Abstract
Hepatitis E virus (HEV) genotype 3 infections in Germany are mainly transmitted zoonotically through the consumption of swine meat. Furthermore, there is evidence that pets might come into contact with HEV, but the relevance of companion animals as possible sources of HEV transmission in Germany still needs to be defined. A monitoring study was therefore carried out on dogs, cats, and horses from Germany. In total 365 serum samples from pets (124 dogs, 119 cats, and 122 horses) were tested for HEV by PCR and for anti-HEV antibodies by a commercial ELISA. The HEV seroprevalence determined by the sero-assay varied significantly between dogs (10%), cats (6%), and horses (2%). Liver injury-related enzymes, alanine transaminase (ALT), and aspartate transaminase (AST) showed no differences between HEV-positive or negative animals. None of the pet serum samples tested positive for PCR. This serological study suggests that dogs and cats are significantly exposed to HEV in Germany, while horses are of minor relevance.
Similar content being viewed by others
Introduction
Hepatitis E virus (HEV) infections occur worldwide1. In tropical developing countries human-associated HEV genotypes 1 and 2 (HEV-1/2) of the genus Paslahepevirus are mostly transmitted through contact with contaminated drinking water leading to epidemic outbreaks. In contrast, genotypes 3 and 4 (HEV-3/4) of the same genus are mostly transmitted zoonotically in industrialized countries mainly from wild boar and pig to humans through ingestion of undercooked meat1. Furthermore, rat-derived HEV strains (HEV-C1) from the genus Rocahepevirus are of particular importance2 as cases of human infections caused by this variant have been diagnosed in Hong Kong and Spain3. Although numerous studies highlighted the role of the natural reservoir hosts especially pig and wild boar but also deer and rabbits on zoonotic transmission of HEV, significantly fewer studies have investigated the relevance of pets as possible hosts and potential sources of infection (Table 1). In general, numerous viruses that can infect companion animals are also infectious to humans4. This raises the question of the significance of HEV infections in companion animals. An in silico analysis of host genetics and HEV genetics identified dogs, and rats as potentially susceptible to Paslahepevirus infections, while cats and dogs were described as susceptible to HEV-C1 infections5. To get more information on HEV infections in German cats, dogs, and horses, a molecular and serological analysis of serum samples provided by veterinary diagnostic laboratories was conducted.
Material and methods
Sampling
All samples were obtained from a routine veterinary laboratory (Synlab, Berlin, Germany). All samples have been collected in April or May 2022. Only serum samples from which sufficient material for PCR and serology (approx. 1 ml) was available after routine diagnostic could be tested. Initially, the goal was to test 120 dogs, 120 cats and 120 horses, but due to availability of samples there were small variations (dogs n = 124, cats n = 119, horses n = 122). Initially, we had tried to achieve an even distribution between animals with elevated liver values and those without elevated liver values in the first 200 or so animals, but since this was not realistically achieved, we switched to unselected serum samples regardless of how high the liver values were.
Basic characteristics such as sex, age, breed, or serum concentrations of enzymes aspartate transaminase (AST) and alanine aminotransferase (ALT) were provided in the far majority of studied pets. Initially, it was tried to include equal numbers of samples with elevated and normal liver values, but this was not successful so we switched to "unselected" study subjects.
No experiments on vertebrates were performed as part of this study. Only retrospective serum samples obtained for diagnostic purposes were retrospectively analyzed.
Therefore, the ARRIVE guidelines do not apply to the study.
The datasets generated and/or analyzed during the current study are not publicly available, because the data were examined completely anonymously and public access would allow individual authors of this manuscript to identify the animals and their serological status. However, the data are available from the corresponding author on reasonable request.
Due to anonymized testing, none of the authors of this paper can currently assign the serological results to individual animals with names and owners, and this anonymity should be preserved as far as possible.
Completeness of the dataset
There were 2555 data collected for the entire study cohort, of which 484 (19%) were missing. In detail, the following were present: anti-HEV IgG value at 100%, OD value at 100%, AST at 90%, ALT at 85%, sex 68%, age 63%, race 62%.
Serology
Serological testing has been performed by the MP Diagnostics HEV ELISA 4.0 (MP Biomedicals Germany GmbH, Eschwege), a commercial test able to detect anti-HEV in mammals.
Since the serum samples were completely anonymized, the personal data of the animal owners were not known during the testing. Therefore, no written consent of the animal owners is available. However, this is also not necessary. According to German laws, there is an obligation to obtain consent and an ethical vote or animal ethical vote for a prospective study and retrospective analyses, but not for retrospective anonymized studies. After consultation with the Ethics Committee of the Hamburg Medical Association, a formal ethics vote is not required for retrospective, anonymized serum analyses.
