Abstract
The aim of this study was to investigate the influence of Bacteroides vulgatus (BV), Clostridium perfringens (CP), Parabacteroides distasonis (PD) and Ruminococcus albus (RA) lysates on secretion of selected cytokines by PBMC, MDM and HT-29 cells, as well as to determine the potential mechanisms of their action in the development of asthma. Enzyme-linked immunosorbent assays were used to analyze the effect of BV, CP, PD and RA lysates on the secretion of IL-1β, IL-6, IL-10 and TNF-α by human PBMC, MDM and HT-29 cells. BV and CP lysates significantly lowered IL-1β secretion by MDM vs. control (p < 0.05 and p < 0.001 respectively) but only at a dose of 400 µg lysate. The secretions of IL-6 by PBMC and MDM were elevated significantly above control values (p < 0.05) after administration of CP and PD lysates. BV, CP and PD lysates (100 µg) significantly increased IL-10 secretion by PBMC vs. control (p < 0.05). CP, PD and RA lysates (400 µg) significantly increased IL-10 secretion by MDM vs. control (p < 0.001). BV lysate (400 µg) also significantly increased IL-10 secretion by MDM as compared to control (p < 0.05). In PBMC and MDM, the production levels of the anti-inflammatory cytokine were increased by all the bacterial lysates used in a dose-dependent manner.
Similar content being viewed by others
Introduction
Asthma is a lung disease associated with chronic inflammation and airway obturation1. The most common asthma symptoms are wheezing, coughing, breath shortness and chest tightness2,3. This disease affected about 262 million people in 20194,5,6. In 2017, the annual costs of asthma treatment were estimated in Europe at about 1900 USD per patient7. Asthma pathology is a multifactorial process associated with chronic airway inflammation which leads to the airflow limitation and also causes bronchial hyper-reactivity8. The main risk factors for developing asthma include genetic predisposition and environmental factors, viral respiratory infections in childhood or diet9.
Gut microbiota has many functions, including: vitamins production, protection from pathogens and enhancement immune response10,11. Several studies confirm that in the first year of a child’s life, microbiological stimulation has a significant impact on the maturation of lymph tissue occurring within the gastrointestinal tract12. Experiments on animal models have proved that stimulation with intestinal bacteria has a significant impact on the diversity of antibodies present in gastrointestinal tract immediately after birth13,14. Studies on GF mice have shown that the development of oral tolerance occurs if the intestinal flora is re-colonized by Bifidobacterium, but no later than in the neonatal period. In addition, bacteria colonize the gut and induce a pronounced immune response to IgA production15,16.
Dysbiosis in early life may predispose to the development of many respiratory diseases, such as asthma, which is associated with the impact of gut microflora on immune system maturation17. In fecal samples of newborns with high asthma development risk reduced amount of Lachnospira, Veillonella, Faecalibacterium, and Rothia strains was observed18. In addition, an increased risk of atopy and asthma was also observed in neonates with an increase in the volume of Streptococcus and Bacteroides spp. and a decrease in the number of Bifidobacterium spp. and Ruminococcus gnavus in fecal samples19. Moreover, lower abundance of Bifidobacteria, Akkermansia and Faecalibacterium spp. in the intestines predisposes to the higher risk of atopy and asthma development in children20. Therefore further intensive research is needed to determine how individual intestinal bacteria may influence the development of asthma, as well as to determine potential mechanisms of their action. The aim of this study was to investigate the influence of Bacteroides vulgatus, Clostridium perfringens, Parabacteroides distasonis and Ruminococcus albus lysates on the secretion of selected cytokines by PBMC, MDM and HT-29 cells, as well as to determine the potential mechanisms of action of these species of intestinal microflora in the development of asthma.
Materials and methods
Bacterial strains
Ruminococcus albus (ATCC 27210, LGC Standards, Teddington, UK) was harvested in ATCC 158 RGCA Medium; Clostridium perfringens (ATCC 13124, LGC Standards, Teddington, UK) was cultivated in ATCC 2107 Modified Reinforced Clostridial medium (LGC Standards, Teddington, UK), Bacteroides vulgatus (ATCC 8482, LGC Standards, Teddington, UK) was harvested in ATCC 2107 Modified Reinforced Clostridial Medium and Parabacteroides distasonis (ATCC 8503, LGC Standards, Teddington, UK) was cultivated in ATCC 1490 Modified Chopped Meat medium in 37 °C in an anaerobic conditions. As a selected medium, ATCC 260 Trypticase soy agar with 5% sheep blood was used.
