Abstract
While chemotherapy alone or in combination with radiotherapy and surgery are important modalities in the treatment of colorectal cancer, their widespread use is not paired with an abundance of diagnostic tools to match individual patients with the most effective standard-of-care chemo- or radiotherapy regimens. Patient-derived organoids are tumour-derived structures that have been shown to retain certain aspects of the tissue of origin. We present here a systematic review of studies that have tested the performance of patient derived organoids to predict the effect of anti-cancer therapies in colorectal cancer, for chemotherapies, targeted drugs, and radiation therapy, and we found overall a positive predictive value of 68% and a negative predictive value of 78% for organoid informed treatment, which outperforms response rates observed with empirically guided treatment selection.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) encompasses cancers of both colon and rectum. Data from the European Cancer Information System1 (ECIS) show that CRC is the second most common cancer and has the second highest mortality in the EU-27 countries. ECIS estimates that there are more than 300 000 new cases and more than 150 000 deaths from CRC every year in EU-27. Average 5-year survival from CRC in this area varies, but is on average slightly above 60% for CRC diagnosed from 2010 to 20142. However, a large proportion of CRC patients present with metastatic disease, which has a substantially lower 5-year survival rate, at 14%3.
Treatment strategies for tumours eligible for curative therapy encompass all major cancer therapy modalities: radiotherapy, chemotherapy, and surgery. Radiation therapy is mainly used in rectal cancer. For colon cancer, chemotherapy is the main adjuvant treatment modality, and can be given both before and/or after surgery, depending on type and extent of disease. Surgery, however, remains the main curative treatment modality for localised colon and rectal cancer.
For advanced-stage disease, where treatment is not typically given with curative intention, chemotherapy is the most important treatment modality. 5-fluorouracil (5-FU), oxaliplatin, and irinotecan are the most commonly used drugs for CRC. They are usually given in one of three different combinations, namely FOLFOX (5-FU and oxaliplatin), FOLFIRI (5-FU and irinotecan), and FOLFOXIRI (5-FU, oxaliplatin and irinotecan). The same regimens are used when chemotherapy is given in a neoadjuvant or adjuvant setting. For rectal cancer, radiation therapy is often used in combination with chemotherapy in a neoadjuvant setting. In addition to this, some targeted therapies have been approved for use in advanced stage colorectal cancer.
Despite a growing arsenal of approved therapeutics, many patients will not experience effective first-line treatment with chemo- or radiotherapy, or even effective treatment at all4. Treatment strategies today are mainly decided empirically, as there are few biomarkers or tests available that reliably match patients with existing treatments. Where biomarkers for therapy selection exist, such as the use of genetic biomarkers to decide on cetuximab therapy in KRAS wild type tumours, these only have modest predictive value5. This means that many patients are exposed to the side-effects of anti-cancer therapies without receiving any of the benefits, and possibly even experiencing progression of disease while on ineffective treatment. In essence, there is a need for better ways of matching patients with effective anti-cancer therapies.
Traditional models of cancers, such as cancer cell lines and xenografted animal models are invaluable tools in cancer research, but neither of these models can bridge the gap between diagnostics and the clinic. Cell lines are inherently homogeneous and not able to accurately represent individual disease, and while xenografted animal models can be derived from patient tumours and as such hoped to faithfully represent individual disease, the process is ethically challenged, and far too time- and resource consuming to be scaled to the level that is needed to be a part of routine clinical decision support6.
A solution that has gained traction is the preclinical testing of chemo- and radiotherapies on patient-derived organoids (PDOs). These are three-dimensional cellular structures that can be cultivated and expanded in vitro from patient-derived material. They can be generated within a clinically relevant timeframe from most solid tissues, both healthy and cancerous, and have been shown to accurately recapitulate characteristics of these tissues genetically, transcriptionally, and in terms of histological architecture. Despite the use of different growth and cultivation protocols, these conclusions have been reached by several research groups7,8,9.
Notably, over the past 3 years, several studies that compare the treatment responses of organoids to those of the patients from whom they were derived have emerged10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29. A visual representation of the typical design of these studies can be seen in Fig. 1. This systematic review is an attempt to summarize the findings of such studies that focus on CRC, and answer the question: “Can cancer organoids predict treatment outcome for anti-cancer therapies in CRC?”.
