Abstract
In the present study, an Iranian natural zeolite (Sabzevar region) was evaluated as a natural adsorbent for the elimination and immobilization of strontium ions from an aqueous solution. For improving the adsorption efficiency of strontium ion, the zeolite surface was modified by the Schiff base ligand of bis (2-hydroxybenzaldehyde)1,2-diaminoethane (H2L). The natural zeolite and zeolite/H2L were characterized using Fourier transform infrared spectroscopy (FT-IR), X-ray diffraction (XRD), X-ray fluorescence (XRF), BET and scanning electron microscope (SEM). Analysis of the natural zeolite showed that the zeolite is from the type of clinoptilolite and has a crystalline structure with the specific surface area 29.74 m2/g. The results showed that strontium adsorption onto modified zeolite increases compared to unmodified zeolite from 64.5% to 97.2% (at pH = 6). The effective parameters pH, adsorbent dosage, initial concentration of strontium ions, contact time, temperature, and interfering ions, were studied and optimized. The maximum adsorption efficiency was confirmed by modified zeolite and found to be 97.5% after 60 min of equilibrium time at pH 6, 0.05g as adsorbent dosage, and at 25 °C. Adsorption of strontium was confirmed by Langmuir model with maximum adsorption capacity of 10.31 mg/g. Kinetic studies showed that the adsorption of strontium ions on the adsorbent follows pseudo-second-order (PSO) model. Also, the thermodynamics of the adsorption process indicated that the adsorption of strontium on zeolite/H2L is an endothermic and spontaneous process, and the adsorption mechanism is a combination of physical and chemical adsorption. Finally, to manage the secondary waste generated from the adsorption process, strontium ions were immobilized in a zeolite structure. The results showed that the stabilization is well done with the thermal preparation process. After thermal treatment at 25–900 °C, modified zeolite satisfactorily retains strontium during back-exchange tests with NaCl solution. According to the results, the amount of strontium released from the adsorbent phase decreases from 52.6 to 1.6% with increasing heat treatment temperature.
Similar content being viewed by others
Introduction
Many nuclear industry activities produce radioactive waste in the environment1. Usually, the complete inhibition of waste production in various sectors, such as the nuclear industry, is not possible despite the use of the best techniques for reducing produced waste, and significant amounts of waste generated during the operation and exploitation. It should be noted that the operation of nuclear waste management is the same general way used everywhere, but the basic principles governing the operation of waste management to preserve humans and the environment from any harmful effects of radioisotopes and ionizing radiations are identical2.
Strontium is an alkaline element and like other members of the family, especially calcium, barium, and radium. Strontium has an atomic radius similar to calcium and easily replaces calcium in minerals. 90Sr is one of the essential components of several nuclear wastes, which is a fusion product of 235U3. This isotope (90Sr) is one of the most dangerous nuclear fission products for humans4. Radioactive strontium can compete with calcium in the biosphere, known as a bone seeker, and may also be transferred to the human body via the food chain due to its long retention time. 90Sr is adsorbed by the digestive system, where it congregates in the bone marrow tissue and damages the blood-producing cells. It may also cause leukemia or skeletal cancer4. So, the separation and removal of strontium ions from aqueous solutions is very important.
Ion exchangers are widely used to treat industrial wastewater and valuable metals are recovered at a lower cost than conventional chemical treatment with significant savings in treatment plant space. Various sorbents/ion exchangers are described in the literature for the adsorption of strontium, cesium, and uranium. Synthetic and natural adsorbents and inorganic ion exchangers, such as sepiolite5, kaolin6, titanosilicates and titanates7,8,9, metal oxides and their mixtures10,11,12,13, and acidic salts of polyvalent metals, salts of heteropolyacids14,15, compared to the known organic resins, have advantages in terms of higher chemical and thermal stability. In addition, they have specific selectivity for some ions.
Zeolites are another valuable inorganic crystalline material that has many industrial applications, especially in the treatment of radioactive liquid wastes because of their structural characteristics such as high mechanical and chemical resistance, porosity, and the presence of alkaline and earth alkaline metal cations. They offer good cation exchange, and catalytic properties and are desirable for analytical purposes4,16,17,18,19,20.
