Abstract
Rock pools are eroded depressions in bedrock providing temporary aquatic habitats with varying morphometric and chemical conditions. Tardigrades have adapted to many habitats with varying and extreme abiotic conditions, including desiccation, but their occurrence in rock pools have rarely been investigated. This study investigated the occurrence of tardigrades and the morphometric and chemical conditions in rock pools by the Baltic Sea in southeast Sweden. Samples of benthic material were collected from rock pools at three sites near the town Karlshamn together with measurements of pool size, pH, temperature, salinity, and dissolved oxygen of the water. Tardigrades occurred in about one fifth of the rock pools and included five eutardigrade genera. Also rotifers and nematodes were observed in the samples. The morphometric and chemical variables varied both within and among the three sites but with few differences between rock pools with or without tardigrades. However, rock pools with tardigrades tended to be overall shallower than pools without tardigrades, indicating that more desiccating-prone rock pools may be more favourable habitats for tardigrades. The study shows that tardigrades are part of the micro-invertebrate fauna in rock pools and this habitat deserves more investigations into the occurrence of this animal group.
Similar content being viewed by others
Introduction
Rock pools are ephemeral aquatic habitats, consisting of depressions in bedrock with occasional water inputs and bottom sediments of trapped unconsolidated material1. They occur on rocky outcrops (e.g., granite, limestone, sandstone) in all major biomes from inland areas (freshwater pools) to shorelines (brackish and marine pools) worldwide. Freshwater pools are mainly rainfed2, while brackish and marine pools also receive seawater through sprays or waves from a neighbouring sea3,4. Rock pools are generally characterized as small-sized habitats with well-defined borders, high local abundance, and as hosts of simple communities and food-webs, making them attractive model systems in ecological and evolutionary research5,6,7. They offer opportunities for both experimental and non-experimental studies about the effects of ephemerality and abiotic stress on biota. Findings in rock pools may also apply to other ephemeral habitats. However, because rock pools often experience some severe abiotic disturbances (e.g., recurring desiccation) and host small populations compared to other habitat types, observed processes and patterns in rock pools must be generalized with care6.
The range of hydroregimes8 in rock pools is as wide as the global distribution of these habitats, and regional variations may also be considerable. For example, reported hydroperiods (time duration from initial filling to desiccation) averaged from several days to little more than a month in pools located in semi-arid regions of southeast Botswana9,10, a few days to about three and a half months in pools of the Korannaberg mountain in central South Africa11, and several days to (at least) five months in pools on islands in the archipelago of southwest Finland12. Variation, frequency, and periodicity of hydroperiods (that is, hydroregime) sets the boundaries for ecological and evolutionary processes operating in rock pools 8. The hydrological selection pressure has resulted in two groups of invertebrate rock pool inhabitants, based on life cycles and dispersal strategies: active and passive dispersers13. Most adult active dispersers (e.g., Insecta, Odonata) only thrive in hydrated pools and migrate to other habitats before desiccation occurs14, while passive dispersers lack active migratory stages and instead disperse as resting stages via other animals, wind, or overflow of water between pools1,15. Passive dispersers represent permanent rock pool inhabitants (so-called ‘rock pool specialists’) with a high extent of endemicity and ability to survive desiccation in situ by adopting drought resistant resting stages2,14 such as diapause (suspended development in response to environmental cues), resting eggs or cysts, or anhydrobiotic states (e.g., Crustacea, Nematoda, Rotifera, Tardigrada)14,16. This permanent fauna normally possesses additional adaptations to abiotic stresses in rock pools. For example, large diurnal and seasonal fluctuations in environmental conditions are common in the pool environment and impose abiotic stress on biota2, especially in shallow pools with low buffering capacity to changes in water variables such as temperature, salinity, pH, and dissolved oxygen10,17,18. Shallow pools with short hydroperiods normally support smaller, less competitive species with a high tolerance to stress, whereas deeper pools with long hydroperiods support larger, predatory species with several developmental stages and less tolerance to stress19,20,21,22, though exceptions to this common observation have been found10. Furthermore, freshwater species usually only appear in freshwater pools, while marine species reside in marine pools, and both freshwater and marine species may occur in brackish pools4,23,24. However, freshwater-tolerant marine species have also been identified25.
