & Hajek, E. Bioclimatología de Chile 129 (Universidad Católica de Chile, 1976).
Carvajal, D. E., Loayza, A. P. & Squeo, F. A. Contrasting responses to water-deficit among Encelia canescens populations distributed along an aridity gradient. Am. J. Bot. 102, 1552–1557. https://doi.org/10.3732/ajb.1500097 (2015).
De Martonne, E. Une nouvelle fonction climatologique: L’indice d’aridité. La Meteorol. 2, 449–458 (1926).
Pérez-Harguindeguy, N. et al. New handbook for standardised measurement of plant functional traits worldwide. Austral. J. Bot. 61, 167–234. https://doi.org/10.1071/bt12225 (2013).
Laliberté, E. & Legendre, P. A distance-based framework for measuring functional diversity from multiple traits. Ecology 91, 299–305. https://doi.org/10.1890/08-2244.1 (2010).
FD: Measuring Functional Diversity from Multiple Traits, and Other Tools for Functional Ecology V. 1.0–12.1 (2014).
Swenson, N. G. Functional and Phylogenetic Ecology in R Vol. XII, 212 (Springer, 2014).
Gotelli, N. J. & Entsminger, G. L. Swap and fill algorithms in null model analysis: Rethinking the knight’s tour. Oecologia 129, 281–291. https://doi.org/10.1007/s004420100717 (2001).
Kembel, S. W. et al. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26, 1463–1464. https://doi.org/10.1093/bioinformatics/btq166 (2010).
EcoSimR: Null Model Analysis for Ecological Data V. R Package Version 0.1.0 (2015).
R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2018).
Acknowledgements
The authors thank all those who assisted with the field data collection, particularly Claus Westphal, Cristian Delpiano Lastra, Paulina Vera, and Natalio Roque. They also thank Rodrigo S. Rios, and two anonymous reviewers for suggestions that improved an earlier version of this manuscript. This research was supported by a Grant from FONDECYT Regular 1151020, Proyecto ANID FB210006 through Institute of Ecology and Biodiversity (IEB). D. E. Carvajal was supported by FONDECYT postdoctoral Grant (Grant Number 3230154).
Author information
Authors and Affiliations
Contributions
D.E.C., A.P.L., and F.A.S. designed the study. D.E.C. collected and analyzed all data and was the primary writer of the manuscript. A.P.L. contributed to writing the manuscript. F.A.S. contributed to revising the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Carvajal, D.E., Loayza, A.P. & Squeo, F.A. Functional diversity and spatial association analyses at different spatial scales reveal no changes in community assembly processes along an aridity gradient in the Atacama Desert. Sci Rep 13, 19905 (2023). https://doi.org/10.1038/s41598-023-47187-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-47187-5
- Springer Nature Limited