Furthermore, if the cation exchange reaction facilitates the treatment of nuclear power plant wastewater, it is evident that the waste problem is subsequently moved to contaminated zeolites, which should be reserved in an appropriate disposal tank as radioactive waste. The greatest conventional processes of stabilization of these wastes include freezing with inorganic reactants, immobilization in a cement matrix, vitrification, and caramelization. Stabilizing the generated waste in an appropriate matrix is typically an effective process to safely and irreversibly entrap the cation. This method has been demonstrated to be proper to eliminate and securely dispose of strontium and cesium ions either through natural or synthetic zeolites21,22,23,24,25,26. Heat treatment at high temperatures causes the zeolite framework to break down and form an amorphous phase, "encapsulating" the unwanted species.
One of the most important characteristics related to nuclear energy is radioactive waste management and disposal. Immobilization of radioactive elements should be planned to ensure that there is no significant release to the environment. So, the present research focused on using clinoptilolite from Iranian zeolites in the Sabzevar region. First, the natural zeolite was prepared and characterized. Then natural zeolite was modified by Schiff base ligand bis (2-hydroxybenzaldehyde)1,2-diaminoethane (H2L) to improve the removal and separation of strontium ions. Adsorption of this ion on the surface of zeolite showed the pH dependency. It is noteworthy that with the modification of zeolite by the H2L ligand, a significant increase in the adsorption percentage of strontium ions from aqueous phases was observed (at pH = 6 from 64.5% to 97.2%). Other parameters affecting to adsorption properties of the modified zeolite were investigated and the adsorption of strontium ions on the adsorbent was evaluated in terms of equilibrium, kinetic, and thermodynamic studies. Then, the strontium ion loaded onto the modified adsorbent was immobilized by the thermal treatment method. The leaching test results showed that the consolidation rate is improved with increasing temperature.
Experimental
Materials and instruments
The zeolite Sabzevar mines were supplied by a company (afrand tooska) in Tehran, Iran. Schiff base ligand bis (2-hydroxybenzaldehyde)1,2-diaminoethane (H2L) was synthesized by using 2-hydroxybenzaldehyde and ethylendiamine (Merck) in methanol solvent for modification of zeolite. To prepare stock solutions of strontium ion (1000 mg/L), an appropriate amount of strontium nitrate salt was dissolved in distilled water. All the other reagents and chemicals used were of analytical grade and were attained from Merck or Aldrich.
The pH is adjusted with a Metrohm (model 780) by adding nitric acid or sodium hydroxide (0.1 M). A water bath incubator shaker Infors AG (model Aquatron) was applied for mixing adsorbent and aqueous solutions. Abstraction of the adsorbent from the solution was conducted by a Sigma centrifuge (model 3-30K). A Varian Liberty 150 XL inductively coupled plasma (ICP) was employed for the analysis of metal ions. The FT-IR spectra natural zeolite and modified zeolite before and after adsorption of strontium ion was recorded using a Brucker Vector 22 spectrophotometer using KBr disks. Adsorbance was measured as a function of the wavenumber (cm−1) between 400 and 4000 cm−1. X-ray powder diffraction (XRD) was accomplished with a 3710 PW Philips in the range of 2θ between 5 and 70°. The surface analysis of natural zeolite before and after modification with H2L ligand were determined using BET (Quantachrome Instruments, version 2.2). Surface morphology natural zeolite and modified zeolite before and after adsorption of strontium ion was visualized by the scanning electron microscopy (SEM, Philips XL-30). The chemical composition of zeolite was also defined by the X-Ray fluorescence (XRF, Oxford ED2000), and a furnace (Exiton, model Atash-1200) was employed for immobilizing the component of loaded strontium onto modified zeolite in different temperatures.
Synthesis and characterization of ligand
The H2L ligand was prepared similarly to the previous methods27. The schematic of H2L ligand synthesis is shown in Fig. 1. 1.23 g 2-hydroxybenzaldehyde (0.01 mol) in 30 mL of ethanol solution was refluxed with 0.39 g of ethylenediamine (0.005 mol) for 2 h. The deposited compound was separated by a filter, and washed with ethanol and water many times: yield, 1.27 g (78.0%). IR: νC=C = 1578 cm−1, νC=N = 1636 cm−1, νC-H = 2902 cm−1, νO-H = 3450 cm−127.