Given the variable and ecologically challenging conditions in rock pools, occurrence of the invertebrate phylum Tardigrada in these habitats is of considerable interest. Tardigrades are aquatic micrometazoans inhabiting all major global ecosystems with more than 1400 species described26. Many tardigrade species have been adapted to live in highly variable environments with respect to water availability and have evolved an ability to enter a reversible suspension of metabolism called cryptobiosis in response to complete desiccation, defined as anhydrobiosis27,28,29. Other abiotic stress factors suggested to induce cryptobiosis are freezing (cryobiosis), lack of oxygen (anoxybiosis), and high or low salinities (osmobiosis)27. Anhydrobiosis results in a high heat tolerance of desiccated tardigrades (up to 100 °C in some species)30, while metabolically active (hydrated) tardigrades are considerably more vulnerable to high temperatures (lethal temperature < 40 °C)31,32,33.
Colonisation skill, parthenogenetic reproduction, and resistant resting stages have likely created the basis for the tardigrades’ worldwide dispersal15, including extreme environments such as cryoconite holes34, deserts35, and the abysses of seas36,37. They are part of meiofaunal communities in aquatic habitats with all types of water and are also abundant in ephemeral terrestrial and limno-terrestrial habitats (thin water layers on e.g., mosses, lichens, leaf litter, or soils)14,38. Occurrence of tardigrades in rock pools has been reported throughout the world (e.g., Israel39, Utah, USA22, Australia40, Ivory Coast41, Italy42, yet specific studies of tardigrades in rock pools are very scarce. Vecchi et al.42 conducted the first study focusing on tardigrades in rock pools and reported a prevalence of tardigrades of 79% in rock pools in an Italian alpine area. The shallowest pools (‘Pans’ < 2 cm depth) had a higher occurrence and taxonomic diversity of tardigrades compared to deeper, less desiccation prone pools (‘Intermediates’ > 2 cm, < 5 cm and ‘Holes’ > 5 cm) in freshwater rock pools of the Italian Apennines. However, tardigrade density was not significantly affected by pool depth and thus hydroperiods. In contrast, Jocqué et al.22 observed high densities of tardigrades (Pseudobiotus sp.) and rotifers in shallow ephemeral freshwater pools with short hydroperiods on a sandflat in the US state Utah. In other taxonomic groups of invertebrates, such as larger microinvertebrates (e.g., crustaceans21,22,23,43) and macroinvertebrates (e.g., insects22,23) densities have been reported to be higher in rock pools with long hydroperiods.
Numerous studies have been reported on animal communities in rock pools related to the Baltic Sea3,17,44,45,46, including the micro- and macrometazoan taxa Rotifera, Nematoda, Turbellaria, Gastropoda, Diptera, Bryozoa, Ostracoda and Crustacea17,47. However, there are no previous reports of tardigrades from these shoreline habitats. The purpose of this study was to investigate the occurrence and taxonomic diversity of tardigrades, and the morphometric and chemical conditions in ephemeral rock pools situated by the Baltic Sea in southeast Sweden. Apart from this specific purpose, the study also aims at highlighting a neglected potential habitat for tardigrades found in many different environments around the world.
Materials and methods
Site description and selection of rock pools
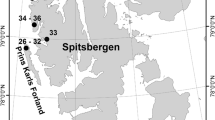
The study was performed in shoreline habitats with exposed rock close to the town Karlshamn at the Baltic Sea in southern Sweden (Fig. 1). The Baltic Sea constitutes an arm of the Atlantic Ocean but with brackish water (ca. 2–8‰). Lack of tide and the low salinity of the adjacent brackish seawater make salinity fluctuations and thus salinity stress on faunal inhabitants rather low in rock pools by the Baltic Sea17, although many pools receive inputs of brackish seawater through sprays or by inundating waves3. Rain showers, evenly distributed over the year, also dilute the pool waters, often exceeding the evaporation rate that increases salinity. Exclusively rain-filled freshwater pools occur further upshore. The atmospheric temperature directly influences the water temperature of rock pools, creating great annual variations from freezing temperatures during winter to summer temperatures above 30 °C17. The predominant wind direction in southern Baltic Sea is west–southwest. Dissolved oxygen content and pH-values are related variables, and closely connected with primary production (mainly algal activity) and community respiration, thus experiencing diurnal and seasonal variations depending on the floral and faunal density and composition of individual pools44.
Location of the study area near Karlshamn, Sweden (©2020 SCB), and the three sites of sampling of rock pools at Gunnön (left), Kofsa nabbe (middle) and Yttervägga (right). The location of rockpools within the sites, with reference to pool number, is also shown. Map background: ‘Map 1:50,000’. ©2020 Lantmäteriet/The Swedish Land Survey.