Human cell lines
Human peripheral blood mononuclear cells (PBMC; SigmaAldrich, Saint Louis, MO, USA) were harvested in Mononuclear Cell Medium (SigmaAldrich, Saint Louis, MO, USA) and human HT-29 cells (LGC Standards, Teddington, UK) were cultivated in McCoy’a 5A (ATCC 30-2007) and fetal bovine serum (500 ml, ATCC 30-2020). Human monocytes were isolated from PBMC (SigmaAldrich, Saint Louis, MO, USA) by incubation for 2 h in RPMI-1640 medium (SigmaAldrich, Saint Louis, MO, USA). Remaining cells were removed from the ones that adhered to the culture flask surface by washing (three times), which allowed monocytes to adhere to the cells that stayed on the surface of the flask. The remaining cells were removed in warm RPMI-1640 medium (SigmaAldrich, Saint Louis, MO, USA). Human monocytes were then harvested in RPMI-1640 medium (SigmaAldrich, Saint Louis, MO, USA) supplemented with 10% FBS (SigmaAldrich, Saint Louis, MO, USA). Each cell line was harvested in T75 flasks in standard conditions (37 °C, 5% CO2, 90% humidity). In the experiment, combination of antibiotics was used (SigmaAldrich, #P4333, Penicilin/Streptomycin; Lonza #195266).
Preparation of Ruminococcus albus, Clostridium perfringens, Bacteroides vulgatus and Parabacteroides distasonis
Intestinal microflora cultures of Ruminococcus albus, Clostridium perfringens, Bacteroides vulgatus and Parabacteroides distasonis were transferred into collection tubes and centrifuged for 5 min, 10,000 rpm at 4 °C. After bacterial cell pellet suspension in 1 ml of distilled water bacterial lysates were prepared by ultrasonic disintegration of cells structure. Samples were then centrifuged at the same conditions and the obtained supernatants were transferred into new collection tubes and stored at − 20 °C.
Stimulation of human PBMC, human MDM and HT-29 cells by Ruminococcus albus, Clostridium perfringens, Bacteroides vulgatus and Parabacteroides distasonis lysates
Human PBMC were seeded on a plate at 2 × 106 cells/well in a 6-well. Human MDM and HT-29 cells were seeded at 0.5 × 106 cells/well. Then, the cell lines were stimulated with Ruminococcus albus, Clostriduim perfringens, Bacteroides vulgatus and Parabacteroides distasonis lysates (two different concentrations: 100 µg and 400 µg) for 24 h. Negative and positive control were the cells incubated respectively with fresh culture medium and with 25 µg/ml dexamethasone. After 24-h stimulation of PBMC, monocytes and HT-29 cells with intestinal microbiota lysates, the samples were centrifuged for 5 min at 1500 rpm and 4 °C. The obtained supernatants were transferred into new collection tubes and stored at − 20 °C.
Analysis of selected secretion of cytokines by PBMC, human MDM and HT-29 cells after incubation with intestinal microbiota lysates
To analyze selected secretion of cytokines by PBMC, MDM and HT-29 cells after incubation with Ruminococcus albus, Clostridium perfringens, Bacteroides vulgatus and Parabacteroides distasonis lysates, enzyme-linked immunosorbent assays were performed. Commercially available ELISA kits from SigmaAldrich (Sain Louis, MO, USA) were used. For IL-1β, IL-6, IL-10 and TNF-α analysis, 100 µl of standards and 100 µl of each sample was added into 96-well ELISA plates in duplicates. The plates were incubated for 2.5 h (ambient temperature, gentle shaking). After the incubation, the plates were washed 4 times with 300 µl/well of Wash solution. Then, 100 µl of detection antibody was added into each well and incubated for 1 h (room temperature, gentle shaking). After the incubation, the plates were washed again 4 times with 300 µl/well of wash solution. Then, 100 µl of Streptavidin solution was added into each well and incubated for 45 min (room temperature, gentle shaking). Next, the plates were washed again 4 times with 300 µl/well of wash solution. Then, 100 µl of Substrate reagent was added to each well and incubated for 30 min at room temperature, in the dark, with gentle shaking. Immediately after adding 50 µl of Stop solution to the plates, absorbance at 450 nm was read on the microplate reader.
