Abstract
Evidence indicates that patients with chronic low back pain (CLBP) have lumbar multifidus muscle (LM) activation deficit which might be caused by changes in cortical excitability. Anodal transcranial direct current stimulation (a-tDCS) can be used to restore cortical excitability. This study aimed to (1) determine the immediate effects of a-tDCS on the cortical excitability and LM activation and (2) explore the relationship between cortical excitability and LM activation. Thirteen participants with CLBP during remission and 11 healthy participants were recruited. Cortical excitability (peak-to-peak motor evoked potential amplitude; P2P and cortical silent period; CSP) and LM activation were measured at pre- and post-intervention. We found significant difference (P < 0.05) in P2P between groups. However, no significant differences (P > 0.05) in P2P, CSP and LM activation were found between pre- and post-intervention in CLBP. The CLBP group demonstrated significant correlation (P = 0.05) between P2P and LM activation. Although our finding demonstrates change in P2P in the CLBP group, one-session of a-tDCS cannot induce changes in cortical excitability and LM activation. However, moderate to strong correlation between P2P and LM activation suggests the involvement of cortical level in LM activation deficit. Therefore, non-significant changes could have been due to inadequate dose of a-tDCS.
Similar content being viewed by others
Introduction
Low back pain (LBP) is a common musculoskeletal problem affecting nearly 60 to 80% of the population throughout their life1. It can occur at any age from adolescents to the elderly and causes individual and social problems1. Once an individual suffers from LBP, a high recurrence rate occurs throughout their lifetime, approximately 60 and 70% yearly2. If left untreated, it might turn into a chronic stage with a more complex mechanism difficult to be treated3,4,5,6. In addition, any intervention that does not address the underlying mechanism could be responsible for a high recurrence rate3,4,5,6. Therefore, the intervention should be designed and focus on a specific underlying mechanism.
Movement control impairment (MCI) has been proposed as a mechanism underlying chronic low back pain (CLBP)4,5,6,7,8. MCI is defined as poor control and coordination of the lumbar and pelvic segments during movements6. One recent study demonstrated greater positive movement control battery tests in patients with CLBP suggesting that CLBP could be caused by MCI5. Several studies also indicate that aberrant movement patterns during clinical movement tests can be used to identify MCI in patients with CLBP5, 6, 8,9,10.
Lumbar multifidus muscle (LM) is responsible for static and dynamic stability of the lumbar spine11,12,13,14. Alteration of the LM can cause an inability to control the lumbar spine resulting in MCI. Electromyography studies reveal decreased bilateral LM activity during functional tasks12, 13. One study investigated LM activation using ultrasound imaging in patients with CLBP suspected to have underlying MCI14. The researchers found lower LM activation in patients with CLBP during remission compared with individuals without LBP14. Although reduced LM activation can be resulted from several factors including muscle atrophy, fatty infiltration, or slow-to-fast muscle transition13, recent studies demonstrated that LM activation deficit may be caused by reduced neural drive from the primary motor cortex (M1)15,16,17,18.
Changes in cortical excitability (motor evoked potential, cortical topography, etc.) in patients with CLBP were supported by several studies15,16,17,18. These changes are also associated with altered movement patterns15. Based on the existing evidence, it could be speculated that insufficient LM activation causing MCI should result from changes in cortical excitability (reduced neural drive from the M1)17, 18. Accordingly, restoration of LM cortical excitability should increase the neural drive to the LM, thereby increasing LM activation.
Anodal transcranial direct current stimulation (a-tDCS) is a non-invasive neuromodulation technique that can be used to enhance cortical excitability19,20,21,22,23,24. The application of a-tDCS to the M1 area showed a favorable result in cortical excitability and pain22, 23, 25. The a-tDCS induces the glutamatergic synapses which in turn enhances the calcium ion influx via N-methyl-D-aspartate (NMDA) receptors19, 24. Calcium ion influx has the ability to induce long term potentiation (LTP) which is necessary for neural plasticity24. In addition, gamma-aminobutyric acid (GABA) activity can cause the neuron less likely to fire an action potential19. By applying a-tDCS, glutamatergic synapses would be activated causing reduced GABA activity, thereby increasing cortical excitability19.