Zeolite modifying
The modification of the natural zeolite was conducted based on the following order: (a) 5 g of the zeolite was dehydrated at 100 °C; (b) then, this amount of adsorbent was refluxed with 1 g ligand in dichloromethane solvent for 24 h; (c) for exiting the excess ligands that were not entered in the structure of the zeolite, the adsorbent produced in the previous step was soxhlet until the solvent was colorless; (d) the adsorbent obtained was filtered and washed many times with distilled water to eliminate the solvent; (e) then, the modified zeolite was dried at 70 °C.
Adsorption procedure
Adsorption runs were performed in polyethylene containers in a shaker water bath using 0.05 g of the adsorbents in 20 mL of sample including metal ion and stirred for 60 min. Filter-separating of solid phase from liquid was followed by centrifuging at 5000 rpm for 10 min. The concentration of cation remaining in the aqueous phase was defined by ICP, and the amount of adsorbed ions was estimated from the change between the initial and final concentrations of cation.
The adsorption and desorption percentage of strontium ion from aqueous solution was calculated as follows28:
where Ci and Cf are the initial and final strontium concentration (mg/L), respectively. “md” and “ma” is the desorption and adsorption of ions from the adsorbent surface (mg), respectively.
Immobilization and leaching tests
The safety test of trapping strontium in modified zeolite was performed in two stages:
-
(a)
Immobilizing test: To test the safety of strontium trapping, first 1 g of the modified adsorbent disperses in 50 mL of 600 mg/L Sr(NO3)2 solution for 24 h. Then adsorber was separated from the liquid phase, and the amount of adsorbed ions by ICP was estimated. 0.2 g pellets of the Sr-zeolites were made using a hydraulic press with a loading pressure of 400 g/cm2 and heated at temperatures of 25, 60, 300, 600, and 900 °C for 4 h.
-
(b)
Leaching test: Thermally treated tablet samples, after being powdered, were stirred for 24 h with 50 ml of a 1 M NaCl solution to study the quantity of back exchangeable strontium. Then, the liquid was removed from the powder by centrifugation and analyzed by ICP.
Results and discussion
Characterization
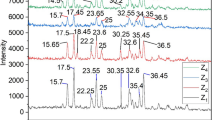
X-ray diffraction spectra of the natural zeolite was shown in Fig. 2. XRD analysis showed that the zeolite is from the type of clinoptilolite (Na–K) and has a crystalline structure. The crystal phase structure and crystal size of samples were examined by X-ray powder diffractometer using Cu Kα radiation (λ = 1.5405 Å). The crystallite size equal to 29.49 nm was defined from the characteristic peak at 2θ = 22.36° for natural zeolites using the Scherrer formula29:
where K, the shape factor = 0.9, λ = wavelength of the X-ray used, and W = width of peak at half-height. Also, XRF analysis of the natural zeolite and the details of their percentage chemical composition were mentioned in Table 1. The specific surface area (SBET) and pore volume of natural zeolite and modified zeolite are reported in Table 2. As shown in the table, the specific surface area of natural zeolite is 29.74 m2/g that after modification of natural zeolite using H2L ligand the specific surface area of natural zeolite was decreased by 12.6%. The modification of natural zeolite may have resulted in pore blockage, leading to a decrease in specific surface area, but this modification also introduced additional adsorption sites, thereby enhancing the adsorption capacity of the modified zeolite19.
Figure 3a,b shows the SEM images of the natural and modified zeolites. The micrographs indicated that there were no significant changes in the surface morphology of natural zeolites after the modification process. Figure 3c shows the surface morphology of modified zeolite after strontium adsorption. Figure 3d,e present the EDX spectrum of modified zeolite before and after strontium adsorption. The results of the EDX analysis show the presence of the C and N elements in the modified zeolite with H2L ligand structure (Fig. 3d). These results provide strong evidence of the successful modification of the natural zeolite surface. The adsorption of strontium on modified zeolite is confirmed by EDX analysis (as presented in Fig. 3e). It shows the presence of strontium at approximately 1.8 keV, confirming the adsorption of strontium onto the surface of modified zeolite.
