Abstract
Ten male cyclists were randomized into four experimental conditions in this randomized, cross-over, double-blind, and sham-controlled study to test the combined effect of acute dark chocolate (DC) ingestion and anodal concurrent dual-site transcranial direct current stimulation (a-tDCS) targeting M1 and left DLPFC on cognitive and whole-body endurance performance in hypoxia after performing a cognitive task. Two hours before the sessions, chocolate was consumed. After arriving at the lab, participants completed an incongruent Stroop task for 30 min in hypoxia (O2 = 13%) to induce mental fatigue, followed by 20 min of tDCS (2 mA) in hypoxia. Then, in hypoxia, they performed a time-to-exhaustion task (TTE) while measuring physiological and psychophysiological responses. Cognitive performance was measured at baseline, after the Stroop task, and during and after TTE. TTE in ‘DC + a-tDCS’ was significantly longer than in ‘white chocolate (WC) + a-tDCS’ and WC + sham-tDCS’. The vastus medialis muscle electromyography amplitude was significantly higher in ‘DC + a-tDCS’ and ‘DC + sham-tDCS’ than in ‘WC + sh-tDCS’. During and after the TTE, choice reaction time was significantly lower in ‘DC + a-tDCS’ compared to ‘WC + sh-tDCS’. Other physiological or psychophysiological variables showed no significant differences. The concurrent use of acute DC consumption and dual-site a-tDCS might improve cognitive and endurance performance in hypoxia.
Similar content being viewed by others
By increasing altitude, the partial pressure of O2 decreases, which would limit oxygen delivery to the exercising muscles1 and the brain2. Numerous studies have shown that exercise performance is impaired in hypoxic conditions3,4,5,6,7. This hypoxia-induced decrement in exercise performance has been attributed to decreased arterial oxygen saturation (SpO2), increased pulmonary ventilation, and also increased afferent feedback from the working muscles which leads to the diminution of motor output from the central nervous system to the periphery6,7,8. In addition, hypoxia-induced impairment in physiological, psychological, cognitive, and perceptual responses to exercise has been reported in most of the previous studies2,7,9,10,11. Interestingly, current studies propose that in both moderate and severe hypoxia, the brain plays a pivotal role in regulating endurance performance, probably via different mechanisms2. The terms like "brain-hypoxic effect" or "the hypoxia-sensitive central component of fatigue" have been used in this regard to accentuating that in severe hypoxia, central mechanisms are the major cause of fatigue and endurance performance deterioration2. It has also been shown that in moderate hypoxia, the central drive from the brain to the working muscles decreases as a result of the hypoxia-induced increase in the discharge of inhibitory muscle afferents leading to a decrement in exercise performance6. The Psychobiological Model of fatigue during endurance exercise provides more support for this claim by assuming that rating of perceived exertion (RPE) and potential motivation, both processed in the brain, are important determinants of endurance performance12. In this regard, it has been reported that the levels of arousal and affective responses also play an important role in exercise performance as they could negatively impact potential motivation and RPE13,14,15 which are important psychophysiological markers for endurance exercise performance16. In fact, it has been demonstrated that the rate of decline in the affective responses is strongly correlated with the time to exhaustion (TTE) in the endurance cycling test16.
Studies have also demonstrated that hypoxia has a detrimental effect on cognitive function (i.e., impaired attentional ability, executive function, and memory function) and the psychophysiological homeostasis of the body17,18. Oxygen delivery to the brain through the neurovascular coupling (NVC) mechanism is one of the most important determinant factors of cognitive functions. It has been shown that the NVC might be negatively affected by hypoxia, culminating in reduced cognitive function19. It has also been indicated that the perturbation in psychophysiological homeostasis caused by hypoxic exposure results in mental fatigue20, which is defined as a psychobiological state characterized by feelings of tiredness/lack of energy at rest and/or increased RPE during subsequent whole-body endurance exercise21,22. Mental fatigue has been experimentally induced by performing a highly demanding cognitive task (e.g., Stroop test) for prolonged periods, in general ranging from 30 to 90 min23. In this context, a systematic review by Van Cutsem et al.23 found that mental fatigue decreased endurance performance (i.e., TTE and self-selected power output), which was associated with an increased RPE. Interestingly, they found no alterations in physiological responses associated with endurance performance [i.e., heart rate (HR), blood lactate, oxygen uptake, cardiac output, maximal aerobic capacity]23. In general, it could be concluded that mental fatigue negatively impacts physical and mental functions21. Accordingly, applying potentially effective strategies to counteract the detrimental effects of hypoxia and mental fatigue on various aspects of exercise performance has become a growing area of interest7,17,20.