Example of study design in included studies. Steps a-d and e–f are conducted in parallel. (a) An eligible patient is selected and included in the study. (b) Tumour material is harvested, either via surgically resected material, or through needle-biopsies. (c) Tumours are processed into organoids and seeded with appropriate culture medium. Organoids are then exposed to the treatments of interest, and their response evaluated. (d) Some tumour material is set aside for further analyses, including histological and genetic analyses. (e) The patient receives appropriate anti-cancer treatment and (f) their response to the assigned therapy is evaluated.
Methods
Search methodology
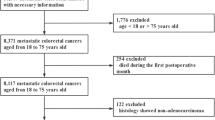
The three databases searched were Medline, Embase, and SCOPUS. The first search was conducted in Medline via PubMed. This database was also used to identify effective keywords, based on some of the already known articles that inspired the review question20,25. Details regarding search terms and concept can be found in Supplementary Tables S1–S4. The search was last updated on the 14th of August 2023. For a detailed description of the search methodology, we refer to the Supplementary Information. Inclusion criteria for the selected studies can be found in Table 1. The study selection process is visualised in Fig. 2.
Inclusion and exclusion criteria
Critical appraisal methodology
To our knowledge, there are no validated tools for the critical appraisal of cell-based studies. We therefore took inspiration from other sources, as well as from our prior knowledge of the field, to compose a list of criteria for the critical appraisal of the included studies. Specifically, inspiration was taken from the 2021 review from Verduin et al.31, and from general evaluation tools32. The criteria were defined to address three main categories: reproducibility, translational potential, and the validity of the results.
Reproducibility was addressed through the two first criteria in Table 2 (detailed description of methods, data availability). There is no consensus on the best method for cultivating organoids, how samples should be exposed to therapy, which readouts should be used, or what threshold should be employed to classify a sample as sensitive or resistant to therapy. A detailed description of the methods used is therefore essential for the reproducibility of the study, and the data should also be readily available for critical assessment.
The translational potential was addressed through criteria three and four in Table 2 (organoid success rate, use of clinically relevant therapies). If organoids are not established successfully, it would not be practically possible to use them to guide treatment decisions. The use of clinically relevant drugs (e.g., standard of care chemotherapies, or approved targeted therapies) will also increase the translational potential of the study. Most importantly, the correlation of organoid response to patient response is necessary for the clinical implementation of organoids as predictors of treatment efficacy. This is addressed in the inclusion criteria.
The validity of the results was addressed through criteria five and six in Table 2 (number of patients included, use/validation of classifier for sensitivity). A high number of included samples/patients results in more robust evidence. The use and validation of a classifier for treatment sensitivity reduces the risk of confirmation bias, while simultaneously increasing the translational potential of the study.
Meta-analysis
Seven of the included studies provided measures of accuracy, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), or the values needed to calculate these, and were thus eligible for our meta-analysis12,16,20,24,25,26,29. Four additional studies likely had these data but did not disclose them in the main text or the supplementary material10,18,22,27. The authors were contacted with a request for these data, but none replied.
Point estimates for each parameter were calculated using a standard confusion matrix where necessary. Confidence intervals (CI) were generated by bootstrapping with the Boot R-package. Further details regarding R-scripts and data tables can be found in the Supplementary Information.
Results
Search results
The final search yielded a total of 2019 references. All references were imported to EndNote 20, and a total of 525 duplicates were removed, leaving 1494 unique references. The titles of these references were surveyed, along with the abstracts of those assumed to be relevant based on the title. We identified 21 references that fitted the inclusion criteria in Table 1, and for 20 of these we were able to obtain the full text. See Fig. 2 for a general overview of the search results and our selection process, and Table 1 for details regarding the inclusion and exclusion criteria. The results are summarized in Table 3, and the critical appraisal of these studies can be found in Table 2.
Organoid cultivation methods
All included references employed similar and comparable cultivation methods, with smaller variations in non-critical steps. The general procedure follows a series of steps: (1) collection of cancer material through biopsy or surgical removal, (2) mechanical and/or enzymatic digestion of the cancer tissue, (3) embedding of the cancer tissue in a suitable supporting growth environment (e.g. Matrigel or other forms of extracellular matrices), (4) addition of more or less chemically defined growth medium to allow formation and growth of organoids, (5) exposure to drugs, and (6) assessing the response. Details regarding methodologies can be found in Supplementary Table S5.