Statistical analysis
Results were presented as MEAN ± SEM. The statistical analysis was performed using one-way ANOVA followed by Tukey multiple range post hoc test and Dunnett’s method. The p < 0.05 was considered as statistically significant.
Results
Evaluation of IL-1β, IL-6, IL-10 and TNF-α concentrations
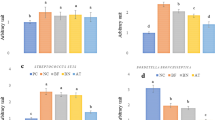
Bacteroides vulgatus and Clostridium perfringens lysates significantly lowered IL-1β secretion by MDM as compared to control (respectively p < 0.05; p < 0.001) but only at a dose of 400 µg lysate (Fig. 1). Ruminococcus albus lysates slightly reduced the IL-1β concentration by MDM vs. control (100 µg of lysate).
The effect of bacterial lysates at a dose of 100 µg (a) and at a dose of 400 µg (b) on IL-1β secretion by human peripheral blood mononuclear cells (PBMC) and human monocyte-derived macrophages (MDM). Tukey’s Test: *p < 0.001 vs. control group; #p < 0.05 vs. control group; $p < 0.05 vs. BV PBMC; ^p < 0.05 vs. PD MDM. BV Bacteroides vulgatus, CP Clostridium perfringens, PD Parabacteroides distasonis, RA Ruminococcus albus.
Clostridium perfringens lysate significantly increased IL-6 secretion by PBMC as compared to control (p < 0.05; Fig. 2). Parabacteroides distasonis lysate significantly increased IL-6 secretion by MDM vs. control (p < 0.05). All other lysates also increased IL-6 secretion by PBMC and MDM as compared to control but without statistical significance (Fig. 2). In contrast, bacterial lysates (100 µg) reduced the IL-6 secretion by HT-29; however, these changes were not significant. Bacteroides vulgatus, Clostridium perfringens and Parabacteroides distasonis lysates (100 µg) significantly increased IL-10 secretion by PBMC as compared to control (p < 0.05). Clostridium perfringens and Parabacteroides distasonis lysates (400 µg) significantly increased IL-10 secretion by PBMC and MDM vs. control (p < 0.05). Clostridium perfringens, Parabacteroides distasonis and Ruminococcus albus lysates (400 µg) significantly increased IL-10 secretion by MDM as compared to control (p < 0.001). Bacteroides vulgatus lysate (400 µg) also significantly increased IL-10 secretion by MDM vs. control (p < 0.05; Fig. 3).
The effect of bacterial lysates at a dose of 100 µg (a) and at a dose of 400 µg (b) on IL-6 secretion by human intestinal epithelial cells (HT-29), human peripheral blood mononuclear cells (PBMC) and human monocyte-derived macrophages (MDM). Data is shown as mean ± S.E.M; Tukey’s Test: #p < 0.05 vs. control group; ^p < 0.05 vs. HT-29. BV Bacteroides vulgatus, CP Clostridium perfringens, PD Parabacteroides distasonis, RA Ruminococcus albus.
The effect of bacterial lysates at a dose of 100 µg (a) and at a dose of 400 µg (b) on IL-10 secretion by human intestinal epithelial cells (HT-29), human peripheral blood mononuclear cells (PBMC) and human monocyte-derived macrophages (MDM). Data is shown as mean ± S.E.M; Dunnett’s Method: *#p < 0.05 vs. control group. BV Bacteroides vulgatus, CP Clostridium perfringens, PD Parabacteroides distasonis, RA Ruminococcus albus.
Bacteroides vulgatus lysate (100 µg) significantly increased TNF-α secretion by MDM as compared to control (p < 0.05). Moreover Bacteroides vulgatus lysate (400 µg) significantly increased TNF-α secretion by HT-29 and MDM as compared to control (p < 0.05). Parabacteroides distasonis lysates (400 µg) significantly increased TNF-α secretion by MDM vs. control (p < 0.05; Fig. 4).