Figure 4 shows the FT-IR spectra of the natural zeolite and zeolite/H2L before and after adsorption of strontium ion. In the 400–600 cm-1 region, the band observed is related to the double ring in the natural zeolite structure. The vibration frequencies at 1050, and 793 cm-1 were allocated to the asymmetric and symmetric stretching related to the external bonds, respectively30,31. The peaks at 3100–3700 and 1400–1700 cm-1 are related to interstitial water and hydroxyl group, and the deformation vibration of the free water molecules, correspondingly32. In the FT-IR spectrum of zeolite/H2L, the intensity of the peaks in the regions of 1633 and 3100–3700 cm−1 has increased, which corresponds to the vibration frequencies of C = N and O–H groups in the ligand structure. After the adsorption of strontium ions on modified zeolite, the peaks at the 1200–1500 cm-1 region almost disappeared. The majority of bands were shifted, confirming the adsorption of strontium onto modified zeolite.
Adsorption runs
Effect of pH
The pH is one of the decisive factors in the amount of adsorption capability. Comparison of the adsorption process of unmodified and modified zeolite showed that in addition to increasing the adsorption of strontium ions with increasing the pH, the presence of Schiff base ligand in zeolite structure was influential on the adsorption efficiency. So that with increasing pH, the adsorption amount increases significantly from 64.5% to 97.2% at pH = 6 (Fig. 5). The results show that the adsorption in an alkaline medium is somewhat higher than in an acidic medium, which is a general phenomenon for most inorganic ion exchangers33. This behavior may arise from the competition between strontium and hydrogen ions for adsorption on the adsorbents. From this figure, it was seen that the adsorption percentage of strontium continuously improves with intensifying pH, and the highest adsorption is attained at pH = 6. Therefore, pH = 6 was selected as the optimal pH, and other factors were investigated using the modified zeolite.
Effect of adsorbent amount
To examine this parameter, 0.005–0.15g of the modified adsorbent was used for the sorption of 20 mg/L of strontium ions from a 20 mL solution. Figure 6 shows that with the increasing amount of adsorbent, the adsorption efficiency of strontium ions increases because of the increase of adsorption parts34. An additional increment in the amount of adsorbent beyond 0.05 g did not change the outcomes, representing surface saturation and equilibrium between the adsorbent and ions. A quantitative uptake (> 97%) of strontium ions was achieved using 0.05 g of the modified zeolite.
Influence of shaking time
To determine the impact of contact time between adsorbent and aqueous solution on strontium adsorption, differences in uptake percentage of strontium against time were sketched. The adsorption of strontium ions from solution with pH = 6 onto modified zeolite (0.05 g) was investigated with various shaking times in 5 to 120 min (Fig. 7). It was seen that the adsorption of strontium ions from aqueous solution via the modified adsorbent was rapid and enhanced continuously with increasing time until equilibrium between the two phases was achieved after 60 min. Hence, this obtained equilibrium time was used for subsequent adsorption tests.
Impact of initial concentration
To investigate the maximum quantity of metal ion adsorbed by a certain extent of sorbent, this variable was investigated with 0.05 g of sorbent for initial concentrations ranging from 10 to 120 mg/L at pH = 6 (Fig. 8). The results show that by increasing the initial concentration up to 20 mg/L, the adsorption percentage increased. However, after the concentration of 20 mg/L, the relative sites available for adsorption on the surface of the adsorbent were low, and consequently, the abstraction percentage of metal ions decreased35.
Temperature dependency
The adsorption mechanism can be influenced by temperature. To evaluate the impact of this factor, the removal of strontium ions onto modified adsorbent was studied in the range of 10–45 °C, while other factors were maintained constant (20 mg/L of strontium in pH = 6). Table 3 shows the adsorption percentage of strontium ions on the adsorbent at different temperatures. This indicates that the uptake of strontium ions increases with increasing temperature from 10 to 25 °C, and the adsorption of strontium ion on adsorbent is endothermic process. Therefore, the high temperature is beneficial for strontium adsorption. The activity of strontium ions in solution increased with increasing temperature, which facilitated the coordination of strontium ions to functional groups of adsorbent surfaces36. The next experiments were performed at 25 °C. Above this temperature, adsorption decreases. These data can be illuminated by studying physical and chemical adsorption mechanisms28.