Common areas of variation were seeding densities of cells/tumour fragments, medium composition, type of extracellular matrix, drugs used, duration of drug exposure, and method of organoid response readout.
The study by Janakiraman et al.15 deviated somewhat from the most common approach to organoid formation, as they used the tumour samples to first establish patient derived xenografts (PDX) in mice. They then used tumour material harvested from the xenografts to cultivate organoids. The authors present this as a way to expand available material from a sparse initial amount, which can be a limiting factor when dealing with core needle biopsies. Other studies used self-fabricated plates to make automation easier11, generated micro organospheres by pumping a Matrigel-cell suspension into oil12, or used alternatives to basement membrane extracts (Matrigel, Geltrex, etc.) for cultivation28.
Use of clinically approved therapies
All studies identified by the literature search tested therapies approved for treatment of colon and/or rectal cancer. Patients were treated according to clinical guidelines, or with off-label or investigative agents, sometimes being included in other clinical trials to get access to drugs. Organoids were treated with both single drugs and clinically approved combinations, as well as radiation therapy. The most frequently tested therapies were 5-FU alone, and its combinations with either oxaliplatin or irinotecan (or its active metabolite SN-38), which comprise the most commonly used standard-of-care chemotherapy regimens in the treatment of CRC. Additionally, several of the included references10,11,15,17,19,21,25 tested targeted therapies (monoclonal antibodies, tyrosine-kinase inhibitors), all of which were either clinically approved for the treatment of CRC or other cancers, or investigative therapies in clinical trials.
Methods of response evaluation
The included studies used different means of comparing patient and organoid response. Patients were evaluated according to established clinical criteria (Response Evaluation Criteria in Solid Tumours, abbreviated RECIST35, and American Joint Committee on Cancer Tumour Regression Grade, abbreviated AJCC-TRG36), progression free survival, and/or tumour circumference change on endoscopy. Organoid response was evaluated most commonly by the CellTiter Glo viability assay, while two of the included studies also used the CellTiter Blue viability assay37. Most studies reported either relative viability compared to vehicle control, or IC50 of the tested drugs.
Interestingly, five of the included studies14,16,22,23,29 applied non-invasive, continuous readouts of organoid viability. These studies used brightfield microscopy images to assess changes in organoid diameter, and calculated the percentage change in growth from seeding, based on the assumption that organoid growth correlates with treatment response. The Pasch et al.23 study additionally utilised optical metabolic imaging to capture NAPDH and FAD fluorescence, which was used to calculate a redox ratio. This provides a measure of cellular metabolic activity without using dyes or disrupting the organoids38, and has been shown to accurately predict drug response in patient derived cellular models39.
Correlation between patient and organoid response
Despite differences in the evaluation of organoid and patient response to therapy, all of the included studies reported that organoid response to treatment was mainly similar to patient response to treatment, with the exception of the 2021 study by Ooft et al.21, where responses to targeted therapies were found to correlate poorly between organoids and patients.
Briefly, Janakiraman et al.15 found that for organoids derived from patients with tumour regression grade (TRG) 0 or 1 (complete and partial response, respectively, as determined by histology assessments) there was a significant difference in viability between drug-treated and control organoids. This was not the case for patients with TRG 2 (minimal response), indicating that organoids from patients with low response to clinically administered therapies also were relatively insensitive to the drug in the paraclinical setup. Ganesh et al.13 found that the area under the curve (AUC) of organoid dose–response curves were inversely correlated with progression free survival of patients, and directly correlated with the change in tumour circumference on endoscopy. Xu et al.28 observed a notable difference in both the AUC and the concentration of drug required to decrease viability of organoids to 50% of control (IC50) in their cellulosic sponge model. This discrepancy was observed when comparing organoids derived from patients with partial response or stable disease to those from patients with progressive disease. Vlachiogannis et al.25 reported that in total, their screens show 100% sensitivity, 93% specificity, 88% positive predictive value, and 100% negative predictive value. It is however not explicitly stated how organoids were classified as drug sensitive or resistant. Narashiman et al.19 found that the drug vandetanib dramatically reduced the viability of organoids from one patient, and this patient was subsequently assigned to the drug. The patient unfortunately showed no signs of response and died four weeks after initiation of treatment. The organoids of another patient showed sensitivity to gemcitabine, and was assigned to this treatment, achieving stable disease for three months. Cui et al.11 followed three patients who experienced tumour shrinkage on FOLFOX treatment, and found that their organoids were sensitive to the same treatment. Hsu et al.14 found that for organoids where a lower dose of radiotherapy was required to reduce the surviving cell fraction to 37%40, the patients from whom they were derived tended to experience larger tumour shrinkage in a clinical setting. Mo et al.18 found that the IC50 of organoids derived from patients with stable disease or partial response to treatment was significantly lower than that of organoids derived from patients with progressive disease. Martini et al.17 used the viability data from their organoid assays to assign two patients to treatment. The first patient received FOLFOX + bevacuzimab and achieved stable disease for 6 months, while the other patient received mitomycine + capecitabine, achieving stable disease for 5 months. These observations are summarized in Table 3.