This study investigated rock pools in 1.8–1.7 billon years old granitic and gneissic bedrock along the shorelines of three capes—Yttervägga, Gunnön and Kofsa nabbe—south of Karlshamn (Fig. 1). The geomorphological formations of the rocky shoreline and archipelago of this area, including frequent sites with a multitude of rock pools, date back to the last glacial period (ca. 115–11.5 thousand years ago), and represent ideal conditions for rock pools studies. The rock pools selected at the three sites represented smaller and shallower pools with expected short hydroperiods while large and deep pools were excluded. The selected pools still varied considerably in size (lengths: 30–240 cm; widths: 12–105 cm) and water depths (1.9–29 cm).
Sampling, extraction and sample analyses
In total 32 rock pools were included in the study. At Yttervägga 8 rock pools were sampled on May 26th in 2021, while at Kofsa nabbe and Gunnön 17 pools and 7 pools, respectively, were sampled on July 9th in 2021. Sampling of benthic material and measurements of the pools were made at the same occasion. All sampling were made at daytime between 9AM and 4PM.
A plastic syringe (100 ml) was used to collect samples of benthic material from different parts of the pools (Fig. 2a), and since benthic material is often concentrated to the deepest parts and bottom crevices of pools these parts were prioritized during sampling. Varying amounts of benthic material were collected from the pools, due to highly different qualities and amounts of benthic material in the pools. Each sample was transferred to a plastic bottle (300 ml) and all bottles were put in a freezer (− 20 °C) in the same day. Analyses of samples took place 1–11 weeks after sampling.
Before extraction of the samples the bottles were thawed in a hot water bath and left standing for a few minutes, allowing the sample material to sediment on the bottom of each bottle. A Pasteur pipette (3 ml) was used to transfer in total 9 ml material from each bottle to petri dishes (3 dishes × 3 ml for bottles containing small or medium amounts of material, or 9 dishes × 1 ml for bottles containing large amounts of or particularly dense material). Distilled water was added to the dishes, which were then analysed for tardigrades under an Olympus SZX9 stereomicroscope, starting at 32 × magnification. The total search time for each dish depended on the absence/occurrence and number of tardigrades found. Every time one or several tardigrades or tardigrade exuvia/eggs were observed, the analysis continued for another 5 mins. If no tardigrades/exuvia/eggs were spotted during the first 5 mins, the analysis was terminated. The upper time limit for one analysis was 90 min. The tardigrades were counted and pipetted to small glass bowls with distilled water and stained with purple-red laceto-aceto-orcein, before mounted on slides in Hoyer’s medium and dried for a few weeks before sealed with transparent nail polish. Mounted specimens were examined under an Olympus BX60 light microscope with up to 1000 × magnification. Tardigrades/exuvia/eggs were photographed with an Olympus UC90 microscope camera and identified to genus level, as it was not possible to identify every specimen to the species level. Taxonomic identification was performed using anatomical descriptions in Nelson et al.48, and identification keys in Pilato and Binda49 and Bingemar and Hohberg50. In addition to tardigrades, the occurrence of rotifers and nematodes in the samples were also documented during the analyses under stereomicroscope, but these animals were not further identified taxonomically.
Measurements of morphometric and chemical variables
The morphometric rock pool variables (length, width, and maximum water depth) were measured with a tape measure. Pool surface area was calculated using the formula to calculate the area of an ellipse (‘A = a × b × π’, where ‘a’ constitutes the minor radius, and ‘b’ the major radius), because most pools had an oblong, round shape. Chemical water variables (temperature, conductivity, pH, and dissolved oxygen) were also recorded for the sampled pools with a portable HACH-HQ40d multimeter with IntelliCAL LDO101 (temperature and dissolved oxygen), IntelliCAL CDC40 (conductivity), and IntelliCAL PHC201 (pH) probes (Fig. 2b). The equipment failed to record the dissolved oxygen content in one pool at Yttervägga and the conductivity in four pools at Kofsa nabbe, thus data for these pools are missing. All conductivities were converted into salinities with the formula ‘TDS = (EC × 0.65)/1000’ (at temp. 25 °C), where ‘TDS’ is the ‰-amount of ‘Total Dissolved Solids’ in the water and ‘EC’ is the conductivity recorded in µS/cm. The conversion factor 0,65 is based on a TDS/EC-relation in natural groundwaters that are minimally affected by anthropogenic activities51.