The effect of interfering ions
Because of the existence of other interfering ions in the nuclear waste, competitive adsorption of Co(II), Fe(III), Ni(II), Cs(I), and Na(I) ions with Sr(II) ion was performed by 0.05 g of adsorbent (Table 4). For this study, 20 ml of solution involving an initial concentration of 0.001 molar from Sr(II), Co(II), and Cs(I) ions, 0.002 molar of Fe(III) and Ni(II), and 0.005 molar of Na(I) ion in the presence of 0.05 g of adsorbent zeolite/H2L at pH = 6 was studied. The results showed that strontium ion has a higher adsorption than other ions, and the selectivity remains unchanged.
Adsorption kinetics
To study the particular rate constants of the current adsorption reactions, we analyzed the kinetic data using pseudo-first-order, (PSO), Erovich, and power-function kinetic approaches. Kinetic factors were evaluated according to the linear graphs of the equations (Table 5). The data attained from the analysis of the current data indicated that strontium adsorption onto zeolite/H2L has been best described (R2 > 0.99) by a (PSO) kinetic equation (Fig. 9).
The (PSO) equation is defined as follows37:
where qe and qt are the equilibrium adsorption capacity and the biosorption capacity at the time of t (mg/g), respectively, K2 is the rate constant of the (PSO) model (g/mg.min).
Adsorption isotherms
Isotherm equations are usually used to describe the adsorption equilibrium between the adsorbed ions and the dissolved ions in the liquid. In the present work, the obtained data were fitted with common isotherm models, including Langmuir, Freundlich, and Temkin. In Table 6, the values of the relevant isotherm parameters and their correlation factors (R2) are presented. High R2 is derived by fitting experimental results into the Langmuir isotherm model (Fig. 10, R2 = 0.956) compared to the Freundlich and Temkin isotherm models. Langmuir approach is often employed for monolayer sorption occurring on a homogeneous surface with identical sorption sites. Its linear type can be explained by the following correlation34:
where qmax is the maximum adsorption capacity (mg/g), qe is the amount of metal ion adsorbed per unit weight of sorbent (mg/g), Ce is the equilibrium concentration of the metal ion (mg/L), and b is the Langmuir constant (L/mg).
Adsorption thermodynamics
To better understand the effect of temperature, the values of the thermodynamic factors, for instance, the Gibbs’ free energy (ΔGº), the standard enthalpy change (ΔHº), and the standard entropy change (ΔSº), were also studied. The enthalpy and entropy changes related to the metal ions adsorption process can be determined using the van't Hoff correlation presented here.
where Kd is the distribution coefficient, T is the absolute temperature (K), and R is the gas constant (0.0083 kJ/K.mol). Figure 11 shows the plot of lnKd vs. 1/T for the adsorption of strontium ions, where a straight line is obtained. To determine enthalpy and entropy, the following Eqs. (7) and (8) are used:
Based on ΔH° and ΔS°, the free energy variation (ΔG°) was also calculated (see Eq. (9)).
The endothermic nature of the adsorption of the strontium ions onto the modified zeolite is suggested by the positive values of ΔH° (0.0138). The increasing randomness at the solid/solution interface during the adsorption process can be seen by the positive values of ΔS° (0.0955). Therefore, the adsorbent's affinity for this ion is indicated by the positive entropy of adsorption, and the values of ∆H° and ∆G° indicate that the adsorption of strontium on H2L/zeolite is an endothermic and spontaneous process (Table 7). The value of ΔG° (− 27.03 kJ/mol at 283 K and − 28.47 kJ/mol at 298 K, respectively) became more negative with the increase of temperature, which indicated that the adsorption process was more favorable at higher temperatures36,38. The values of − 400 < ΔG° < − 80 kJ/mol describes chemical adsorption while, the value of − 20 < ΔG° < 0 kJ/mol describes physical adsorption, and the value of − 80 < ΔG° < − 20 kJ/mol describes combination of physical and chemical adsorption39,40,41. Therefore, the values of calculated ΔG° confirmed the physicochemical adsorption property.