Enhanced cortical excitability using a-tDCS should lead to increase in neural drive to the LM. However, this proposed mechanism has not been systematically investigated. In addition, some studies found changes in cortical excitability15,16,17,18, while others found LM activation deficits in patients with CLBP12,13,14. However, no study has investigated the relationship between cortical excitability and LM activation.
Therefore, this study aimed to investigate the effects of a single session of a-tDCS on cortical excitability and LM activation in patients with CLBP (MCI subgroup) during remission. We also explored the relationship between cortical excitability and LM activation. We hypothesized that a-tDCS would enhance LM cortical excitability, thereby increasing LM activation. We also expected that cortical excitability would be correlated with LM activation in patients with CLBP.
Methods
Study design
The study employed a quasi-experimental design to investigate the effect of single session a-tDCS on cortical excitability and LM activation in patients with CLBP (MCI subgroup) during remission.
Participants
This study used a convenience sampling recruited from university physical therapy clinic and surrounding communities. The inclusion criteria for individuals with CLBP (CLBP) were age between 18 and 40 years, having at least three recurrent episodes for more than three months that interfered with activities of daily living, but currently pain-free, and having more than two positive results of six movement control battery tests to identify MCI including (1) waiter’s bow, (2) standing with posterior pelvic tilt, (3) single leg stance, (4) sitting with knee extension, (5) quadruped rocking forward/backward, and (6) prone with knee flexion5. Studies demonstrated people who had higher frequency of aberrant movement patterns during clinical movement tests were likely to have MCI which might be responsible for persistence/recurrence of low back symptoms5, 8, 10.
We acknowledged that central pain processing can suppress the LM maximum contraction, and a-tDCS could inhibit this central pain process enabling greater LM contraction13, 23, 25. We aimed to prevent the occurrence of this mechanism to investigate our proposed concept. In this study, we investigated patients with CLBP during remission.
The inclusion criteria for individuals without LBP (NoLBP) were no low back pain for the past 6 months and presenting less than two positive movement control battery tests. The exclusion criteria were presence of specific LBP conditions (e.g. degenerative spine, spondylosis, or spinal stenosis), red flags (e.g. infection, tumors, fracture, radicular syndrome, or inflammatory disease), having a diagnosis of neurological, musculoskeletal, or cardiac abnormalities (e.g. scoliosis, myelopathy, atrial fibrillation), receiving motor control training exercises for the past six months, or body mass index greater than 30 kg/m2. This study was a part of 6-week intervention study with a pre-specified sample size. Therefore, we did not perform a sample size calculation. However, effect size and the required sample size were calculated for future replications of this study. All participants provided a written informed consent before data collection.
Instruments
Transcranial magnetic stimulation (TMS; Magstim 200, Magstim Co., UK) was used to stimulate the motor cortex area using a figure-of-eight coil. This system was used to measure the cortical excitability (motor evoked potential; MEP and cortical silent period; CSP) of the LM18, 22, 26, 27. One study found moderate to excellent test–retest reliability (ICC3,1 = 0.58 to 0.94) to determine cortical excitability27. Our pilot data (unpublished work) related to reliability of measurement indicates excellent within-session (ICC3,k = 0.95) and moderate between-session (ICC2,k = 0.67) test–retest reliability. Therefore, TMS was sufficiently reliable to measure cortical excitability.
Rehabilitative ultrasound imaging device (RUSI; model Affiniti 50, Philips, NV, USA) with a broadband curvy linear array (model C6-2) probe was used to measure LM activation at the L4-5 facet joint (2 cm lateral to the lower half of the L4 spinous process). Studies suggest that the relative LM thickness change could represent LM activation28,29,30. Our previous study demonstrated excellent intra-rater reliability (ICC3,1 ranging between 0.91 and 0.99), and inter-rater reliability (ICC2,k = 0.95) for LM thickness measurements30. Moreover, 95% confidence minimal detectable change was 0.11 cm30. Therefore, RUSI was valid and reliable to measure LM activation.