Nutritional interventions are the most common strategies to counteract hypoxia-induced impairments in exercise performance24. In this regard, polyphenols are a nutritional component that has been proposed to have antioxidant, anti-inflammatory, and metabolic properties, and their ingestions might be beneficial for cognitive performance, brain functions, brain plasticity, and neuroprotection25,26. Alternatively, polyphenols can also have vasoactive effects likely due to their capacity to enhance the bioavailability and bioactivity of nitric oxide (NO), which could present interesting effects on increasing blood flow to the muscles and active brain areas during exercise that could improve exercise performance27,28. Cocoa is an important source of dietary polyphenols because its seeds contain high levels of polyphenols, which are bioactive compounds, besides fatty acids, vitamins, minerals, fiber, and several methylxanthine alkaloids29,30. Dark chocolate (DC) contains high amounts of cocoa, which makes it easier for daily consumption31. A recent review by Martín et al.26 has found that acute ingestion of DC improves several domains of cognitive performance in young adults, which are accompanied by increased cerebral blood flow and oxygenation. The efficacy of consuming DC in exercise performance, cognitive function, and reduction of fatigue in hypoxic conditions has also been reported32,33. Shaw et al.33 demonstrated that the consumption of DC caused a decrease in lactate levels and fatigue at the simulated altitude, with no change in endurance performance. Decroix et al.32 showed that consuming cocoa flavanols had a beneficial effect on prefrontal oxygenation during moderate-intensity exercise in both normoxia and hypoxia. In addition, several studies have reported that polyphenolic compounds can have a positive effect on cognitive function, especially executive function, attention, working memory, and processing speed34,35,36,37,38. However, the effect of acute DC ingestion on cognitive and endurance performance after performing a prolonged cognitive effort in hypoxia remains to be tested.
Moreover, anodal transcranial direct current stimulation (a-tDCS) has recently been suggested to boost exercise performance39 and cognitive function40,41,42. tDCS is a non-invasive, relatively inexpensive, portable, and easy to use, brain-modulating technique that involves applying a weak electrical current (up to 4 mA) to the scalp over a brain area of interest that can modulate spontaneous neuronal activity and excitability, in general, in a polarity-dependent manner39,43. The primary motor cortex (M1) and dorsolateral prefrontal cortex (DLPFC) are the most common areas investigated in previous studies mostly because of their substantial role in regulating exercise endurance performance and cognitive function39,44. Indeed, various studies have shown that stimulating either M1 or DLPFC improves various aspects of exercise performance39,45,46,47,48,49. The underlying mechanisms in this context have been reported to be increasing corticospinal excitability and neural drive to the periphery50, improving blood flow to the brain51, reducing RPE49, improving the psychophysiological responses39, and optimizing the information processing in the brain52. However, the effect of tDCS may depend on stimulation parameters (e.g., electrode montage, size, number, current intensity, and density), and previous studies using tDCS to boost physical performance used electrode montages either targeting M1 or DLPFC separately43,46,53. In this context, the anodal unihemispheric concurrent dual-site tDCS (a-tDCSUHCDS) was recently proposed as an effective strategy for simultaneously stimulating M1 and DLPFC yielding a greater increase in corticospinal excitability compared to the isolated stimulation of M1 and DLPFC54,55. It is noteworthy that this increase in corticospinal excitability lasted for 24 h after a-tDCSUHCDS54. Talimkhani et al.56 provided further support for the effectiveness of a-tDCSUHCDS by showing its positive effect on the acquisition of cognitive skills and functions in healthy subjects. However, from a practical point of view, if this greater corticospinal excitability translates into improved performance in whole-body endurance exercise (specifically under hypoxic conditions) also remains to be tested.
Thus, considering the detrimental effect of both hypoxia and mental fatigue on cognitive and physical performance, and the overlapping between its underlying mechanisms with the action mechanisms of DC and tDCS, it is possible to speculate that the combination of DC and a-tDCSUHCDS would have a synergistic effect to counteract the detrimental effects of hypoxia and mental fatigue on cognitive and exercise performance. Therefore, we aimed to analyze the acute effect of combined DC supplementation and a-tDCSUHCDS on cognitive performance, whole-body endurance exercise performance, physiological, and psychophysiological responses under hypoxia after performing a mentally demanding task.