Immobilization and leaching experiments
First, 1 g of the modified adsorbent was dispersed in 50 mL of 600 mg/L Sr(NO3)2 solution and stirred for 24 h. Then the adsorbent was separated and dried. Using a hydraulic press, 5 tablets (0.2 g of the Sr-zeolite/H2L) were prepared and heated for 4 h at various temperatures of 25, 60, 300, 600, and 900 °C. With increasing temperature, the appearance of the pellets changed and darkened (Fig. 12). The change in the appearance of the samples at temperature of 25–300 °C is related to the release of water molecules that are into the zeolite structure, and the appearance change of the samples at temperatures higher than 300 °C is related to the burning, and structural decomposition of the Schiff base ligand.
Leaching tests were performed following the preparation of the pellets at various temperatures. To test the safety of immobilized samples, pellets were powdered and stirred for 24 h with 50 ml of a 1M NaCl solution. Then the amount of released strontium was evaluated. The results showed that the amount of strontium released from the adsorbed phase decreases with increasing heat treatment temperature (Table 8).
Conclusions
Studies conducted on natural zeolite of the Sabzevar region show that this type of zeolite has a crystalline structure and is the type of sodium clinoptilolite, and its particle size is equal to 29.49 nm. Examining the ion exchange properties of the zeolite showed that it can be used as an ion exchange of strontium from aqueous solutions. The Adsorption process was dependent on the environmental pH and in the range of 4–6 showed the best performance. To improve the performance of zeolite adsorption from Schiff base ligand synthesized to encapsulate in the zeolite pores was used. To compare the efficiency of adsorption of strontium ions onto unmodified and modified zeolite showed that the ligand present in the zeolite structure significantly increases the adsorption efficiency of strontium ions. So that the strontium ion in optimum conditions is almost completely removed from the medium. Kinetic, isotherm, and thermometric studies revealed that the removal process follows the (PSO) model, Langmuir adsorption isotherm, and endothermic process, respectively. Given that the adsorption of strontium ion on modified zeolite was a significant amount, to verify that the adsorbent can also be used as an appropriate medium to stabilize the strontium ion, the experiments to stabilize the strontium ion were performed at different temperatures. The results showed that Sr-zeolite/H2L retains strontium after heat treatment at 900 °C and can be used as a suitable matrix for stabilizing and maintaining strontium ions. Hence the differences in zeolite structure and composition determine the final safety of the strontium retention. Using the results of this study and similar works, these resources can be used in wastewater purification, especially wastes resulting from nuclear activities. The main advantages of the use of zeolite is easy to use, inexpensive, and optimal compatibility with the environment and the lack of contaminating the environment.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Nan, S., Tel, H. & Alta, Y. Adsorption studies of strontium on hydrous zirconium dioxide. J. Radional. Nucl. Chem. 267, 615–621 (2006).
Fuleihan, G. E. Strontium ranelate—A novel therapy for osteoporosis or a permutation of the same?. N. Engl. J. Med. 350, 504–506 (2004).
Hari, P. et al. Adsorptive removal of strontium from water by using chemically modified orange juice residue. Separat. Sci. Technol. 49, 1244–1250 (2014).
Sadeghi, M., Yekta, S., Ghaedi, H. & Babanezhad, E. Effective removal of radioactive 90Sr by CuO NPs/Agclinoptilolite zeolite composite adsorbent from water sample: Isotherm, kinetic and thermodynamic reactions study. Int. J. Ind. Chem. 7, 315–331 (2016).
Kilislioglu, A. & Aras, G. Adsorption of uranium from aqueous solution on heat and acid treated sepiolites. Appl. Radiat. Isot. 68, 2016–2020 (2010).
Wang, G., Wang, X., Chai, X., Liu, J. & Deng, N. Adsorption of uranium(VI) from aqueous solution on calcined and acid-activated kaolin. Appl. Clay Sci. 47, 448–451 (2010).
Noh, Y. D., Komarneni, S. & Mackenzie, K. J. D. Titanosilicates: Giant exchange capacity and selectivity for Sr and Ba. Sep. Purif. Technol. 95, 222–226 (2012).