The remaining studies all devised a classifier for organoid sensitivity, i.e. a threshold below which an organoid is deemed sensitive to the given treatment, by measuring organoid growth or viability and comparing these data to patient responses. Pasch et al.23 utilised their classifiers for both diameter change and redox ratio to successfully predict one patient’s response to the FOLFOX chemotherapy regimen prospectively. Ding et al.12 found that organoid response matched patient responses in six out of eight cases. The 2019 study by Ooft et al.20, Yao et al.29, both the 2021 and 2023 study by Wang et al.26,27, Park et al.22, Cho et al.10, Lv et al.16, and Tang et al.24 applied their classifier to larger cohorts of patients. Ooft et al. and Wang et al. both used an IC50 cut-off value, while Yao et al. used an organoid diameter change cut-off. Yao et al. and Ooft et al. compared patient and organoid responses to find a mean optimal cut-off, which best separated responsive PDOs and responsive patients from resistant PDOs and non-responsive patients. In contrast, Wang et al. first conducted a pilot study with a smaller cohort of patients to establish their classifier threshold, which they then validated in a larger cohort in a blinded study. All three studies concluded that their classifiers performed well. Park et al. found that the surviving cell fraction was best able to differentiate between sensitive and resistant samples, and that it accurately classified responders- and non-responders. Cho et al. found that an organoid score of 2.5 (see Table 3 for definition) accurately differentiated between sensitive and resistant organoids, and that a lower score correlated with longer progression free survival. Lv et al. found that there was a match rate between patient and organoid response of 73.8%, 71.3%, and 66.3% for irinotecan, radiation, and 5-FU respectively. Tang et al. found that a lower IC50 for the FOLFOX regimen was associated with better patient outcomes. Finally, Wang et al. found that the drug sensitivity of organoids could predict the probability of a patient experiencing a 1-year progression free survival or not.
Meta-analysis of organoid assays
Several of the included studies12,16,20,24,25,26,29 provided measures of (or the numbers needed to calculate) accuracy, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of the organoid assays, when compared to patient responses. These studies were included in our meta-analysis to find predictive performances for individual studies and for all studies combined, as shown in Figs. 3 and 4. Overall, the organoid assays of the included studies show high predictive performance in identifying patients that respond to a given therapy, with some variation. Bootstrapping was applied to calculate confidence intervals. Point estimates with confidence intervals for individual studies are summarised in Supplementary Table S6. We found an overall test accuracy of 0.76 (95% CI 0.72–0.80), a sensitivity of 0.79 (95% CI 0.71–0.84), and a specificity of 0.75 (95% CI 0.69–0.78). Combined, the assays had both positive and negative predictive values exceeding the pre-test likelihood of response to treatment of 47%, with gathered means of 0.68 (95% CI 0.61–0.72) for PPV and 0.78 (95% CI 0.72–0.82) for NPV.
Positive and negative predictive values for the organoid assays. Point estimates and 95% confidence intervals are shown. Size of the dots correspond to the number of patients. Vertical red lines represent the pre-test likelihood of a patient responding to treatment (for PPV) and not responsding to treatment (for NPV).
Discussion
In this review we present an overview of the growing field of translational organoid research, with the main finding being that organoids hold great potential in predicting patient responses to radio- and chemotherapy in CRC.
Although the use of organoids in translational medicine is still very much in its early stages, several reviews have been published on the subject, including some that align with the focus of the present review31,41,42. Our systematic review provides a more comprehensive meta-analysis compared to previous reviews, where we have also included the computation of PPV and NPV.