Results
tDCS-induced sensations and blinding
All 10 participants received the experimental conditions according to the randomization. The participants reported no serious side or adverse effects. The most common sensations reported were itching and burning. No other sensation beyond the ones listed in the questionnaire was reported. All participants reported that these sensations were located on the head, starting and ending at the beginning of the stimulation (Table 1). There was no significant difference in sensations intensity, beginning and end of the sensations, and perceived effect on performance among conditions. All participants reported these sensations positively affected their performance ranging from slightly to considerably. The percentage of correct guesses regarding the tDCS condition differed among conditions (χ22 = 30.0; p < 0.001), with the active tDCS conditions (DC + a-tDCS = 100% and WC + a-tDCS = 100%) significantly higher than sham conditions (DC + sh-tDCS = 0% and WC + sh-tDCS = 0%; all ps = 0.002). This was because all individuals (100%) thought they had been stimulated in all four conditions. Hence, considering the similar tDCS-induced sensations and active guess rate, it can be assumed that the study blinding protocol was effective.
The mean value of the study variables under four experimental conditions is presented in Table 2.
Effect of the demanding cognitive task
Performing the incongruent only Stroop task for 30 min did not induce a state of mental fatigue as we found no significant main effect of time (F(1,72) = 0.635; p = 0.428), condition (F(3,72)= 0.335; p = 0.8), or time x condition interaction (F(3,72)= 0.088; p = 0.967) on CRT (Fig. 1A).
Reaction time changes in the four experimental conditions. Comparison of choice reaction time performance (A) at baseline in normoxia and after performing a modified Stroop task (incongruent only) for 30 min in hypoxia among experimental conditions. Choice reaction time performance (B) before, during, and immediately after the time to exhaustion test (TTE) under hypoxia (O2 = 13%) after performing an incongruent-only Stroop task for 30 min and receiving anodal or sham Transcranial Direct Current Stimulation for 20 min in hypoxia. * = “DC + a-tDCS" significantly higher than “WC + sh-tDCS” (p = 0.003). DC dark chocolate; WC white chocolate; a-tDCS anodal transcranial direct current stimulation; sh-tDCS sham transcranial direct current stimulation.
Effect of DC and a-tDCSUHCDS on cognitive performance
Our results showed a significant condition x time interaction (F(6,54)= 2.39, p=0.04, ɳ2p= 0.210) on CRT, but no main effect of either time or condition separately. Hence, we explored this result to analyze the main effect of the condition at each time point. The results demonstrated that there was no significant difference in CRT at baseline among experimental conditions (F(3,27)= 0.112, p = 0.95, ɳ2p= 0.012; Fig. 1A). Inversely, there was a significant effect of condition on CRT during TTE in hypoxia (F(3,27)= 9.42, p = 0.0001, ɳ2p= 0.512; Fig. 1B), which was significantly lower in ‘DC + a-tDCS’ compared to ‘WC + sh-tDCS’ condition (p = 0.008, dav= 2.4, Δ = − 28.93%) with no significant differences among other conditions (p ˃ 0.05). There was also a significant effect of condition on CRT after exhaustion in hypoxia (F(3,27)= 4.61, p = 0.01, ɳ2p= 0.339; Fig. 1B), which was significantly lower in ‘DC + a-tDCS’ compared to ‘WC + sh-tDCS’ condition (p = 0.016, dav= 1.04, Δ= − 23.19%) with no significant among other conditions (p ˃ 0.05).
Effect of DC and a-tDCSUHCDS on endurance performance
The concomitant use of DC and a-tDCSUHCDS improved endurance performance in hypoxia, as there was a significant effect of the conditions (F(3,27)=7.34, p=0.001, ɳ2p=0.449; Fig. 2A), with a significantly higher TTE in ‘DC + a-tDCS’ than in ‘WC + a-tDCS’ and ‘WC + sh-tDCS’ conditions (p=0.002, dav=0.77, Δ=46.7%; p=0.038, dav=0.85, Δ=49.3%, respectively). No significant differences were observed among other conditions (p˃0.05).