The effect of bacterial lysates at a dose of 100 µg (a) and at a dose of 400 µg (b) on TNF-α secretion by human intestinal epithelial cells (HT-29), human peripheral blood mononuclear cells (PBMC) and human monocyte-derived macrophages (MDM). Data is shown as mean ± S.E.M; Dunnett’s Method: #p < 0.05 vs. control group. BV Bacteroides vulgatus, CP Clostridium perfringens, PD Parabacteroides distasonis, RA Ruminococcus albus.
Discussion
The role of chronic inflammation and airway remodeling in the pathogenesis of bronchial hyperresponsiveness may change during the clinical course of asthma. In the initial period, inflammation plays the dominant role, then bronchial hyperresponsiveness depends on tissue remodeling. Moreover, as the disease progresses, bronchial structural cells play an increasingly important role in the amplification of the inflammation by releasing inflammatory mediators. They are an important source of chemokines, cytokines and other inflammatory mediators maintaining the inflammatory process. The bronchial epithelium plays an active role in the pathogenesis of asthma. Interaction with environmental factors leads to the release of thymic stromal lymphopoietin and interleukins: 25 and 33. The epithelium is a rich source of pro-inflammatory cytokines such as TNF-α, chemokines, growth factors and interferons. In asthma patients the infectious agent induces lower production of interferon-γ by inflammatory cells and lower production of interferon P and X by respiratory epithelial cells, which makes it difficult to generate effective defense reaction against viruses.
The role of gut microbiota is still underestimated. Our present findings indicate that B. vulgatus and C. perfringens lysates at a dose of 400 µg significantly lowered IL-1β secretion by MDM vs. control. Whereas, R. albus lysates at a dose of 100 µg only slightly reduced the IL-1β concentration by MDM compared to control.
The role of Bacteroides in the course of various diseases has been described in many studies, including alleviating obesity21, preventing atherosclerosis22, protecting against colitis23 and inhibiting cancer24. Unfortunately, there are no similar studies linking BV and asthma.
The results of the Liu et al. study25 showed that administration of B. vulgatus Bv46 reduces the expression of interleukins 1β, 6 and TNF-α in the colon in dextran sodium sulfate-induced colitis in vivo and diminishes the secretion of interleukins- 1β, 6 and TNF-α in macrophages stimulated by LPS in vitro. What's more BV downregulates Ccl19, Cd19, Cd22, Cd40 and Cxcr5 genes in the colon of mice, which are mainly involved in the regulation of B cell responses. Additionally, BV in combination with B. dorei (BD) reduces the concentration of lipopolysaccharides in serum and feces, prevents cytokine induction and inhibits atherosclerosis. Scientists have shown that treatment with live BV and BD can help prevent coronary artery disease22.
However, some studies in other experimental models report increased IL-1β secretion. Fasina’s research indicated that after the CP challenge, the expression level of interleukin 1β was upregulated26. Another study also demonstrated that high dose of CP significantly increased pro-inflammatory factors such as interleukins 1β, 6 and TNF-α27. Tang et al.28 report that CP challenge can cause an upregulation on pro-inflammatory mediator genes including TNF-α and interleukins: 1β, 8 in the intestine of broilers.
Our results suggest that C. perfringens lysates significantly increased IL-6 secretion by PBMC and by MDM compared to control. All other lysates also increased IL-6 secretion by these cells; however, these changes were not significant. In contrast, bacterial lysates caused a statistically insignificant decrease in interleukin 6 concentration in HT-29 cells.
Guo et al. described that CP infection elevated levels of interleukins- 6, 829. In the next study30, a homopolysaccharide fraction isolated from culture broth of PD exerted an immunostimulatory effect by promoting the secretion of interleukins-1β, 6 and TNF-α.
However, another studies report significant decrease in IL-6 secretion. Some authors have shown that PD has anti-inflammatory properties31. In 2021, scientists published data on the protective role of selected strains of B. vulgates against colitis caused by dextran sodium sulfate (DSS). Among the tested strains, B. vulgatus 7K1 turned out to be the most effective in significantly inhibiting the increase in IL-6 and TNF-α concentrations and increasing IL-10 concentrations to the level found in the control group32.