Procedure
This human research followed the principles of the Declaration of Helsiki. Informed consent for publication of identifying information/images in an online open-access publication has also been obtained. The funders played no role in the design, conduct, or reporting of this study. Participants underwent the screening process along with TMS screening questions for eligibility. Eligible participants were asked to fill out demographic data forms. Then, a researcher identified bilateral LM (1 cm lateral to the spinous process of L5)23 and prepared the skin using a 70% alcohol swab to reduce skin impedance. EMG electrodes (Telemyo 2400 T G2, Noraxon USA Inc., AZ, USA) were attached on the pain-free side, while TMS electrodes were attached on the painful side based on history of LBP (Fig. 1A). If the history indicated bilateral LBP, a more painful or dominant side was selected. A ground electrode was placed over the posterior superior iliac spine.
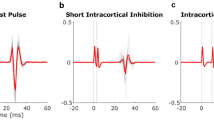
Example of transcranial magnetic stimulation (TMS) protocol to measure cortical excitability of right lumbar multifidus muscle including TMS electrode (A, right) and electromyography (A, left) placements, pre-marked 5X7 cm swimming cap (B), and coil placement during stimulation with visual feedback (C). Peak-to-peak motor evoked potential (MEP) amplitude and cortical silent period derived from MEP time-series data (D).
The participants were asked to performed two repetitions of three-second maximum voluntary isometric contraction of back extension in the prone with a one-minute rest between repetitions. The averaged MVIC was used to set real-time visual target at 10% MVIC during TMS measurement15, 18.
The participants were asked to sit in a TMS chair with their feet flat on the floor and wear a swimming cap marked with a 5 × 7 cm grid relative to the vertex (Fig. 1B). The navigation system along with the 5 × 7 grid was used to consistently position the TMS coil. Then, the participants performed leaning forward to activate the LM until EMG visual feedback reached the 10% MVIC target (Fig. 1C).
A researcher placed a figure-of-eight coil parallel to the scalp site contralateral to the painful side hemisphere in a posterior to anterior direction (Fig. 1C). The researcher used a single-pulse monophasic mode to stimulate each point over the 5 × 7 grid and identified the LM hotspot for each participant15. This hotspot was simultaneously registered to the navigation system for repositioning the TMS coil after applying tDCS.
After identifying the hotspot, an active motor threshold was determined using the lowest stimulus intensity to elicit MEP (Fig. 1D) at the hotspot15, 18. A MEP amplitude ≥ 200 μV more than 50% of the number of stimuli was needed to activate muscles at 10% MVIC15, 18. The intensity of MEP was set at 120% of the active motor threshold stimulus intensity and twenty MEPs were recorded. These data were further used to derive peak-to-peak MEP amplitude (P2P) and cortical silent period (CSP)15, 22, 26. CSP was defined as time between the end of the MEP to the point when the LM EMG activity recurred15, 22, 26.
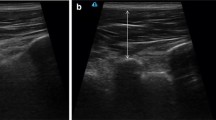
For RUSI measurement, the participant was asked to lie in the prone position with a towel roll below the abdomen to reduce lumbar lordosis (lumbosacral angle < 10 degrees). The thorax (T3) and pelvis (S2) were securely stabilized to the treatment bed using straps. Then, the researcher placed the RUSI transducer over the painful side of the LM (2 cm lateral to the lower half of the L4 spinous process; Fig. 2A)14, 30. Several EMG and MRI studies showed no significant difference between painful and non-painful sides11,12,13. One RUSI study also found no significant difference between painful and non-painful sides in unilateral CLBP, as well as between right and left sides in bilateral CLBP31.