Endurance performance and physiological responses in the four experimental conditions. Time to exhaustion test (A), heart rate (B), blood oxygen saturation (SpO2; C), and electromyographic (EMG) amplitude of the vastus lateralis (D), vastus medialis (E), and rectus femoris (F) during the endurance cycling task under four experimental conditions in hypoxia. * = significantly different from WC + sh-tDCS (p < 0.05); # = significantly different from WC + a-tDCS (p < 0.05). DC dark chocolate; WC white chocolate; a-tDCS anodal transcranial direct current stimulation; sh-tDCS sham transcranial direct current stimulation.
Effect of DC and a-tDCSUHCDS on physiological responses
DC and/or a-tDCSUHCDS increased EMG amplitude during TTE in hypoxia, as there was a significant effect of the condition on the EMG amplitude of VM muscle during TTE in hypoxia (F(3,27)= 4.7, p = 0.009, ɳ2p= 0.344; Fig. 2E). A significantly higher VM EMG amplitude in ‘DC + a-tDCS’ (p = 0.049, dav= 1.59, Δ = 64.3%) and ‘DC + sh-tDCS’ (p = 0.045, dav= 1.5, Δ=95.8%) compared to the ‘WC + sh-tDCS’ condition, with no other significant difference (p˃0.05). However, there was no significant difference in the EMG amplitude of RF (F(1.46,13.14)= 0.180, p = 0.7, ɳ2p= 0.02; Fig. 2F) and VL muscles (F(3,27)= 0.315, p = 0.8, ɳ2p= 0.034; Fig. 2D). Similarly, there was no significant effect of the conditions on HR (F(3,27)= 0.141, p = 0.93, ɳ2p= 0.015; Fig. 2B) and SpO2 (F(3,27)= 2.08, p = 0.12, ɳ2p= 0.188; Fig. 2C) during TTE in hypoxia among conditions.
Effect of DC and a-tDCSUHCDS on psychophysiological responses
DC and/or a-tDCSUHCDS did not change psychophysiological responses during TTE in hypoxia, as there was no significant effect of condition on RPE (F(3,27)= 1.41, p = 0.25, ɳ2p= 0.114), affective responses (F(3,27)= 0.64, p = 0.59, ɳ2p= 0.066), or FAS (F(3,27)= 1.08, p = 0.37, ɳ2p= 0.108) during TTE in hypoxia (Fig. 3A–C).
Psychophysiological responses to exercise in the four experimental conditions. Ratings of perceived exertion (RPE; A), affective responses (B), and felt arousal (C) during the endurance cycling task under four experimental conditions in hypoxia. DC dark chocolate; WC white chocolate; a-tDCS anodal transcranial direct current stimulation; sh-tDCS sham transcranial direct current stimulation.
Discussion
The main results of the present study were that (1) ‘DC + a-tDCS’ resulted in longer TTE in hypoxia than ‘WC + a-tDCS’ and ‘WC + sh-tDCS’ conditions, (2) with greater EMG activity of the VM muscle, and (3) improved cognitive performance during and after TTE. These results suggest the possibility of a synergistic effect between DC and a-tDCSUHCDS. To the best of the authors’ knowledge, this is the first study to test the combined effect of DC and tDCS on cognitive and endurance performance in hypoxia after performing a prolonged cognitive effort, and its related physiological and psychophysiological responses.
First, it is important to note that despite performing the modified incongruent Stroop task for 30 min in hypoxia, cognitive performance was unchanged over time (Fig. 1A). This indicates that a state of mental fatigue was not induced in the present study. One could argue that this result was due to chocolate consumption 2 h before the experimental sessions, but no difference was found between DC and WC (‘placebo’) conditions. It is noteworthy that various studies succeed in inducing mental fatigue using the same cognitive task and duration57,58,59, including in professional road cyclists60. Hence, despite the potential negative effect of hypoxia on cognitive performance61,62, which would have exacerbated the induction of mental fatigue in the current study63,64, it did not occur. Therefore, we did not assume that mental fatigue played a role in this study, and we used the term "prior cognitive effort" to refer to the attempt to induce mental fatigue. When attempting to induce mental fatigue in hypoxia in endurance-trained athletes, future studies should use longer durations of cognitive effort.