It is a growing concern for many that there are no standardised methods for the cultivation of organoids, a fact that has led to a lack of reproducibility of data originating for experiments with 3D models, as well as heterogeneity in both organoid morphology and response to treatments43,44. Although our included references mainly retained the same key steps in their cultivation protocols, they too differ in the details. Despite these differences, all the references included in our paper report a positive correlation between organoid and patient response to the same treatment, with the exception of the 2021 study by Ooft et al.21. There is however considerable variability of these results, even for studies that use near identical protocols, such as Lv et al.16 and Yao et al.29. Although it is likely that standardisation of cultivation protocols will lead to more consistent data across different labs, the methods of today are still able to capture the predictive ability of organoid models. We take the fact that research groups using near identical cultivation protocols report different results to imply that there are likely other factors than differences in cultivation protocols that also contribute to the observed heterogeneity in the predictive power of organoid assays.
Most of the identified references focus on standard-of-care chemotherapies (5-FU, oxaliplatin, irinotecan, radiation). This is also true for the studies included in our meta-analysis, with the exception of the Vlachiogannis et al.25 study, which utilised a broad panel of both chemotherapies and targeted inhibitors. The use of standard-of-care and clinically approved therapies likely makes the road to clinical trials and possible clinical implementation of organoid-based diagnostics shorter than for therapies which are not routinely used in the clinic.
Several of the papers also delved into genotype–phenotype investigations, exploring how known genetic alterations affect PDO response to targeted therapies for specific alterations. Vlachiogannis et al.25 showed how specific amplifications and activating mutations in their organoids correlated with higher sensitivity to therapies specifically targeting these alterations. Interestingly, they found that this was not true for all mutations included in their next generation sequencing panel, which could highlight the importance of the PDOs as a preclinical model that circumvents the intricacies of using genomic data to match patients with effective therapies5. Additionally, the studies by Ganesh et al.13 and Janakiraman et al.15 specifically compared organoid KRAS mutation status to cetuximab response. Both found that KRAS-mutation conferred a lack of response to treatment with the EGFR-inhibiting antibody, as would be expected from clinical observations.
Interestingly, Ooft et al.20 found that their PDOs could predict sensitivity to irinotecan and FOLFIRI but not FOLFOX. The study by Xu et al.28 directly addressed this observation. They suggest that Ooft et al. were unable to predict the activity of FOLFOX due to Matrigel inducing the epithelial-to-mesenchymal transition (EMT) in the organoids, as evident by reduced expression of E-cadherin on Matrigel-cultivated PDOs. Reduced E-cadherin expression is a commonly recognized marker of EMT45. They find that by using their cellulosic sponge model, PDOs maintain expression of E-cadherin, while simultaneously accurately modelling patient response to FOLFOX. However, several of the other included studies cultivated their PDOs in Matrigel, without taking measures described by Xu et al. and still found a correlation between patient and PDO response to FOLFOX, which indicates that also other factors could contribute to the lack of correlation found by Ooft et al. Their medium composition, for example, is different from most other included studies, shared only with Tang et al., which had one of the lowest predictive performances of our included studies. These discrepancies call for further explorations into medium composition and its influences on drug responses.
Our meta-analysis (Fig. 4) shows a pooled PPV of 68% (95% CI 0.61–0.72) and NPV of 78% (95% CI 0.72–0.82). The combined response rate of patients in our included studies was 47%, which matches clinically observed response rates in meta-analyses of standard colon cancer therapy46. This means that using organoids to guide treatment decisions could significantly increase response rates.
A common drawback for several of the included studies is their retrospective nature, i.e., that organoid drug screens were not used to assign patients to specific treatment regimens, but rather PDO and patient sensitivity to treatment are compared after therapy is given. As such, retrospective studies of organoid responses could be said to model, rather than predict, treatment responses. Four of the included studies did however make use of the PDO assays to assign patients to treatment17,19,21,23, and for the majority of these successfully so. Other studies included follow-up data for their patients, showing how organoid responses correlated positively with the progression free survival of included patients10,16,24,27, further strengthening the predictive potential of PDOs.