Duff, M. C. et al. Mechanisms of strontium and uranium removal from high-level radioactive waste simulant solutions by the sorbent monosodium titanate. Environ. Sci. Technol. 38, 5201–5210 (2004).
Saberi, R., Nilchi, A., Rasouli Garmarodi, S. & Zarghami, R. Adsorption characteristic of 137Cs from aqueous solution using PAN-based sodium titanosilicate composite. J. Radioanal. Nucl. Chem. 284, 461–469 (2010).
Tel, H. et al. Preparation of ZrO2 and ZrO2 –TiO2 microspheres by the sol–gel method and an experimental design approach to their strontium adsorption behaviours. Chem. Eng. J. 161, 151–160 (2010).
Inan, S. & Altas, Y. Preparation of zirconium-manganese oxide/polyacrylonitrile (Zr-Mn oxide/PAN) composite spheres and the investigation of Sr(II) sorption by experimental design. Chem. Eng. J. 168, 1263–1270 (2011).
Nilchi, A., Dehaghan, T. S. & Rasouli Garmarodi, S. Kinetics isotherm and thermodynamics for uranium and thorium ions adsorption from aqueous solutions by crystalline tin oxide nanoparticles. Desalination 321, 67–71 (2013).
Li, D., Zhang, B. & Xuan, F. The sequestration of Sr(II) and Cs(I) from aqueous solutions by magnetic graphene oxides. J. Mol. Liq. 209, 508–514 (2015).
Ghanadi Maragheh, M., Husain, S. W. & Khanchi, A. R. Selective sorption of radioactive cesium and strontium on stannic molybdophosphate ion exchanger. Appl. Rad. Isotop. 50, 459–465 (1999).
Kubica, B., Tuteja-Krysa, M. & Misiak, R. The behavior of Ba and Sr on inorganic and organic ion-exchangers from sulphuric acid solutions. J. Radioanal. Nucl. Chem. 258, 167–170 (2003).
Yusan, S. & Erenturk, S. Adsorption characterization of strontium on PAN/Zeolite composite adsorbent, world. J. Nucl. Sci. Technol. 1, 6–12 (2011).
Yuna, Z. Review of the natural, modified, and synthetic zeolites for heavy metals removal from wastewater. Environ. Eng. Sci. 33, 1–12 (2016).
Medykowska, M., Wisniewska, M., Szewczuk-Karpisz, K. & Panek, R. Interaction mechanism of heavy metal ions with the nanostructured zeolites surface–Adsorption, electrokinetic and XPS studies. J. Mol. Liq. 357, 119144–119154 (2022).
Alotaibi, A. M., Ismail, A. F. & Aziman, E. S. Ultra-effective modified clinoptilolite adsorbent for selective thorium removal from radioactive residue. Sci. Rep. 13, 9316 (2023).
Praipipat, P., Ngamsurach, P. & Roopkhan, N. Zeolite A powder and beads from sugarcane bagasse fly ash modified with iron(III) oxide-hydroxide for lead adsorption. Sci. Rep. 13, 1873 (2023).
Liguori, B., Caputo, D., Iucolano, F., Aprea, P. & De Gennaro, B. Entrapping of Cs and Sr in heat-treated zeolite matrices. J. Nucl. Mater. 435, 196–201 (2013).
Li, L. et al. Immobilization of strontium and cesium by aluminosilicate ceramics derived from metakaolin geopolymer-zeolite A composites via 1100 C heating treatment. Ceram. Int. 48, 15236–15242 (2022).
Mukiza, E. et al. Co-immobilization of cesium and strontium containing waste by metakaolin-based geopolymer: Microstructure, mineralogy and mechanical properties. J. Nucl. Mater. 585, 154639 (2023).
Park, M., Kim, S., Takahashi, Y. & Jeong, H. Y. Thermal stabilization of extraframework Cs+ in zeolite 13X. J. Nucl. Mater. 572, 154078 (2022).
Lee, K. & Kim, J. Immobilization of 137Cs as a crystalline pollucite surrounded by amorphous aluminosilicate. Environ. Res. 221, 115309 (2023).