Statistical analyses
The potential differences in rock pool size, water depth, and chemical conditions between pools with and without tardigrades (pooling data from all three study sites) were analysed with one-way ANOVA (IBM SPSS Statistics v. 27). No statistical tests were used to compare these two groups within the sites, since the number of rock pools with tardigrades were too small (n < 5) at the sites.
Results
Occurrence of tardigrades in rock pools
Tardigrades were found in 19% (6/32) of the sampled rock pools and tended to be more common at Gunnön (57%, 4/7), than at Kofsa nabbe (6%, 1/17), and Yttervägga (13%, 1/8) (Table 1). The highest tardigrade abundance was, however, observed in samples from one of the pools at Yttervägga, with > 100 specimens of the genus Ramazzottius, while the other pools with tardigrades had < 10 specimens of various genera. In total, 5 genera from 5 families of the class Eutardigrada were identified. Isohypsibius and Macrobiotus were the most common genera in the pools with tardigrades (50%, 3/6) and were present in pools at Kofsa nabbe and Gunnön, while Ramazzottius was present in pools at Yttervägga and Gunnön. The two remaining genera, Milnesium and Hypsibius, were only found in pools at Gunnön.
Rotifers occurred in several pools at all three sites (56%, 18/32) and were often abundant in the samples, while nematodes were present in few pools (9%, 3/32) at Yttervägga and Gunnön and generally rare in the samples. All six pools with tardigrades also contained rotifers and two of them contained nematodes, while one pool contained both rotifers and nematodes but no tardigrades.
Relationship between tardigrade occurrence and rock pool conditions
The morphometric and chemical conditions of all rock pools are presented in Supplementary Table S1, and statistical summaries for pools with and without tardigrades at the three sampled areas are given in Table 2. Combining the data from all sites, rock pools with tardigrades were overall significantly shallower (4.6 ± 2.8 cm) compared to the pools without tardigrades (11.1 ± 7.3 cm) (F1,30 = 4.47, P = 0.043), and tended to have smaller surface area (0.2 ± 0.2 m2) than pools without tardigrades (0.5 ± 0.4 m2) but not significantly so (F1,30 = 1.78, P = 0.19). The recorded estimates of temperatures and dissolved oxygen were similar in pools with and without tardigrades at all three sites, and combining data from all sites there were no significant differences between pools with tardigrades and pools without tardigrades (temperature: F1,30 = 0.011, P = 0.92; oxygen: F1,29 = 3.74, P = 0.063).
All pools with tardigrades were freshwater pools (salinity < 0.5‰). The pools without tardigrades, on the other hand, showed greater variation in their salinities and contained both freshwater and brackish water (0.04 ± 0.006‰ at Gunnön, 1.8 ± 3.6‰ at Kofsa nabbe, and 3.7 ± 3.2‰ at Yttervägga). However, there was no significant difference in salinity between pools with and without tardigrades (F1,26 = 2.31, P = 0.14).
A large range of pH-values below and above neutral pH 7.0 were found in the pools at the respective sites. The pH-values of the single pools with tardigrades at Kofsa nabbe (pH 8.9) and Yttervägga (pH 7.9) were close to the average pH-values of the pools without tardigrades (8.8 ± 1.0 at Kofsa nabbe, 8.3 ± 0.8 at Yttervägga), while the average pH-values differed 1.4 units between pools with (6.7 ± 0.5) and without (8.1 ± 0.9) tardigrades at Gunnön. Overall, pools with tardigrades had significantly lower pH than pools where no tardigrades were found (F1,30 = 8.36, P = 0.007).
Discussion
Occurrence of tardigrades
This study is the second that specifically investigated the occurrence of tardigrades in rock pools (following the first by Vecchi et al.42), and the first to document tardigrades in rock pools by the Baltic Sea. Tardigrades were found in pools at all three sites and in nearly 20% of the samples, suggesting that tardigrades represent a relatively common component of the meiofaunal communities and biodiversity of rock pools of this archipelagic region. The prevalence of tardigrades in this study is however considerably lower than in the study by Vecchi et al.42 of Italian alpine rock pools (79%). In all except one of the pools in our study the number of specimens found was low, but the benthic material analysed from each pool only represented a small proportion of the total material in the pools, making it likely that the total number of tardigrades in the pools were higher. The presence of exuvia and eggs also indicates that these tardigrades have resident populations in the rock pools.
Rotifers were more common and abundant in the pools, while nematodes were few and less common than tardigrades. Both rotifers and nematodes have been reported from Baltic rock pools before, but nematodes were found much less frequently in these pools17,47, in line with the results of this study. Nematodes in rock pools on islands of the southern archipelago of Finland appeared to be immigrants to the pools from nearby intertidal algae, terrestrial moss, or grass carpets47.