Molecular biology
All animals have been tested by the RealStar® HEV RT-PCR Kit 2.0 (Altona Diagnostics, Hamburg Germany). Serological positive serum samples have been re-tested by a second independent SYBR Green-based nested in-house broad range reverse-transcriptase (RT-) PCR, which targets a highly conserved region of the RNA-dependent RNA Polymerase within the ORF1 covering all genera of the subfamily Hepevirinae including genus Rocahepevirus6.
Statistical analysis
Continuous variables with a non-normal distribution were expressed as median and interquartile range (IQR). Groups were compared using the Mann–Whitney U test. Categorical variables were expressed as a number (%) and compared with Fisher’s exact test. p values less than 0.05 were considered statistically significant. Statistical analyses were performed using SPSS, version 21.0 (IBM Corp., Armonk, NY, USA).
Results
In total 365 serum samples of companion animals have been studied. In 246/365 (67%) the sex was known and 55% of these were male (n = 135). In 230/365 the age was known (63%), in 328/365 the AST was known (90%) and in 309/365 the ALT was known (85%) Characteristics of anti-HEV positive and negative animals are depicted in Table 2.
The anti-HEV seroprevalence determined by the MP assay varied largely between 10% in dogs (12/124), 6% in cats (7/119), and 2% in horses (2/122) (Fig. 1). The difference between seroprevalence rates in dogs vs. horses (p = 0.01) reached statistical significance (Chi-square test).
Anti-HEV ELISA OD values were significantly higher in dogs in comparison to cats (p = 0.008) or horses (p < 0.001) and in cats compared to horses (p = 0.008, C, Mann–Whitney test) (Fig. 2). None of the animal serum samples tested PCR positive. All serologic-positive animals were re-tested with a broad range of RT-PCR covering tall genera of the Orthohepevirinae family but did not uncover any positive result.
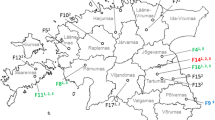
An analysis of the samples by zip code of origin did not indicate a regional clustering of positive samples. The area of submission covers the whole of Germany and the positive samples came from Baden-Württemberg (n = 2), Bavaria (n = 4), Berlin (n = 2), Brandenburg (n = 2), Hessen (n = 2), Lower Saxony (n = 3), North Rhine-Westphalia (n = 3), Schleswig–Holstein (n = 2).
Discussion
While the relevance of pork consumption for transmission of HEV genotype 3 infections in industrialized nations is well established, the role of domestic animals in the transmission of HEV genotype 3 infections in industrialized nations is still unclear.
The current study demonstrates that the risk of anti-HEV carriage and thus prior exposure to HEV in cats and dogs is between 6 and 10% Similar values of 9.9% for dogs and 2.2% for cats were found in a study from Spain7 using the same commercial ELISA. Such data, based on a veterinary serological assay, provide a clear picture of the relevance of HEV contacts in companion animals, whereas there is a wide variation in results for assays not specifically designed for animals: In dogs, the reported prevalences range from 19% up to 56.6%8 when the most often used Wantai ELISA was applied and 0% up to 23% for in-house ELISA (Table 1). A similar heterogeneous finding is seen in cats when using the Wantai ELISA (3.1%9 over 14.9%10 up to 32.3%10 or in-house ELISA from 2%11 over 11%12 up to 33%13 (Table 1).
The first finding of anti-HEV seropositive horses in Germany highlights the possible infection of equids with HEV. Seroprevalence from other horse studies shows a high variation from 0.4% up to 18.18% (Table 1). The lower seroprevalence in horses compared to dogs and cats may be explained by the fact that dogs and cats live directly in the same household as their owners and are carnivores, making transmission from the owner's meat products conceivable.
A so far underestimated risk could be the increasingly popular BARF diet (“Biologically Appropriate Raw Food”), i.e. feeding raw meat to dogs and cats, which could be a source of infection for parasites, bacteria, and viruses such as Feline and Canine Calicivirus and even then HEV14.
Although this pilot study provides a very good overview of the exposure of dogs, cats, and horses in Germany to HEV, no reliable conclusions can be made concerning individual dog, cat, or horse breeds because this information was missing in 38% of the animals. Prospective larger cohorts are needed if this aspect is to be studied in more detail.
While no significant difference regarding to transaminases could be found in HEV-negative vs. positive animals (Table 2), individual HEV-positive animals display increased liver enzyme values (Table 3). Although these could be interpreted as an expression of ongoing liver inflammation in the context of hepatitis E viremia that has just healed, there are numerous other possible causes. This includes Pancreatitis, Diabetes but also normal age-related changes, that reflect adaptations during the transition from young to adult individuals15,16.