This paper shows that B. vulgatus, C. perfringens and P. distasonis lysates (100 µg) significantly increased IL-10 secretion by PBMC compared to control. CP and PD lysates (400 µg) significantly increased IL-10 secretion by PBMC and MDM compared to control. C. perfringens, P. distasonis and R. albus lysates (400 µg) significantly increased IL-10 secretion by MDM compared to control. B. vulgatus lysate (400 µg) also significantly increased IL-10 secretion by MDM compared to control.
Similar results, that PD supplementation leads to an increase in IL-10 levels were reported by Kverka et al.33. The authors proved that PD, has the ability to reduce the intestinal inflammation by inducing the anti-inflammatory cytokine IL-10 and suppressing the secretion of inflammatory interleukins- 6 and 17. The results of recent studies confirm that B. vulgatus FTJS7K1 supplementation can significantly increase the level of mRNA of the anti-inflammatory factor IL-10 compared to the LPS groups. This bacterial strain reduces acute inflammation and intestinal injury in mice by modulating the gut microbial community. The authors showed that the genes responsible for the secretion of SCFA are responsible for the anti-inflammatory effect34.
Probiotic strains of Bifidobacteria in BFM may interfere with the intestinal inflammatory response. The authors described the effect of probiotic strains on IL-10 secretion by peripheral blood mononuclear cells and IL-8 production by intestinal epithelial cells. B. vulgatus, B. fragilis and B. thetaiotaomicron reduced the transcription of poly(I:C)-induced inflammatory genes35.
Our results show that B. vulgatus lysate (100 µg) significantly increased TNF-α secretion by MDM compared to control. Moreover, B. vulgatus lysate at a dose of 400 µg significantly increased TNF-α secretion by HT-29 and MDM compared to control. PD lysates (400 µg) also significantly increased TNF-alpha secretion by MDM as compared to control.
In 2022, Chamarande et al.36 published data on the properties of P. distasonis strains. In PBMC, the production levels of the proinflammatory cytokines, such as interleukins 1β, 6 and TNF-α were increased by almost all the PD strains tested. Moreover, the PD treatment during E. coli LPS-induced inflammation did not reduce the proinflammatory cytokine production of PBMC. These results, contrary to HT-29 results, suggest a proinflammatory response of the immune system cells due to P. distasonis stimulation, although with strain variability.
However, some studies report a decrease in TNF-α secretion. The authors described that oral treatment with B. vulgatus might reduce dysbiosis of the colonic microbiota by reducing LPS/TLR-4/p-NF-κB signaling pathway in the colon and serum TNF-α to attenuate lumbar bone loss in ovariectomized mice. In the same study, no differences were observed for interleukins 1β, 6 and 837.
Papers published more than a decade ago have already indicated a relationship between the microbiome and the asthma symptoms occurring. Correlation between Bacteroides and IgE/IgG immune response in allergic children was investigated by Fukuda et al.38. A higher IgG titer to B. vulgaris was found in the children with allergic symptoms. Kirjavainen et al.39 indicated that in infants intolerant to extensively hydrolyzed whey formula, serum total IgE correlates with faecal Bacteroides counts at 4–6 months of age. Another study40 also confirms that the faecal counts of Bacteroides at age 5 years correlate positively with the serum IgE concentration. Sudo et al.41 proved that kanamycin-induced elevation of the serum IgE levels in mice was improved by the inoculation with BV. An increased ratio of Bacteroides fragilis to Bifidobacterium bacteria in the faeces of adults allergic to pollen during the pollen season has been demonstrated. This increase was prevented by Bifidobacterium administration. Scientists, using PBMC of patients with pollen allergy also showed that B. fragilis strains induced more Th2 cytokines but fewer Th1 cytokines compared with Bifidobacterium strains42.