In the present study the pools hosted a diversity of eutardigrade genera, indicating tardigrade dispersal to the pools from nearby limnic or limno-terrestrial habitats (e.g., lichens, soils, grass turfs). The idea of short-distance dispersal from surrounding habitats to rock pools is supported by Jensen47 for nematodes, and also by Vecchi et al.42 who found a close correspondence between the examined tardigrade community composition of freshwater rock pools in the Italian Apennines and the community composition previously recorded in the same region for mosses, lichens, and freshwater habitats.
Many tardigrades, rotifers and nematodes possess resistant resting stages and an ability to colonise rapidly (e.g., by wind dispersal) and reproduce asexually by parthenogenesis15, which are common characteristics of passive dispersers2,13,14 and especially favoured in ephemeral habitats such as rock pools. Drought resistant resting stages16,27,28,29 allow these phyla to withstand recurring desiccation, which excludes more drought-sensitive taxa from ephemeral pools (e.g., species of Insecta or Odonata14). Our finding of a higher occurrence of tardigrades in shallow Baltic rock pools compared to deeper pools is consistent with earlier records of tardigrades in freshwater pools22,42. This pattern may relate to the ability of tardigrades to survive severe and recurrent desiccation by anhydrobiosis. Larger, predatory invertebrates generally inhabit deeper pools with longer hydroperiods that allow longer life cycles of species19,20,21,22,23,43. These potential predators and competitors of tardigrades are thus excluded from shallow rock pools with short hydroperiods and exposure to dry conditions, which may create more favourable conditions for tardigrades to persist and proliferate.
Morphometric and chemical conditions
Large diurnal and seasonal fluctuations in morphometric and chemical conditions are often recorded in ephemeral rock pools with a low buffering capacity to changes in the pool environment2,10,17,18. The measurements performed in this study only provide a snapshot of the conditions of the investigated pools, since the study was restricted to just one day of field measurements at each of the three selected sites. To get a more detailed picture of how pool conditions fluctuate over time, monitoring of pools during several hydroperiods is necessary. However, recordings of many pools at a single time can still provide information on natural variation in pool conditions among different rock pools. In our study, the existence of such variation was clear for salinity, pH, and dissolved oxygen.
Baltic rock pools are considered to have low salinity fluctuations due to the low salinity of the adjacent brackish seawater that may reach pools through sprays or by inundating waves3,17. Recurring rainfalls over the year also counteract evaporation and dilute the water in pool by the Baltic Sea. This study mainly recorded low salinities that represented freshwater in the pools, yet some of the pools at Yttervägga and Kofsa nabbe contained brackish water. Tardigrades only occurred in freshwater pools at all three sites, and this may be expected since genera of mainly limnic and limno-terrestrial tardigrades were identified, and freshwater invertebrates usually only appear in freshwater pools23,24. Some limno-terrestrial tardigrades have been documented for a high tolerance to osmotic stress and may well survive saline conditions52, but whether they may sustain populations under low saline/brackish conditions is unknown, and the presence of tardigrades in the brackish water of the Baltic Sea is largely unexplored.
The temperate climate of the Baltic region makes annual variations in the atmospheric temperature large and constitute one of the main regulating factors of Baltic rock pool communities17. The recorded pool temperatures in this study were close to the ambient air temperature and similar in the pools at the respective sites, around 16 °C at Yttervägga in May, and 20–23 °C at Kofsa nabbe and Gunnön in July. Despite a tolerance of tardigrades to temperatures up to 100 °C in the desiccated anhydrobiotic state30, under active hydrated conditions tardigrades do not tolerate temperatures over 40 °C31,32,33. Summer temperatures of Baltic Sea rock pools may exceed 30 °C17 and may well reach hazardous levels for tardigrades under heat waves, especially in small and shallow rock pools. Low temperatures, on the other hand, is likely to be much less of a problem for tardigrades inhabiting rock pools since many tardigrades are able to withstand very low freezing temperatures53. Shallow rock pools are more likely to freeze to the bottom during the winter than deeper ones, and tolerance to freezing is therefore a requirement of invertebrates that maintain permanent populations in Baltic Sea rock pools, particularly in the northern parts of the sea. Interestingly, in a recent study on tolerance to repeated freeze–thaw cycles in 12 different tardigrade species from different habitats, specimens of the genus Ramazzottius collected from Italian mountain rock pools were reported to survive the highest number of cycles54, indicating that tardigrades living in habitats with frequent freeze–thaw and/or desiccation-hydration cycles such as rock pools are also well adapted to survive such conditions.