The LM thickness was measured during resting (Fig. 2B) twice. Then, the participants were asked to place their hands behind the neck and perform two repetitions of five-second MVIC of back extension with rotation to the opposite side against the external force, while the researcher was recording LM thickness (Fig. 2C)32. One-minute rest was provided between repetitions to prevent muscle fatigue. The distance between the tip of the L4-5 facet joint and thoracolumbar fascia was measured for both conditions14, 30. Percent LM thickness change from resting was used to represent LM activation. Our previous study indicated excellent intra-rater (ICC3,1 = 0.987–0.996) and inter-rater reliability (ICC2,k = 0.978–0.997)30. Moreover, 95% confidence minimal detectable change was 0.11 cm30.
After baseline collection, only the CLBP group received anodal tDCS (a-tDCS) using 5X7 cm electrodes in which the anodal electrode was placed on the hotspot representing the LM in the M1 area contralateral to the painful side, while the cathodal electrode was attached to the ipsilateral supraorbital area22, 23. The a-tDCS was applied to the participants in sitting position. Participants were asked to stay awake during stimulation. The intensity was set at 2 mA with 10-s fade in/out22, 23. The participants were stimulated by a-tDCS for 20 min. Then, TMS and RUSI measurements were performed again to obtain post-intervention data.
Statistical analysis
All statistical analyses were performed using SPSS Software (IBM SPSS Statistics 23 for Windows). Descriptive statistics was used to analyze participant characteristics, and the Shapiro–Wilk test was used to determine normality of the data. We found that our data were normally distributed; therefore, an independent t test was used to compare cortical excitability (P2P and CSP) and LM activation between groups, while a paired t-test was used to compare the differences between pre- and post-intervention for the CLBP group. In addition, Pearson’s correlation coefficient was used to examine the association between cortical excitability and LM activation. We separately analyzed correlations for each group because healthy individuals might not have any impairment; therefore, there should not be any correlation between cortical excitability and LM activation. We also performed correlation at post-intervention in the CLBP group to ensure whether we found similar correlation compared with pre-intervention.
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of Mahidol University (COA No. MU-CIRB 2021/184.0309) and registered in clinical trial (ClinicalTrials.gov ID: NCT05156242).
Results
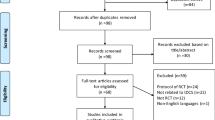
Thirteen healthy participants and 38 participants with low back pain were screened for eligibility. Of 13 healthy participants, 2 participants had more than 2 positive movement control battery tests; therefore, 11 participants without LBP were included for this study. For participants with low back pain, 13 participants did not meet movement control screening and 12 participants did not meet the definition of CLBP. Therefore, 13 participants with CLBP were included in this study. No significant differences (P > 0.05) were found in participant characteristics between groups, except frequency of positive movement control battery tests in which the CLBP group had significantly greater frequency (P < 0.05) than that of the NoLBP group (mean frequency = 0.9 and 3.8, respectively). The participant characteristics are presented in Table 1.
Figure 3 demonstrates example of MEPs from individual without LBP (A) and individual with CLBP (B). Statistical analysis demonstrated significant difference in P2P between groups. However, no significant differences were found in P2P, CSP, and LM activation between pre- and post-intervention. Between groups and within-group comparisons are presented in Table 2. In addition, a trend indicating moderate positive correlation was observed between P2P and LM activation (r = 0.55, P = 0.05) in CLBP group pre-intervention (Table 3), while a strong positive correlation (r = 0.72, P = 0.006) was found post-intervention. No significant correlations (P > 0.05) were found between CSP and LM activation in the CLBP group. The NoLBP group did not show any significant correlation (P > 0.05) between cortical excitability and LM activation. In addition, no participants reported serious adverse effects of a-tDCS and only one participant reported an itchy sensation in the forehead during a-tDCS application. However, it did not last more than one day.
Discussion
Our study was designed to investigate the immediate effects of a-tDCS on cortical excitability and LM activation and explore the relationship between cortical excitability and LM activation in participants with CLBP during remission. We first compared whether our participants with CLBP actually had changes in cortical excitability and LM activation. P2P finding supports that those participants with CLBP had decreased MEP amplitude. Although we found trends in which participants with CLBP had shorter CSP and lower LM activation, these findings did not yield statistical significance.