An interesting study conducted in the U.S. suggests that the "high risk group" of developing allergic reactions was characterized by a relatively lower number of Bifidobacterium, Akmermansia and Faecalibacterium and a higher number of Candida and Rhodotorula20. Similar publications proved that patients diagnosed with severe asthma are characterized by a significantly lower number of Bifidobacterium bacteria in the intestinal flora43. It is worth noting that the formation of microflora is a gradual phenomenon. Thus, it is possible to influence its development through a proper diet or probiotics/synbiotics supplementation. A full understanding of the “gut-lung” axis may be helpful in the asthma treating and preventing. The mechanism by which these two systems can affect each other is not well understood, however, inflammation that has been initiated in the intestines may result in the lungs inflammation as a result of the destructive effects of the over-stimulated immune system.
To investigate new, potentially therapeutic applications of probiotic bacteria in relieving asthma symptoms, researchers evaluated the effects of long-term use of Bifidobacterium breve M-16 V and Lactobacillus rhamnosus NutRes1 with glucocorticoid therapy as a reference medicine. The study showed that L. rhamnosus is as effective as budesonide in alleviating the inflammatory response and reducing airway resistance in diseased animals. In addition, both probiotic microorganisms successfully alleviated chronic allergic inflammation by weakening the total number of inflammatory cells and individual BAL fluid cell counts44. Moreover, studies on animal models have confirmed the adverse role of antibiotics as one of the reasons for the increased incidence of asthma, which is consistent with the “hygiene hypothesis”45,46,47.
It turns out that, unfortunately, there are not enough research projects with a similar methodology to draw clear conclusions. Without systematizing research techniques, conclusions from experiments will remain ambiguous. Supplementing the information on a detailed therapy regimen and the selection of specific strains for specific groups of patients is necessary to fully use the potential of microorganisms in the treatment of asthma. The effectiveness of selected microorganisms requires a detailed knowledge of the entire human microbiome, as well as checking, among others: the impact of modified bacteria on the microflora in the long term. Not only the effectiveness, but also the safety of the species/strains used should be carefully assessed, which, may represent a unique opportunity for many patients with bronchial asthma in the future.
Abbreviations
- BV:
-
Bacteroides vulgatus
- CP:
-
Clostridium perfringens
- MDM:
-
Human monocyte-derived macrophages
- PD:
-
Parabacteroides distasonis
- PBMC:
-
Peripheral blood mononuclear cells
- RA:
-
Ruminococcus albus
References
Akkoc, T., O’Mahony, L., Ferstl, R., Akdis, C. & Akkoc, T. Mouse models of asthma: Characteristics, limitations and future perspectives on clinical translation. In Cell Biology and Translational Medicine Vol. 15 (ed. Turksen, K.) 119–33 (Springer International Publishing, 2021).
Centers for Disease Control and Prevention. Vital Signs: Asthma Prevalence, Disease Characteristics, and Self-Management Education --- United States, 2001--2009 [Internet], [cited 2022 Nov 29] (2011).
Holgate, S. T. et al. Understanding the pathophysiology of severe asthma to generate new therapeutic opportunities. J. Allergy Clin. Immunol. 117(3), 496–506 (2006).
Our World in Data. Asthma prevalence, 1990 to 2019 [Internet] (2022).
The Global Asthma Report 2022. The international journal of tuberculosis and lung disease. Int. J. Tuberc. Lung Dis. 26(1), 1–104 (2022).
World Health Organization. Asthma [Internet] (2022).
Nunes, C., Pereira, A. M. & Morais-Almeida, M. Asthma costs and social impact. Asthma Res. Pract. 3(1), 1 (2017).
Mims, J. W. Asthma: Definitions and pathophysiology: Asthma: Definitions and pathophysiology. Int. Forum Allergy Rhinol. 5(S1), S2-6 (2015).
Kaufman, G. Asthma: Pathophysiology, diagnosis and management. Nurs. Stand. 26(5), 48–57 (2011).
Barcik, W., Boutin, R. C. T., Sokolowska, M. & Finlay, B. B. The role of lung and gut microbiota in the pathology of asthma. Immunity 52(2), 241–255 (2020).
Hillman, E. T., Lu, H., Yao, T. & Nakatsu, C. H. Microbial ecology along the gastrointestinal tract. Microbes Environ. 32(4), 300–313 (2017).
Ulluwishewa, D. et al. Regulation of tight junction permeability by intestinal bacteria and dietary components. J. Nutr. 141, 769–776 (2011).