Small ephemeral rock pools are also characterized by large diurnal and seasonal variations in dissolved oxygen content and pH, which mainly depend on primary production and community respiration in pools10,17,44. Although levels of dissolved oxygen varied considerably among rock pools in this study, pools with and without tardigrades did not differ significantly in oxygen level, while pH values were significantly lower and closer to neutral pH 7.0 in rock pools with tardigrades compared to pools without tardigrades. The limited scope of our study with relatively small number of rock pools investigated (limiting the power of the statistical tests) does not allow us to draw any firm conclusions from these results, but both oxygen levels and pH have been reported to show considerable variation at a daily basis in Baltic Sea rock pools17, which makes it worthwhile to more closely investigate how tardigrades respond to variation in these factors.
Conclusion
In conclusion, this study shows that tardigrades are part of the meiofauna in rock pools of the Baltic Sea shoreline, although present in only one fifth of the rock pools. Rock pools where tardigrades were found tended to be shallower and have lower pH, while other measured parameters did not show a relationship with presence of tardigrades. Invertebrate communities of rock pools deserve more studies in view of the extreme variability of abiotic conditions in this extreme habitat, and tardigrades represent a particularly interesting organism to investigate due to its documented tolerance to some of the extreme conditions that rock pools provide. Rock pools offer a range of opportunities for both descriptive and experimental studies on responses to abiotic stress, population dynamics, and dispersal of tardigrades in this small-scale natural habitat type that occurs in many different ecosystems. Due to their small scale and well-defined borders, rock pools are easy to measure and manipulate, and their abundance and morphometric and chemical variability make them ideal as model systems for ecological and evolutionary research.
Data availability
All data underlying this article are presented in the article and in its online supplementary information.
References
Brendonck, L., Lanfranco, S., Timms, B. & Vanschoenwinkel, B. Invertebrates in rock pools. In Invertebrates in Freshwater Wetlands (eds Batzer, D. & Boix, D.) 25–53 (Springer, 2016).
Jocqué, M., Vanschoenwinkel, B. & Brendonck, L. Freshwater rock pools: A review of habitat characteristics, faunal diversity and conservation value. Freshw. Biol. 55, 1587–1602 (2010).
Ranta, E. Animal communities in rock pools. Ann. Zool. Fenn. 19, 337–347 (1982).
Dethier, M. N. Disturbance and recovery in intertidal pools: Maintenance of mosaic patterns. Ecol. Monogr. 54, 99–118 (1984).
Blaustein, L. & Schwartz, S. S. Why study ecology in temporary rock pools?. Isr. J. Zool. 47, 303–312 (2001).
Srivastava, D. S. et al. Are natural microcosms useful model systems for ecology?. Trends Ecol. Evol. 19, 379–384 (2004).
Brendonck, L., Jocqué, M., Hulsmans, A. & Vanschoenwinkel, B. Pools ‘on the rocks’: Freshwater rock pools as model system in ecological and evolutionary research. Limnetica 29, 25–40 (2010).
Hulsmans, A., Vanschoenwinkel, B., Pyke, C., Riddoch, B. J. & Brendonck, L. Quantifying the hydroregime of a temporary pool habitat: A modelling approach for ephemeral rock pools in SE Botswana. Ecosystems 11, 89–100 (2008).
Brendonck, L., Riddoch, B. J., Van de Weghe, V. & Van Dooren, T. J. M. The maintenance of egg banks in very short-lived pools: A case study with anostracans (Branchiopoda). Arch. Hydrobiol. 52, 141–161 (1998).
Brendonck, L., Hamer, M. L., Riddoch, B. J. & Seaman, M. T. Branchipodopsis species: Specialists of ephemeral rock pools. Afr. J. Aquat. Sci. 25, 98–104 (2000).
Vanschoenwinkel, B. et al. Community structure in temporary freshwater pools: Disentangling effects of habitat size and hydroregime and the impact of dispersal mode. Freshw. Biol. 54, 1487–1500 (2009).
Altermatt, F., Pajunen, V. I. & Ebert, D. Desiccation of rock pool habitats and its influence on population persistence in a Daphnia metacommunity. PLoS ONE 4, e4703. https://doi.org/10.1371/journal.pone.0004703 (2009).