Several studies reported changes in cortical excitability in patients with CLBP15,16,17,18. Researchers suggest that these changes can be caused by reflex inhibition response to pain/injury16, 33. This reflex inhibition is reflected as muscle activation and movement pattern alterations that may aim to minimize the load on injured structures16, 33. In addition, the repetition of adapted muscle activation and movements over time results in neuroplastic changes that can be represented as altered cortical excitability within the motor cortex15,16,17,18.
The non-significant increase in MEP amplitude after a-tDCS intervention did not support our hypothesis in which we expected that tDCS can modulate cortical excitability. This finding is inconsistent with the study reporting the immediate effect of a-tDCS on cortical excitability22. However, those studies applied a-tDCS in healthy individuals which could cause different responses compared with those of patients with CLBP22.
Another potential explanation is the reversed effect of a-tDCS. Theoretically, a-tDCS should induce increased MEP amplitude by depolarization of the resting membrane potential causing the long-term potentiation (LTP)-like plasticity effect19, 24. However, a-tDCS might induce long-term depression (LTD)-like plasticity resulting in inhibiting the excitability34, 35. Another mechanism is that a-tDCS can induce increase in cortical excitability by opening the Ca+ influx19, 24. However, if an overwhelming Ca+ occurs in the voltage channel, the counter regulation mechanism will take place in this process which in turn blocks the N-methyl-D-aspartate (NMDA) receptor and could be eventually result in decreased cortical excitability34.
Although between-participant variability with different underlying neurophysiological mechanisms could be responsible for non-significant changes in cortical excitability in other studies36, 37, our study specifically included participants with the same underlying MCI. Therefore, the effect of variability in our participants on non-significant changes is unlikely.
The intra-subject differences under their neurophysiological mechanism such as emotional changes, stress, fatigue, variation over time and hormonal changes occurred in our study38. Although we controlled the participant characteristics and time of the day for the investigation, we did not control their neurophysiological conditions that might have affected the response to a-tDCS38.
Significant difference in MEP amplitude between healthy individuals and participants with CLBP indicated participants with CLBP actually experienced a change in cortical excitability. This would suggest that our one session of a-tDCS at 2 mA for 20 min might not have been sufficient to increase cortical excitability. Therefore, further study is needed to determine appropriate doses and explore more possible outcomes by considering and controlling possible neurophysiological mechanisms using a true experimental study design.
No significant change in CSP post-intervention also did not support our hypothesis. This could have been due to cortical inhibition caused by GABA activity24, 39. The a-tDCS should activate NMDA receptors and induce calcium-dependent plasticity; however, excessive Ca+ might trigger GABA activity to counter regulate by blocking the NMDA receptor resulting in unchanged CSP24, 39. Another explanation might be our participants did not experience CSP change compared with their NoLBP counterpart. In addition, several studies found high variability in CSP and suggested that CSP might be unreliable measurement to investigate neurophysiological response in participants with CLBP37, 38.
Our finding did not show increase in LM activation after a-tDCS intervention as we expected. No study has investigated the effect of a-tDCS on LM activation; therefore, we were unable to compare and contrast with other studies. However, studies commonly used a-tDCS to reduce pain23, 25. The presence of pain can cause reflex inhibition to LM leading to inability to perform maximum contraction14, 30. One of proposed effects of a-tDCS is to reduce pain which could prevent reflex inhibition, thereby indirectly increasing LM activation. Our study recruited participants with CLBP during remission, and non-significant difference in LM activation between groups suggests no LM activation deficit in our participants with CLBP. Therefore, this mechanism might not have taken place in our study.