Venkatesan, S., Hassan, M. & Ryu, H. J. Adsorption and immobilization of radioactive ionic-corrosion-products using magnetic hydroxyapatite and cold-sintering for nuclear waste management applications. J. Nucl. Mater. 514, 40–49 (2019).
Shiri-Yekta, Z. & Yaftian, M. R. Anion control selectivity of neutral N4-type schiff base extractants towards transition metal ions. Iran. J. Chem. Chem. Eng. 29, 11–17 (2010).
Shiri-Yekta, Z., Yaftian, M. R. & Nilchi, A. Silica nanoparticles modified with a Schiff base ligand: An efficient adsorbent for Th(IV), U(VI) and Eu(III) ions. Korean J. Chem. Eng. 30, 1644–1651 (2013).
Cullity, B. D. & Stock, S. R. Elements of X-ray Diffraction 3rd edn, 388 (Prentice Hall, 2001).
Breck, D. W. Zeolite Molecular Sieves (Wiley, 1974).
Bulbulian, S. & Bosch, P. Vitrification of gamma irradiated 60Co2+ zeolites. J. Nucl. Mater. 295, 64–72 (2001).
Nilchi, A. et al. Ion exchangers in radioactive waste management: Natural Iranian zeolites. Appl. Radiat. Isotopes 64, 138–143 (2006).
Ahmadi, S. J., Akbari, N., Shiri-Yekta, Z., Mashhadizadeh, M. H. & Pourmatin, A. Adsorption of strontium ions from aqueous solution using hydrous, amorphous MnO2–ZrO2 composite: A new inorganic ion exchanger. J. Radioanal. Nucl. Chem. 299, 1701–1707 (2014).
Taheri, M., Khajenoori, M., Shiri-Yekta, Z. & Zahakifar, F. Application of plantain leaves as a bio-adsorbent for biosorption of U(VI) ions from wastewater. Radiochim. Acta 111, 513–524 (2023).
Ahmadi, S. J., Akbari, N., Shiri-Yekta, Z., Mashhadizadeh, M. H. & Hosseinpour, M. Removal of strontium ions from nuclear waste using synthesized MnO2-ZrO2 nano-composite by hydrothermal method in supercritical condition. Korean J. Chem. Eng. 32, 478–485 (2015).
Li, X., Li, F., Jin, Y. & Jiang, C. The uptake of uranium by tea wastes investigated by batch, spectroscopic and modeling techniques. J. Mol. Liq. 209, 413–418 (2015).
Yousefi, T., Moazami, H. R., Mahmudian, H. R., Torab-Mostaedi, M. & Moosavian, M. A. Modification of natural zeolite for effective removal of Cd(II) from wastewater. J. Water Environ. Nanotechnol. 3, 150–156 (2018).
Inan, S., Tel, H. & Alta, Y. Sorption studies of strontium on hydrous zirconium dioxide. J. Radioanal. Nucl. Chem. 267, 615–621 (2006).
Abdel-Mohsen, A. M., Jancar, J., Kalina, L. & Hassan, A. F. Comparative study of chitosan and silk fibroin staple microfibers on removal of chromium (VI): Fabrication, kinetics and thermodynamic studies. Carbohydr. Polym 234, 115861 (2020).
Li, Q., Yue, Q. Y., Su, Y., Gao, B. Y. & Sun, H. J. Equilibrium, thermodynamics and process design to minimize adsorbent amount for the adsorption of acid dyes onto cationic polymer-loaded bentonite. Chem. Eng. J. 158, 489–497 (2010).
Abbasi, A. et al. Development of nanoporous alumino-borosilicate as a novel matrix for the sorption and stable immobilization of cesium ions. J. Inorg. Organomet. Polym. Mater. 30, 369–378 (2020).
Author information
Authors and Affiliations
Contributions
M.F. performed the experiments. Z.S.Y. wrote the manuscript. H.S. and M.R.: data analysis. M.H. edited the manuscript. S.Z. prepared figures. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fayezi, M., Shiri-Yekta, Z., Sepehrian, H. et al. Adsorption and safe immobilization of Sr ions in modified zeolite matrices. Sci Rep 13, 19087 (2023). https://doi.org/10.1038/s41598-023-46381-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-46381-9
- Springer Nature Limited