An additional finding from the present study is—similar to all previous reports- that no viral RNA could be detected, which is a strong indication, that these animals can not be considered to be reservoir hosts so far. However, due to their close contact with humans, these animals can be regarded as sentinels, indicating that they share a common source of infection with their owners. Thus risk patients (e.g. transplant recipients) should be informed that dogs and cats—and to a minor degree horses—can indicate and determine risks of HEV exposure for humans.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available, because the data were examined completely anonymously and public access would allow individual authors of this manuscript to identify the animals and their serological status. However, the data are available from the corresponding author on reasonable request. Due to anonymized testing, none of the authors of this paper can currently assign the serological results to individual animals with names and owners, and this anonymity should be preserved as far as possible.
Change history
24 November 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41598-023-47523-9
References
Horvatits, T., Schulze Zur Wiesch, J., Lutgehetmann, M., Lohse, A. W. & Pischke, S. The clinical perspective on hepatitis E. Viruses 11(7), 617 (2019).
Andonov, A. et al. Rat hepatitis E virus linked to severe acute hepatitis in an immunocompetent patient. J. Infect. Dis. 220(6), 951–955 (2019).
Rivero-Juarez, A. et al. Orthohepevirus C infection as an emerging cause of acute hepatitis in Spain: First report in Europe. J. Hepatol. 77, 326–331 (2022).
Reperant, L. A. et al. Companion animals as a source of viruses for human beings and food production animals. J. Comp. Pathol. 155(1 Suppl 1), S41–S53 (2016).
Li, B., Wu, H., Miao, Z. & Lu, Y. Using codon usage analysis to speculate potential animal hosts of hepatitis E virus: An exploratory study. Infect. Genet. Evol. 101, 105284 (2022).
Vina-Rodriguez, A. et al. Hepatitis E virus genotype 3 diversity: Phylogenetic analysis and presence of subtype 3b in wild boar in Europe. Viruses 7(5), 2704–2726 (2015).
Caballero-Gomez, J. et al. Serological and molecular survey of hepatitis E virus in cats and dogs in Spain. Transbound. Emerg. Dis. 69(2), 240–248 (2022).
Dahnert, L., Conraths, F. J., Reimer, N., Groschup, M. H. & Eiden, M. Molecular and serological surveillance of Hepatitis E virus in wild and domestic carnivores in Brandenburg, Germany. Transbound. Emerg. Dis. 65(5), 1377–1380 (2018).
Capozza, P. et al. A surveillance study of hepatitis E virus infection in household cats. Res. Vet. Sci. 137, 40–43 (2021).
Li, Y. et al. Hepatitis E virus seroprevalence in pets in the Netherlands and the permissiveness of canine liver cells to the infection. Ir. Vet. J. 73, 6 (2020).
Mochizuki, M. et al. Epidemiological study of hepatitis E virus infection of dogs and cats in Japan. Vet. Rec. 159(25), 853–854 (2006).
Peralta, B. et al. Anti-HEV antibodies in domestic animal species and rodents from Spain using a genotype 3-based ELISA. Vet. Microbiol. 137(1–2), 66–73 (2009).
Okamoto, H., Takahashi, M., Nishizawa, T., Usui, R. & Kobayashi, E. Presence of antibodies to hepatitis E virus in Japanese pet cats. Infection 32(1), 57–58 (2004).
Ahmed, F. et al. Raw meat based diet (RMBD) for household pets as potential door opener to parasitic load of domestic and urban environment. Revival of understated zoonotic hazards? A review. One Health 13, 100327 (2021).
Alvarez, L. & Whittemore, J. Liver enzyme elevations in dogs: physiology and pathophysiology. Compend. Contin. Educ. Vet. 31(9), 408–410, 12–3 (quiz 14) (2009).
Center, S. A. Interpretation of liver enzymes. Vet. Clin. North Am Small Anim. Pract. 37(2), 297–333 (2007).
Arankalle, V. A. et al. Prevalence of anti-hepatitis E virus antibodies in different Indian animal species. J. Viral Hepat. 8(3), 223–227 (2001).
Vitral, C. L. et al. Serological evidence of hepatitis E virus infection in different animal species from the Southeast of Brazil. Mem. Inst. Oswaldo Cruz 100(2), 117–122 (2005).
Saad, M. D. et al. Hepatitis E virus infection in work horses in Egypt. Infect. Genet. Evol. 7(3), 368–373 (2007).