The correlation between P2P and LM activation in participants with CLBP partially supports our hypothesis in which decreased MEP amplitude would result in LM activation deficit. Some studies demonstrated changes in cortical excitability15,16,17,18, while others found LM activation deficits in patients with CLBP12,13,14. Those researchers assumed a relationship existed between cortical excitability and LM activation. Our moderate (pre-intervention) and strong (post-intervention) positive correlations did support the existence of this relationship. These correlations suggest that cortical excitability may play a critical role in LM activation; therefore, intervention should include a neuromodulator to enhance cortical excitability. One study reported no relationship between changes in cortical excitability and LM activation in patients with CLBP15. The discrepancy between studies could be because our study included participants with CLBP with underlying MCI as a homogeneous group, while their study might have included CLBP with different underlying mechanisms.
Although our study did not show improved cortical excitability and LM activation after a-tDCS intervention, we found moderate to strong positive correlations between MEP amplitude and LM activation. These findings at least suggest the involvement of the cortical level in CLBP. Therefore, clinicians should include interventions involving neuromodulation to treat patients with CLBP with underlying movement control impairment.
Limitations
Limitations were encountered in this study. Our participants with CLBP were specific to pain-free condition, age between 20 and 40 years, and presenting at least three positive movement control tests. These characteristics would limit generalization in a CLBP population. We were able to recruit only a small sample size and our study used a quasi-experimental design due to difficulty in recruiting participants with specific criteria. However, we used healthy individuals as reference to compare at baseline to ensure the existence of changes in cortical excitability and LM activation in participants with CLBP. Replication of our study employing a larger sample and randomized controlled trial is needed. We performed post-hoc power analysis for our correlation findings (r = 0.55 and 0.72) with 95% confidence and sample size of 13 (CLBP group). We found achieved power of 0.58 and 0.92, respectively. In addition, a minimum sample size of 21 and 10 participants would be required to replicate our study using these correlation coefficients, 80% power and confidence level of 0.05. Decreased LM activation could be resulted from other factors, such as muscle atrophy, fatty infiltration, or slow-to-fast muscle transition. Therefore, future study should take these potential confounding factors into consideration. We used single-pulse TMS to investigate excitability of motor cortex corresponding to painful side only. This would limit the investigation of intra-cortical connections within a specific brain region and inter-cortical connections between different brain regions. Therefore, future study may use paired-pulse paradigm to provide a more comprehensive understanding of excitatory and inhibitory mechanisms.
Data availability
The datasets used and/or analyzed during this study would be available from corresponding author upon reasonable request.
References
Hoy, D. et al. A systematic review of the global prevalence of low back pain. Arthritis Rheum. 64(6), 2028–2037 (2012).
da Silva, T. et al. Recurrence of low back pain is common: A prospective inception cohort study. J. Physiother. 65(3), 159–165 (2019).
Hartvigsen, J. et al. What low back pain is and why we need to pay attention. Lancet 391(10137), 2356–2367 (2018).
Hides, J. A. et al. Convergence and divergence of exercise-based approaches that incorporate motor control for the management of low back pain. J. Orthop. Sports Phys. Ther. 49(6), 437–452 (2019).
Luomajoki, H., Kool, J., de Bruin, E. D. & Airaksinen, O. Movement control tests of the low back; evaluation of the difference between patients with low back pain and healthy controls. BMC Musculoskelet. Disord. 9, 170 (2008).
O’Sullivan, P. Diagnosis and classification of chronic low back pain disorders: maladaptive movement and motor control impairments as underlying mechanism. Man. Ther. 10(4), 242–255 (2005).
George, S. Z. et al. Interventions for the management of acute and chronic low back pain: Revision 2021. J. Orthop. Sports Phys. Ther. 51(11), Cpg1–Cpg60 (2021).
Wattananon, P. et al. Kinematic characterization of clinically observed aberrant movement patterns in patients with non-specific low back pain: A cross-sectional study. BMC Musculoskelet. Disord. 18(1), 455 (2017).