Several of the included studies point to larger prospective studies in the vein of Pasch et al.23 as a natural next step. Ooft et al.21, following up on their work from 2019, used PDO screens to prospectively assign 6 patients to single agent off label targeted therapies. Unfortunately, the patients did not respond to the assigned treatments, despite their organoids being classified as sensitive, for the particular drugs tested. However, it is important to note that monotherapy with targeted agents in CRC has previously been shown to be suboptimal, as the effect can be circumvented by upregulation of alternate cellular signalling pathways47. This should serve as a reminder that while organoids are a huge step forward compared to simpler cellular models, they still cannot recapitulate the entire complexity of the cancer disease.
Concluding remarks
To answer our initial question, on whether cancer organoids can predict treatment outcome for anti-cancer therapies in CRC, we conclude that our meta-analysis demonstrate a large potential for the use of PDOs to assist treatment decisions. The included studies have focused mostly on standard-of-care chemotherapy regimens, along with radiation therapy, which is an area with a severely unmet clinical need in patient treatment response prediction. Although the influence of a publication bias cannot be excluded, seeing as near all the included studies report positive correlations, it does indeed seem like cancer organoids are well suited to predict treatment outcomes for anti-cancer therapies in CRC. Larger and prospective studies are needed, as well as establishing standardised cultivation protocols, organoid viability assays, and one or several validated classifiers for organoid sensitivity that accurately predicts treatment response. Several of the included studies point to prospective studies using PDOs to assign patients to standard-of-care systemic therapies as their next step, something that would be of great benefit for patients with metastatic colorectal cancer, relying on systemic treatment for survival.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files. For further clarification, information, or other remaining questions regarding the data and analysis thereof, the corresponding author can be contacted.
References
ECIS - European Cancer Information System. From https://ecis.jrc.ec.europa.eu Accessed on 20/11/2021.
Allemani, C. et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 391, 1023–1075. https://doi.org/10.1016/s0140-6736(17)33326-3 (2018).
Wang, J. et al. Metastatic patterns and survival outcomes in patients with stage IV colon cancer: A population-based analysis. Cancer Med. 9, 361–373. https://doi.org/10.1002/cam4.2673 (2020).
Grothey, A., Sargent, D., Goldberg, R. M. & Schmoll, H. J. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J. Clin. Oncol. 22, 1209–1214. https://doi.org/10.1200/jco.2004.11.037 (2004).
De Roock, W. et al. KRAS wild-type state predicts survival and is associated to early radiological response in metastatic colorectal cancer treated with cetuximab. Ann. Oncol. 19, 508–515. https://doi.org/10.1093/annonc/mdm496 (2008).
Pampaloni, F., Reynaud, E. G. & Stelzer, E. H. K. The third dimension bridges the gap between cell culture and live tissue. Nat. Rev. Mol. Cell Biol. 8, 839–845. https://doi.org/10.1038/nrm2236 (2007).
Kondo, J. et al. High-throughput screening in colorectal cancer tissue-originated spheroids. Cancer Sci. 110, 345–355. https://doi.org/10.1111/cas.13843 (2019).
Sachs, N. et al. A living biobank of breast cancer organoids captures disease heterogeneity. Cell 172, 373-386.e310. https://doi.org/10.1016/j.cell.2017.11.010 (2018).
van de Wetering, M. et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell 161, 933–945. https://doi.org/10.1016/j.cell.2015.03.053 (2015).
Cho, Y. W. et al. Patient-derived organoids as a preclinical platform for precision medicine in colorectal cancer. Mol. Oncol. 16, 2396–2412. https://doi.org/10.1002/1878-0261.13144 (2022).
Cui, Y. et al. Establishment of organoid models based on a nested array chip for fast and reproducible drug testing in colorectal cancer therapy. Bio-Design Manuf. 5, 674–686. https://doi.org/10.1007/s42242-022-00206-2 (2022).
Ding, S. et al. Patient-derived micro-organospheres enable clinical precision oncology. Cell Stem Cell 29, 905-917.e906. https://doi.org/10.1016/j.stem.2022.04.006 (2022).
Ganesh, K. et al. A rectal cancer organoid platform to study individual responses to chemoradiation. Nat. Med. 25, 1607–1614 (2019).
Hsu, K. S. et al. Colorectal cancer develops inherent radiosensitivity that can be predicted using patient-derived organoids. Cancer Res. 82, 2298–2312. https://doi.org/10.1158/0008-5472.CAN-21-4128 (2022).