On the other hand, DC + a-tDCS improved cognitive performance during and after TTE in hypoxia. This is partially in line with previous studies showing cognitive improvements at rest with either DC26,34,65 or tDCS isolated40,41,42. Talimkhani et al.56 also showed that a-tDCS decreased the reaction time. However, different from previous studies we combined a-tDCS and DC and also assessed cognitive performance during and after an exhaustive endurance task, which is a novel contribution of the current study. This result is particularly important since cognitive performance has been deemed paramount for endurance exercise regulation and a determinant for the occurrence of exercise-induced fatigue23,57. Interestingly, the improved cognitive performance with DC + a-tDCS was accompanied by improved exercise performance (i.e., longer TTE duration) and, despite sustaining exercise for longer, cognitive performance was still preserved during and immediately after exhaustion. Hence, one possible explanation for improved endurance performance could be due to a preserved and/or improved cognitive performance that would be associated with greater top-down control of exercise performance.
The improved cognitive performance with DC + a-tDCS might be due to increased blood flow and oxygenation to the brain26. The consumption of cocoa flavonols, which are present in DC, a subgroup of polyphenols with antioxidant capacity, may cause NO-dependent vasodilation66. The regulation of NO metabolism through nutritional interventions can improve sports performance and reduce fatigue in both normoxia and hypoxia conditions66,67. Indeed, Maschelin et al.68 showed improvement in SpO2 and TTE after maximal exercise tests in hypoxic conditions (O2 = 11%) with 6-day consumption of nutritional supplements aiming at increasing plasma NO. However, we measured neither brain blood flow nor oxygenation or variables related to NO and, thus, this possible mechanism is speculative.
We found improved endurance performance (i.e., longer TTE) in hypoxia with DC + a-tDCS. This is also a novel finding of the present study. It should be noted that only DC + a-tDCS improved cognitive and endurance performance. Thus, we propose that this could be a synergistic effect since neither DC alone (DC + sh-tDCS) nor tDCS alone (WC + a-tDCS) improved cognitive or endurance performance. Despite previous studies have shown that a-tDCS targeting M1 or DLPFC separately improved endurance performance in cycling39,69, other studies found no change in endurance performance targeting the same brain regions43,70, which aligns with our finding that tDCS alone did not improved endurance performance. It is noteworthy that all previous studies have evaluated the effect of tDCS on exercise performance under normal ambient conditions and only a single recent exception assessed the effect of single-site tDCS on exercise performance in hypoxia and found improved endurance performance with DLPFC but not M171. This is particularly important given that hypoxia provides important environmental changes that differ substantially from normoxia conditions. Considering DC, no study has shown so far that acute ingestion of DC improves endurance performance either in normoxia or in hypoxia72, which is also in line with our findings that DC alone did not improve endurance performance.
One possible mechanism for this synergistic effect is that the possible vascular effect of DC supplementation (i.e., vasodilation) allowed for the neuromodulatory effect of a-tDCS to take place. Theoretically, a tDCS-induced increase in corticospinal excitability and the change in the use of motor units can lead to an improvement in TTE73. Interestingly, a-tDCSUHCDS was shown to induce greater corticospinal excitability compared to the isolated stimulation of M1 and DLPFC54, which lasted for 24 h after a-tDCS. However, it seems to have only taken place when associated with DC in the current study. Some studies suggest that the mechanisms associated with longer exercise tolerance mediated by a-tDCS stimulation are increased intracortical facilitation and M1 excitability74,75,76. Hence, the form of tDCS used in the present study targeting to stimulate simultaneously M1 and DLPFC seems to be a promising montage for endurance performance enhancement.
We found an increased EMG of the VM muscle under both DC + a-tDCS and DC + sh-tDCS compared to WC + sh-tDCS, but no difference in EMG of the RF and VL. Few studies analyzed the effect of tDCS on EMG during an endurance task, which limits comparisons. Vitor-Costa et al.45 found no change in EMG of the VL and RF during a TTE test after a-tDCS targeting M1 despite improved endurance performance. It should be noted that the EMG of VM muscle was not measured in that study, which limits comparison45. Previous studies showed that the greatest activity for the VL and VM occurs during the propulsion phase in cycling (knee extension)77, which is the most important phase of the cycling cycle. EMG of a given muscle represents motor units’ recruitment and/or rate of discharge of the active motor units, thus, being a surrogate measure of the descending neural drive from motor areas of the brain, especially M1. Hence, we could speculate that both DC + a-tDCS and DC + sh-tDCS increased the neural drive to the quadriceps muscle, especially the VM, during the propulsion phase. The same mechanisms explained above might be involved in these results, however, our outcome measures do not allow us to firmly establish that.