Wiggins, G. B., Mackay, R. J. & Smith, I. M. Evolutionary and ecological strategies of animals in annual temporary pools. Arch. Hydrobiol. 58, 97–206 (1980).
Williams, D. D. The Biology of Temporary Waters (Oxford University Press, 2006).
Fontaneto, D. Long-distance passive dispersal in microscopic aquatic animals. Mov. Ecol. 7, 1–10 (2019).
Hibshman, J. D., Clegg, J. S. & Goldstein, B. Mechanisms of desiccation tolerance: Themes and variations in brine shrimp, roundworms, and tardigrades. Front. Physiol. 11, 592016. https://doi.org/10.3389/fphys.2020.592016 (2020).
Ganning, B. Studies on chemical, physical and biological conditions in Swedish rockpool ecosystems. Ophelia 9, 51–105 (1971).
Legrand, E. et al. Ecological characterization of intertidal rockpools: Seasonal and diurnal monitoring of physico-chemical parameters. Reg. Stud. Mar. Sci. 17, 1–10 (2018).
Tevis, L. Unsuccessful breeding by desert toads (Bufo punctatus) at the limit of their ecological tolerance. Ecology 47, 766–775 (1966).
Jeffries, M. Invertebrate communities and turnover in wetland ponds affected by drought. Freshw. Biol. 32, 603–612 (1994).
King, J. L., Simovich, M. A. & Brusca, R. C. Species richness, endemism and ecology of crustacean assemblages in northern California vernal pools. Hydrobiologia 328, 85–116 (1996).
Jocqué, M., Graham, T. & Brendonck, L. Local structuring factors of invertebrate communities in ephemeral freshwater rock pools and the influence of more permanent water bodies in the region. Hydrobiologia 592, 271–280 (2007).
Therriault, T. W. & Kolasa, J. Desiccation frequency reduces species diversity and predictability of community structure in coastal rock pools. Isr. J. Zool. 47, 477–489 (2001).
Therriault, T. W. Temporary aquatic habitats: Constraints and opportunities. Aquat. Ecol. 36, 529–540 (2002).
Aarnio, S., Teittinen, A. & Soininen, J. High diatom species turnover in a Baltic Sea rock pool metacommunity. Mar. Biodivers. 49, 2887–2899 (2019).
Degma, P. & Guidetti, R. Actual checklist of Tardigrada species (2009–2023), 42th Edition, 2023). https://doi.org/10.25431/11380_1178608.
Keilin, D. The Leeuwenhoek lecture: The problem of anabiosis or latent life: History and current concept. Proc. R. Soc. B. 150, 149–191 (1959).
Wright, J. C. The significance of four xeric parameters in the ecology of terrestrial tardigrada. J. Zool. 224, 59–77 (1991).
Schill, R. O. & Hengherr, S. Environmental adaptations: Desiccation tolerance. In Water Bears: The Biology of Tardigrades (ed. Schill, R. O.) 273–293 (Springer, 2018).
Hengherr, S., Worland, M. R., Reuner, A., Brümmer, F. & Schill, R. O. High-temperature tolerance in anhydrobiotic tardigrades is limited by glass transition. Physiol. Biochem. Zool. 82, 749–755 (2009).
Li, X. & Wang, L. Effect of thermal acclimation on preferred temperature, avoidance temperature and lethal thermal maximum of Macrobiotus harmsworthi Murray (Tardigrada, Macrobiotidae). J. Therm. Biol. 30, 443–448 (2005).
Rebecchi, L. et al. Stress response of a boreo-alpine species of tardigrade, Borealibius zetlandicus (Eutardigrada, Hypsibiidae). J. Limnol. 68, 64–70 (2009).
Neves, R. C., Hvidepil, L. K. B., Sørensen-Hygum, T. L., Stuart, R. M. & Møbjerg, N. Thermotolerance experiments on active and desiccated states of Ramazzottius varieornatus emphasize that tardigrades are sensitive to high temperatures. Sci. Rep. 10, 94. https://doi.org/10.1038/s41598-019-56965-z (2020).
Zawierucha, K. et al. A hole in the nematosphere: Tardigrades and rotifers dominate the cryoconite hole environment, whereas nematodes are missing. J. Zool. 313, 18–36 (2020).
Neher, D. A., Lewins, S. A., Weicht, T. R. & Darby, B. J. Microarthropod communities associated with biological soil crusts in the Colorado Plateau and Chihuahuan deserts. J. Arid Environ. 73, 672–677 (2009).