Zhang, Y. et al. Impacts of gut bacteria on human health and diseases. Int. J. Mol. Sci. 16, 7493–7519 (2015).
Lanning, D. K., Rhee, K. J. & Knight, K. L. Intestinal bacteria and development of the P- lymphocyte repertoire. Trends Immunol. 26, 419–425 (2005).
Tsuji, M., Suzuki, K., Kinoshita, K. & Fagarasan, S. Dynamic interactions between bacteria and immune cells leading to intestinal IgA synthesis. Semin. Immunol. 20, 59–66 (2008).
Matamoros, S., Gras-Leguen, C., LeVacon, F., Potel, G. & DeLaCochetiere, M. F. Development of intestinal microbiota in infants and its impact on health. Trends Microbiol. 21, 167–173 (2013).
Sokolowska, M., Frei, R., Lunjani, N., Akdis, C. A. & O’Mahony, L. Microbiome and asthma. Asthma Res. Pract. 4(1), 1 (2018).
Arrieta, M. C. et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci. Transl. Med. 7(307), 307ra152 (2015).
Arrieta, M. C. et al. Associations between infant fungal and bacterial dysbiosis and childhood atopic wheeze in a nonindustrialized setting. J. Allergy Clin. Immunol. 142(2), 424-434.e10 (2018).
Fujimura, K. E. et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat. Med. 22(10), 1187–1191. https://doi.org/10.1038/nm.4176 (2016).
López-Almela, I. et al. Bacteroides uniformis combined with fiber amplifies metabolic and immune benefits in obese mice. Gut Microbes 13(1), 1–20. https://doi.org/10.1080/19490976.2020.1865706 (2021).
Yoshida, N. et al. Bacteroides vulgatus and Bacteroides dorei reduce gut microbial lipopolysaccharide production and inhibit atherosclerosis. Circulation 138(22), 2486–2498 (2018).
Chan, J. L. et al. Non-toxigenic Bacteroides fragilis (NTBF) administration reduces bacteria-driven chronic colitis and tumor development independent of polysaccharide A. Mucosal Immunol. 12(1), 164–177. https://doi.org/10.1038/s41385-018-0085-5 (2019).
Vétizou, M. et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 350(6264), 1079–1084. https://doi.org/10.1126/science.aad1329 (2015).
Liu, L. et al. Bacteroides vulgatus attenuates experimental mice colitis through modulating gut microbiota and immune responses. Front. Immunol. 13, 1036196 (2022).
Fasina, Y. O. & Lillehoj, H. S. Characterization of intestinal immune response to Clostridium perfringens infection in broiler chickens. Poult. Sci. 98(1), 188–198. https://doi.org/10.3382/ps/pey390 (2019).
Jiang, Z. et al. Effect of porcine Clostridium perfringens on intestinal barrier, immunity, and quantitative analysis of intestinal bacterial communities in Mice. Front. Vet. Sci. 9, 881878 (2022).
Tang, Y. et al. Dietary ellagic acid ameliorated Clostridium perfringens-induced subclinical necrotic enteritis in broilers via regulating inflammation and cecal microbiota. J. Anim. Sci. Biotechnol. 13(1), 47. https://doi.org/10.1186/s40104-022-00694-3 (2022).
Guo, S., Li, C., Liu, D. & Guo, Y. Inflammatory responses to a Clostridium perfringens type A strain and α-toxin in primary intestinal epithelial cells of chicken embryos. Avian Pathol. 44(2), 81–91. https://doi.org/10.1080/03079457.2015.1005573 (2015).
Zhu, Y. et al. Structural analysis and immunomodulatory activity of a homopolysaccharide isolated from Parabacteroides distasonis. Arab. J. Chem. 15(5), 103755 (2022).
Pfalzer, A. C. et al. Diet- and genetically-induced obesity differentially affect the fecal microbiome and metabolome in Apc1638N mice. PLoS One 10(8), e0135758 (2015).
Li, S. et al. Evaluation of the effects of different Bacteroides vulgatus strains against DSS-induced colitis. J. Immunol. Res. 2021, 9117805. https://doi.org/10.1155/2021/9117805 (2021).