Janakiraman, H. et al. Modeling rectal cancer to advance neoadjuvant precision therapy. Int. J. Cancer 147, 1405–1418. https://doi.org/10.1002/ijc.32876 (2020).
Lv, T. et al. Patient-derived tumor organoids predict responses to irinotecan-based neoadjuvant chemoradiotherapy in patients with locally advanced rectal cancer. Int. J. Cancer 152, 524–535. https://doi.org/10.1002/ijc.34302 (2023).
Martini, G. et al. Establishment of patient-derived tumor organoids to functionally inform treatment decisions in metastatic colorectal cancer. ESMO Open 8, 101198. https://doi.org/10.1016/j.esmoop.2023.101198 (2023).
Mo, S. et al. Patient-derived organoids from colorectal cancer with paired liver metastasis reveal tumor heterogeneity and predict response to chemotherapy. Adv. Sci. (Weinh) 9, e2204097. https://doi.org/10.1002/advs.202204097 (2022).
Narasimhan, V. et al. Medium-throughput drug screening of patient-derived organoids from colorectal peritoneal metastases to direct personalized therapy. Clin. Cancer Res. 26, 3662–3670. https://doi.org/10.1158/1078-0432.Ccr-20-0073 (2020).
Ooft, S. N. et al. Patient-derived organoids can predict response to chemotherapy in metastatic colorectal cancer patients. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.aay2574 (2019).
Ooft, S. N. et al. Prospective experimental treatment of colorectal cancer patients based on organoid drug responses. ESMO Open 6, 100103. https://doi.org/10.1016/j.esmoop.2021.100103 (2021).
Park, M. et al. A patient-derived organoid-based radiosensitivity model for the prediction of radiation responses in patients with rectal cancer. Cancers (Basel) 13, 3760. https://doi.org/10.3390/cancers13153760 (2021).
Pasch, C. A. et al. Patient-derived cancer organoid cultures to predict sensitivity to chemotherapy and radiation. Clin. Cancer Res. 25, 5376–5387. https://doi.org/10.1158/1078-0432.CCR-18-3590 (2019).
Tang, Y. et al. Cutoff value of IC(50) for drug sensitivity in patient-derived tumor organoids in colorectal cancer. iScience 26, 107116. https://doi.org/10.1016/j.isci.2023.107116 (2023).
Vlachogiannis, G. et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science 359, 920–926. https://doi.org/10.1126/science.aao2774 (2018).
Wang, T. et al. Accuracy of using a patient-derived tumor organoid culture model to predict the response to chemotherapy regimens in stage IV colorectal cancer: A blinded study. Dis. Colon Rectum https://doi.org/10.1097/dcr.0000000000001971 (2021).
Wang, T. et al. Patient-derived tumor organoids can predict the progression-free survival of patients with stage IV colorectal cancer after surgery. Dis. Colon Rectum 66, 733–743. https://doi.org/10.1097/dcr.0000000000002511 (2023).
Xu, Y. et al. Patient-derived organoids in cellulosic sponge model chemotherapy response of metastatic colorectal cancer. Clin. Transl. Med. https://doi.org/10.1002/ctm2.285 (2021).
Yao, Y. et al. Patient-derived organoids predict chemoradiation responses of locally advanced rectal cancer. Cell Stem Cell 26, 17-26.e16. https://doi.org/10.1016/j.stem.2019.10.010 (2020).
Page, M. J. et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 372, n71. https://doi.org/10.1136/bmj.n71 (2021).
Verduin, M., Hoeben, A., De Ruysscher, D. & Vooijs, M. Patient-derived cancer organoids as predictors of treatment response. Front. Oncol. https://doi.org/10.3389/fonc.2021.641980 (2021).
Will, R. Students 4 best evidence. In Critical Appraisal: A Checklist
Hafner, M., Niepel, M., Chung, M. & Sorger, P. K. Growth rate inhibition metrics correct for confounders in measuring sensitivity to cancer drugs. Nat. Methods 13, 521–527. https://doi.org/10.1038/nmeth.3853 (2016).
Glass, G. V., McGraw, B. & Smith, M. L. Meta-Analysis in Social Research (Sage, 1981).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247. https://doi.org/10.1016/j.ejca.2008.10.026 (2009).