Luomajoki, H. A., Bonet Beltran, M. B., Careddu, S. & Bauer, C. M. Effectiveness of movement control exercise on patients with non-specific low back pain and movement control impairment: A systematic review and meta-analysis. Musculoskelet. Sci. Pract. 36, 1–11 (2018).
van Dieën, J. H., Reeves, N. P., Kawchuk, G., van Dillen, L. R. & Hodges, P. W. Motor control changes in low back pain: Divergence in presentations and mechanisms. J. Orthop. Sports Phys. Ther. 49(6), 370–379 (2019).
Beneck, G. J. & Kulig, K. Multifidus atrophy is localized and bilateral in active persons with chronic unilateral low back pain. Arch. Phys. Med. Rehabil. 93(2), 300–306 (2012).
Danneels, L. A. et al. Differences in electromyographic activity in the multifidus muscle and the iliocostalis lumborum between healthy subjects and patients with sub-acute and chronic low back pain. Eur. Spine J. 11(1), 13–19 (2002).
Hodges, P. W. & Danneels, L. Changes in structure and function of the back muscles in low back pain: Different time points, observations, and mechanisms. J. Orthop. Sports Phys. Ther. 49(6), 464–476 (2019).
Sungnak, P. et al. Individuals with impaired lumbopelvic control demonstrate lumbar multifidus muscle activation deficit using ultrasound imaging in conjunction with electrical stimulation: A cross-sectional study. Arch. Phys. Med. Rehabil. 103(10), 1951–1957 (2022).
Massé-Alarie, H., Beaulieu, L. D., Preuss, R. & Schneider, C. Corticomotor control of lumbar multifidus muscles is impaired in chronic low back pain: Concurrent evidence from ultrasound imaging and double-pulse transcranial magnetic stimulation. Exp. Brain Res. 234(4), 1033–1045 (2016).
Silfies, S. P., Vendemia, J. M. C., Beattie, P. F., Stewart, J. C. & Jordon, M. Changes in brain structure and activation may augment abnormal movement patterns: An emerging challenge in musculoskeletal rehabilitation. Pain Med. 18(11), 2051–2054 (2017).
Strutton, P. H., Theodorou, S., Catley, M., McGregor, A. H. & Davey, N. J. Corticospinal excitability in patients with chronic low back pain. J. Spinal Disord. Tech. 18(5), 420–424 (2005).
Tsao, H., Danneels, L. A. & Hodges, P. W. ISSLS prize winner: Smudging the motor brain in young adults with recurrent low back pain. Spine 36(21), 1721–7 (2011).
Nitsche, M. A. & Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 527(Pt 3), 633–9 (2000).
Nitsche, M. A. & Paulus, W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 57(10), 1899–1901 (2001).
Nitsche, M. A. & Paulus, W. Transcranial direct current stimulation–update 2011. Restor. Neurol. Neurosci. 29(6), 463–492 (2011).
Schabrun, S. M., Burns, E., Thapa, T. & Hodges, P. The response of the primary motor cortex to neuromodulation is altered in chronic low back pain: A preliminary study. Pain Med. 19(6), 1227–1236 (2018).
Schabrun, S. M., Jones, E., Elgueta Cancino, E. L. & Hodges, P. W. Targeting chronic recurrent low back pain from the top-down and the bottom-up: A combined transcranial direct current stimulation and peripheral electrical stimulation intervention. Brain Stimul. 7(3), 451–459 (2014).
Stagg, C. J. & Nitsche, M. A. Physiological basis of transcranial direct current stimulation. Neuroscientist 17(1), 37–53 (2011).
Jiang, N. et al. Effect of dry-electrode-based transcranial direct current stimulation on chronic low back pain and low back muscle activities: A double-blind sham-controlled study. Restor. Neurol. Neurosci. 38(1), 41–54 (2020).
Burns, E., Chipchase, L. S. & Schabrun, S. M. Temporal and spatial characteristics of post-silent period electromyographic bursting in low back muscles: Comparison between persons with and without low back pain. Int. J. Neurosci. 127(12), 1074–1081 (2017).