Kverka, M. et al. Oral administration of Parabacteroides distasonis antigens attenuates experimental murine colitis through modulation of immunity and microbiota composition. Clin. Exp. Immunol. 163(2), 250–259. https://doi.org/10.1111/j.1365-2249.2010.04286.x (2011).
Wang, C. et al. Protective effects of different Bacteroides vulgatus strains against lipopolysaccharide-induced acute intestinal injury, and their underlying functional genes. J. Adv. Res. 36, 27–37. https://doi.org/10.1016/j.jare.2021.06.012 (2021).
Pathmanathan, S. G., Lawley, B., McConnell, M., Baird, M. A. & Tannock, G. W. Gut bacteria characteristic of the infant microbiota down-regulate inflammatory transcriptional responses in HT-29 cells. Anaerobe 61, 102112. https://doi.org/10.1016/j.anaerobe.2019.102112 (2020).
Chamarande, J., Cunat, L., Pavlov, N., Alauzet, C. & Cailliez-Grimal, C. Parabacteroides distasonis properties linked to the selection of new biotherapeutics. Nutrients 14(19), 4176. https://doi.org/10.3390/nu14194176 (2022).
Yuan, S. & Shen, J. Bacteroides vulgatus diminishes colonic microbiota dysbiosis ameliorating lumbar bone loss in ovariectomized mice. Bone 142, 115710 (2021).
Fukuda, S. et al. Allergic symptoms and microflora in schoolchildren. J. Adolesc. Health 35, 156–158 (2004).
Kirjavainen, P. V., Arvola, T., Salminen, S. J. & Isolauri, E. Aberrant composition of gut microbiota of allergic infants: A target of bifidobacterial therapy at weaning?. Gut 51, 51–55 (2002).
Sepp, E., Julge, K., Mikelsaar, M. & Bjorksten, B. Intestinal microbiota and immunoglobulin E responses in 5-year-old Estonian children. Clin. Exp. Allergy 35, 1141–1146 (2005).
Sudo, N. et al. An oral introduction of intestinal bacteria prevents the development of a long-term Th2-skewed immunological memory induced by neonatal antibiotic treatment in mice. Clin. Exp. Allergy 32, 1112–1116 (2002).
Odamaki, T. et al. Fluctuation of fecal microbiota in individuals with Japanese cedar pollinosis during the pollen season and influence of probiotic intake. J. Investig. Allergol. Clin. Immunol. 17, 92–100 (2007).
Hevia, A. et al. Allergic patients with long-term asthma display low levels of bifidobacterium adolescentis. PLoS One 11(2), e0147809 (2016).
Sagar, S., Morgan, M. E., Chen, S., Vos, A. P. & Garssen, J. Bifidobacterium breve and Lactobacillus rhamnosus treatment is as effective as budesonide at reducing inflammation in a murine model for chronic asthma. Respir. Res. 15, 46 (2014).
Hill, D. A. et al. Commensal bacteria-derived signals regulate basophil hematopoiesis and allergic inf lammation. Nat. Med. 18, 538–546 (2012).
Russell, S. L. et al. Perinatal antibiotic treatment affects murine microbiota, immune responses and allergic asthma. Gut Microbes 4(2), 158–164 (2013).
Marra, F. et al. Antibiotic use in children is associated with increased risk of asthma. Pediatrics 123, 1003–1010 (2009).
Funding
This work was funded by a Polpharma Scientific Foundation grant no. 0706-BCWN-2021 to R.P. and by a grant 503/0-149-03/503-01-001-19-00 from the Medical University of Lodz.
Author information
Authors and Affiliations
Contributions
P.K. writing-original draft, review and editing, visualization; PN.K-S. performing experiments, writing-original draft, visualization; A.H. conceptualization, methodology/Study design; R.P. conceptualization, supervision, funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kleniewska, P., Kopa-Stojak, P.N., Hoffmann, A. et al. The potential immunomodulatory role of the gut microbiota in the pathogenesis of asthma: an in vitro study. Sci Rep 13, 19721 (2023). https://doi.org/10.1038/s41598-023-47003-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-47003-0
- Springer Nature Limited