Amin, M. B. et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 67, 93–99. https://doi.org/10.3322/caac.21388 (2017).
Riss TL, M. R., Niles AL, et al. in Assay Guidance Manual (ed Grossman A Markossian S, Brimacombe K, et al.) (Eli Lilly & Company and the National Center for Advancing Translational Sciences, 2013.
Skala, M. C. et al. In vivo multiphoton fluorescence lifetime imaging of protein-bound and free nicotinamide adenine dinucleotide in normal and precancerous epithelia. J. Biomed. Opt. 12, 024014. https://doi.org/10.1117/1.2717503 (2007).
Walsh, A. J. et al. Quantitative optical imaging of primary tumor organoid metabolism predicts drug response in breast cancer. Cancer Res. 74, 5184–5194. https://doi.org/10.1158/0008-5472.Can-14-0663 (2014).
Nomiya, T. Discussions on target theory: Past and present. J. Radiat. Res. 54, 1161–1163. https://doi.org/10.1093/jrr/rrt075 (2013).
Le Compte, M. et al. Patient-derived organoids as individual patient models for chemoradiation response prediction in gastrointestinal malignancies. Crit. Rev. Oncol. Hematol. 157, 103190. https://doi.org/10.1016/j.critrevonc.2020.103190 (2021).
Wensink, G. E. et al. Patient-derived organoids as a predictive biomarker for treatment response in cancer patients. npj Precis. Oncol. 5, 30. https://doi.org/10.1038/s41698-021-00168-1 (2021).
Zanoni, M. et al. 3D tumor spheroid models for in vitro therapeutic screening: A systematic approach to enhance the biological relevance of data obtained. Sci. Rep. 6, 19103. https://doi.org/10.1038/srep19103 (2016).
Tosca, E. M., Ronchi, D., Facciolo, D. & Magni, P. Replacement, reduction, and refinement of animal experiments in anticancer drug development: The contribution of 3D in vitro cancer models in the drug efficacy assessment. Biomedicines 11, 1058 (2023).
Zeisberg, M. & Neilson, E. G. Biomarkers for epithelial-mesenchymal transitions. J. Clin. Investig. 119, 1429–1437. https://doi.org/10.1172/JCI36183 (2009).
Hoang, T., Sohn, D. K., Kim, B. C., Cha, Y. & Kim, J. Efficacy and safety of systemic treatments among colorectal cancer patients: A network meta-analysis of randomized controlled trials. Front. Oncol. 11, 756214. https://doi.org/10.3389/fonc.2021.756214 (2021).
Kopetz, S. et al. Encorafenib, binimetinib, and cetuximab in BRAF V600E–mutated colorectal cancer. N. Engl. J. Med. 381, 1632–1643. https://doi.org/10.1056/NEJMoa1908075 (2019).
Funding
Open access funding provided by Norwegian University of Science and Technology. This work was funded by Liaison Committee between the Central Norway Regional Health Authority (Samarbeidsorganet) and the Norwegian University of Science and Technology (NTNU), Norges Forskningsråd, 310160. The Research Council of Norway, under the framework of the European Research Area (ERA) PerMed program, 32509. The NTNU Strategic Research Area NTNU Health.
Author information
Authors and Affiliations
Contributions
All authors contributed to concept formation and discussion of the included references. B.C.S. conducted the literature search, did meta-analysis, and wrote the manuscript. Å.F. contributed to the meta-analysis and writing of the manuscript. Remaining authors contributed to the revision of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The corresponding author has received honoraria from pharmaceutical companies Bayer, Novartis, Pfizer, Pierre Fabre, Amgen, and regularly interacts with major pharmaceutical companies in Norway in the CONNECT consortium (https://oslocancercluster.no/connect/), but we do not see that this should influence the interpretation of our findings. The other authors declare no conflicts of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sakshaug, B.C., Folkesson, E., Haukaas, T.H. et al. Systematic review: predictive value of organoids in colorectal cancer. Sci Rep 13, 18124 (2023). https://doi.org/10.1038/s41598-023-45297-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-45297-8
- Springer Nature Limited
This article is cited by
-
Organoids as a biomarker for personalized treatment in metastatic colorectal cancer: drug screen optimization and correlation with patient response
Journal of Experimental & Clinical Cancer Research (2024)