Christensen, P. B. et al. Time trend of the prevalence of hepatitis E antibodies among farmers and blood donors: A potential zoonosis in Denmark. Clin. Infect. Dis. 47(8), 1026–1031 (2008).
Zhang, W. et al. Hepatitis E virus infection among domestic animals in eastern China. Zoonoses Public Health 55(6), 291–298 (2008).
Liu, J. et al. Prevalence of antibody to hepatitis E virus among pet dogs in the Jiang-Zhe area of China. Scand. J. Infect. Dis. 41(4), 291–295 (2009).
Shao, Z. J. et al. An investigation on hepatitis E virus infection status among livestock in Xi’an area. Zhonghua Liu Xing Bing Xue Za Zhi 29(2), 158–160 (2008).
Song, Y. J. et al. Analysis of complete genome sequences of swine hepatitis E virus and possible risk factors for transmission of HEV to humans in Korea. J. Med. Virol. 82(4), 583–591 (2010).
Dong, C. et al. Restricted enzooticity of hepatitis E virus genotypes 1 to 4 in the United States. J. Clin. Microbiol. 49(12), 4164–4172 (2011).
Geng, J. et al. Potential risk of zoonotic transmission from young swine to human: Seroepidemiological and genetic characterization of hepatitis E virus in human and various animals in Beijing, China. J. Viral Hepat. 18(10), e583–e590 (2011).
Mesquita, J. R., Valente-Gomes, G., Conceicao-Neto, N. & Nascimento, M. S. Pet veterinarians have no increased risk of hepatitis E compared to the general population. J. Med. Virol. 86(6), 954–956 (2014).
McElroy, A. et al. Detection of hepatitis E virus antibodies in dogs in the United Kingdom. PLoS One 10(6), e0128703 (2015).
Wang, L. et al. Seroprevalence of hepatitis E virus infection among dogs in several developed cities in the Guangdong province of China. J. Med. Virol. 88(8), 1404–1407 (2016).
Yonemitsu, K. et al. Simple and specific method for detection of antibodies against hepatitis E virus in mammalian species. J. Virol. Methods 238, 56–61 (2016).
Zeng, M. Y. et al. High hepatitis E virus antibody positive rates in dogs and humans exposed to dogs in the south-west of China. Zoonoses Public Health 64(8), 684–688 (2017).
Mooij, S. H. et al. Risk factors for hepatitis E virus seropositivity in Dutch blood donors. BMC Infect. Dis. 18(1), 173 (2018).
Garcia-Bocanegra, I. et al. Hepatitis E virus infection in equines in Spain. Transbound. Emerg. Dis. 66(1), 66–71 (2019).
Lyoo, K. S., Yang, S. J., Na, W. & Song, D. Detection of antibodies against hepatitis E virus in pet veterinarians and pet dogs in South Korea. Ir. Vet. J. 72, 8 (2019).
Veronesi, R. et al. Seroprevalence of hepatitis E virus in dogs in Switzerland. Zoonoses Public Health 68(1), 8–11 (2021).
Bernardini, A. et al. Serological, virological investigation and hepatic injury evaluation for hepatitis E virus in hunting dogs. Pathogens 11(10), 1123 (2022).
Yoon, J. et al. Surveillance of hepatitis E virus in the horse population of Korea: A serological and molecular approach. Infect. Genet. Evol. 103, 105317 (2022).
Funding
Open Access funding enabled and organized by Projekt DEAL. This research was funded by the German Center for Infection Research (DZIF), Grant number DZIF TI 07.001, and the Else-Kröner-Fresenius-Stiftung, grant number 2019:EKFS10. We acknowledge financial support from the Open Access Publication Fund of UKE - Universitätsklinikum Hamburg-Eppendorf and DFG – German Research Foundation.
Author information
Authors and Affiliations
Contributions
S.P., E.V.K., M.H.G. and M.E. conceived of the presented idea. S.P., T.H. and J.S. designed the details of the project. S.P. and A.W. performed the computations. M.M., L.K. and K.W. performed the testing. A.W., J.S. and B.C. encouraged S.P. to investigate the relevance of subgroups according to age and sex. B.K. [a specific aspect] and supervised the findings of this work. All authors discussed the concept of the study before starting, discussed the results and contributed to the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The Funding section in the original version of this Article was incomplete. Full information regarding the corrections made can be found in the correction for this Article.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pischke, S., Knoop, E.V., Mader, M. et al. Anti-HEV seroprevalence and rate of viremia in a German cohort of dogs, cats, and horses. Sci Rep 13, 19240 (2023). https://doi.org/10.1038/s41598-023-46009-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-46009-y
- Springer Nature Limited