Livingston, S. C. & Ingersoll, C. D. Intra-rater reliability of a transcranial magnetic stimulation technique to obtain motor evoked potentials. Int. J. Neurosci. 118(2), 239–256 (2008).
Djordjevic, O., Konstantinovic, L., Miljkovic, N. & Bijelic, G. Relationship between electromyographic signal amplitude and thickness change of the trunk muscles in patients with and without low back pain. Clin. J. Pain 31(10), 893–902 (2015).
Kim, C. Y. et al. Comparison between muscle activation measured by electromyography and muscle thickness measured using ultrasonography for effective muscle assessment. J. Electromyogr. Kinesiol. 24(5), 614–620 (2014).
Wattananon, P. et al. Using neuromuscular electrical stimulation in conjunction with ultrasound imaging technique to investigate lumbar multifidus muscle activation deficit. Musculoskelet. Sci. Pract. 50, 102215 (2020).
Thu, K. W., Maharjan, S., Sornkaew, K., Kongoun, S. & Wattananon, P. Multifidus muscle contractility deficit was not specific to the painful side in patients with chronic low back pain during remission: A cross-sectional study. J. Pain Res. 15, 1457–1463 (2022).
Kiesel, K. B., Uhl, T. L., Underwood, F. B., Rodd, D. W. & Nitz, A. J. Measurement of lumbar multifidus muscle contraction with rehabilitative ultrasound imaging. Man. Ther. 12(2), 161–166 (2007).
Horvath, J. C., Forte, J. D. & Carter, O. Evidence that transcranial direct current stimulation (tDCS) generates little-to-no reliable neurophysiologic effect beyond MEP amplitude modulation in healthy human subjects: A systematic review. Neuropsychologia 66, 213–236 (2015).
Hassanzahraee, M., Nitsche, M. A., Zoghi, M. & Jaberzadeh, S. Determination of anodal tDCS duration threshold for reversal of corticospinal excitability: An investigation for induction of counter-regulatory mechanisms. Brain Stimul. 13(3), 832–839 (2020).
Mosayebi Samani, M., Agboada, D., Jamil, A., Kuo, M. F. & Nitsche, M. A. Titrating the neuroplastic effects of cathodal transcranial direct current stimulation (tDCS) over the primary motor cortex. Cortex 119, 350–361 (2019).
Bashir, S. et al. Effects of anodal transcranial direct current stimulation on motor evoked potentials variability in humans. Physiol. Rep. 7(13), e14087 (2019).
Wiethoff, S., Hamada, M. & Rothwell, J. C. Variability in response to transcranial direct current stimulation of the motor cortex. Brain Stimul. 7(3), 468–475 (2014).
Horvath, J. C., Carter, O. & Forte, J. D. Transcranial direct current stimulation: Five important issues we aren’t discussing (but probably should be). Front. Syst. Neurosci. 8, 2 (2014).
Werhahn, K. J., Kunesch, E., Noachtar, S., Benecke, R. & Classen, J. Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. J. Physiol. 517(2), 591–597 (1999).
Acknowledgements
We would like to thank the Spine Biomechanics Laboratory, Faculty of Physical Therapy, Mahidol University, for providing the data collection space and equipment. We would also like to thank all participants in this study.
Funding
This study was partially funded by National Research Council of Thailand (N42A650360, 2020) and the Faculty of Physical Therapy, Mahidol University (Research Assistant Fund).
Author information
Authors and Affiliations
Contributions
P.W. and K.W.T. have contributed to the conception, research design, data analysis, and drafting the manuscript, and editing and revising the manuscript. K.W.T., S.M. and K.S. have substantially contributed to research design and data collection. H.K.W. has contributed to the conception and research design.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wattananon, P., Thu, K.W., Maharjan, S. et al. Cortical excitability and multifidus activation responses to transcranial direct current stimulation in patients with chronic low back pain during remission. Sci Rep 13, 16242 (2023). https://doi.org/10.1038/s41598-023-43597-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-43597-7
- Springer Nature Limited