Bussau, C. New deep-sea Tardigrada (Arthrotardigrada, Halechiniscidae) from a manganese nodule area of the eastern South Pacific. Zool. Scr. 21, 79–92 (1992).
Kharkevych, K. O. & Sergeeva, N. G. Deep-water Tardigrada of the Istanbul Strait’s (Bosporus) outlet area of the Black Sea. Vestn. Zool. 47, 17–27 (2013).
Nelson, D. R., Bartels, P. J. & Guil, N. Tardigrade Ecology in Water Bears: The Biology of Tardigrades 163–210 (Springer, 2018).
Spencer, M., Blaustein, L., Schwartz, S. S. & Cohen, J. E. Species richness and the proportion of predatory animal species in temporary freshwater pools: Relationships with habitat size and permanence. Ecol. Lett. 2, 157–166 (1999).
Boix, D. et al. Invertebrates of freshwater temporary ponds Mediterranean climates. In Invertebrates in Freshwater Wetlands (eds Batzer, D. & Boix, D.) 141–189 (Springer, 2016).
Snoeks, J. M., Driesen, M., Porembski, S., Aristizábal-Botero, Á. & Vanschoenwinkel, B. Contrasting biodiversity and food web structure of three temporary freshwater habitats in a tropical biodiversity hotspot. Aquat. Conserv. Mar. Freshw. Ecosys. 31, 1–18 (2021).
Vecchi, M., Ferrari, C., Stec, D. & Calhim, S. Desiccation risk favours prevalence and diversity of tardigrade communities and influences their trophic structure in alpine ephemeral rock pools. Hydrobiologia 849, 1995–2007 (2022).
Eitam, A., Blaustein, L., Van Damme, K., Dumont, H. J. & Martens, K. Crustacean species richness in temporary pools: Relationships with habitat traits. Hydrobiologia 525, 125–130 (2004).
Ganning, B. & Wulff, F. Measurements of community metabolism in some Baltic brackish water rockpools by means of diel oxygen curves. Oikos 21, 292–298 (1970).
Arnér, M. Organisms and Food Webs in Rock Pools: Responses to Environmental Stress and Trophic Manipulation (PhD thesis, Stockholm University, 1997).
Pajunen, V. I. & Pajunen, I. Long-term dynamics in rock pool Daphnia metapopulations. Ecography 26, 731–738 (2003).
Jensen, P. The absence of a permanent nematode fauna in Baltic rock pool ecosystems. Estuar. Coast. Mar. Sci. 9, 797–800 (1979).
Nelson, D. R., Guidetti, R. & Rebecchi, L. Phylum Tardigrada. In Ecology and General Biology: Thorp and Covich’s Freshwater Invertebrates (eds Thorp, J. & Rogers, D. C.) 347–380 (Academic Press, 2015).
Pilato, G. & Binda, M. G. Definition of families, subfamilies, genera and subgenera of the Eutardigrada, and keys to their identification. Zootaxa 2404, 1–54 (2010).
Bingemar, J. & Hohberg, K. An illustrated identification key to the eutardigrade species (Tardigrada, Eutardigrada) presently known from European soils. Soil Org. 89, 127–149 (2017).
Thirumalini, S. & Joseph, K. Correlation between electrical conductivity and total dissolved solids in natural waters. Malay. J. Sci. 28, 55–61 (2009).
Heidemann, N. W. T. et al. Osmotic stress tolerance in semi-terrestrial tardigrades. Zool. J. Linn. Soc. 178, 912–918 (2016).
Hengherr, S. & Schill, R. O. Environmental adaptations: Cryobiosis. In Water Bears: The Biology of Tardigrades (ed. Schill, R. O.) 295–310 (Springer, 2018).
Zawierucha, K., Vecchi, M., Takeuchi, N., Ono, M. & Calhim, S. Negative impact of freeze-thaw cycles on the survival of tardigrades. Ecol. Indic. 154, 110460 (2023).
Funding
Open access funding provided by Kristianstad University.
Author information
Authors and Affiliations
Contributions
S.T.: Conceptualization, collection of data, data analyses, writing—original draft. K.I.J.: Conceptualization, collection of data, writing—review & editing final draft, supervision of data analyses and writing. All authors accepted the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Troell, S., Jönsson, K.I. Occurrence of tardigrades and morphometric and chemical conditions in rock pools by the Baltic Sea. Sci Rep 13, 19776 (2023). https://doi.org/10.1038/s41598-023-46697-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-46697-6
- Springer Nature Limited