On other hand, there was no change in HR, SpO2, and RPE in the present study. These results align with previous studies with acute or sub-acute DC supplementation that found no changes in the mentioned physiological parameters78,79,80. Studies with tDCS have shown contrasting results, with some studies finding significantly lower HR and RPE at lower intensities or at intermediate time points49,69, while others found no changes43,70. Considering that studies with tDCS were performed in normoxia, SpO2 was not measured. The difference between studies might be related, for instance, to the participant’s characteristics such as training and/or fitness status and/or tDCS parameter used. In addition, considering that HR and RPE were analyzed considering the mean of the entire test duration, we suggest that a TTE lasting ~ 45% longer in DC + a-tDCS condition with similar HR and RPE should be considered a result of modulation in these variables as well. For instance, despite we did not perform this analysis, if we consider HR and RPE relative to each TTE duration the values in DC + a-tDCS condition would be probably lower than the other conditions81,82. Finally, the fact that SpO2 was not changed by any intervention could be explained by the type of measure performed. Considering that blood flow is precisely regulated to either increase or decrease according to the tissue needs, the fingertip pulse oximeter is not able to detect changes that would occur in the active muscles or the brain. It could only measure systemic changes or the fingertip level. Other measures of muscle and brain blood flow and oxygenation are suggested for future investigations. Finally, we found no difference in the affective response (FS) or perceived activation (FAS) during the TTE test. To the best of the authors' knowledge, despite the rate of decline in the affective responses being strongly correlated with TTE in cycling16, no study assessed the effect of DC or tDCS on these variables, which limits the comparisons. A similar rationale used to RPE (i.e., perceptual response relative to TTE duration) could explain the FS and FAS responses.
The interpretation and generalization of the findings of the present study should be made with caution since the participants were endurance-trained male cyclists. In addition, the lack of a measure of brain activity (e.g., EEG, fNIRS), corticospinal excitability, muscle, and brain blood flow, and oxygenation does not allow us to fully understand the mechanisms involved in the present findings. Future studies should consider these limitations and also expand the present findings, for instance, to higher levels of hypoxia. On the other hand, the strengths of the present study include a large array of outcome variables assessed and a complex experimental design testing the isolated and combined effects of DC and tDCS on cognitive and endurance performance in hypoxia and its related physiological and psychophysiological responses in professional athletes. From a practical perspective, if consistently replicated, the present findings indicate that athletes and coaches can use DC supplementation and tDCS to boost endurance performance in hypoxia.
The present study showed a possible acute synergistic effect of a-tDCS targeting concurrently M1 and left DLPFC along with the consumption of dark chocolate on improving whole-body endurance performance after prior cognitive effort, with greater EMG of VM muscle, cognitive performance during and after endurance TTE under hypoxia, despite unchanged HR, SpO2, and RPE, affective responses, and arousal in professional male cyclists. Therefore, these two types of interventions can be used in conditions of hypoxia and mental fatigue to improve cognitive and physiological factors in trained individuals.
Methods
Participants
Ten endurance-trained male cyclists voluntarily participated in this randomized, sham-controlled, and double-blind trial. The general characteristics of the participants are presented in Table 3. Participants were recruited from the cycling clubs of the city of residence, all of which participated in regular training at a high level and were involved in most of the competitions at regional and national levels for ≥ 3 years, with most being sponsored. The sample size was calculated using G*Power (Version 3.1.9.2, Kiel, Germany) for a repeated-measure ANOVA within factors as follows: α = 0.05; power (1-β err prob): 0.80; effect size f: 0.4; the number of groups = 1; correlation among repeated measures = 0.5, and non-sphericity correction = 1. The expected effect size for calculating the sample size was based on the meta-analysis by Machado et al.39. Accordingly, a sample of 10 participants was required. Considering the dropout rate of 20%, 12 participants were recruited for the present study, but 2 participants withdrew from the study for personal reasons. The inclusion criteria were as follows: professional male cyclists, no stay in altitude > 2000 m and no blood donation in the previous two months, no history of neurological diseases, no implanted medical devices or pacemakers in the body, no cardiovascular or orthopedic disease, no use of tobacco, drugs, and alcohol. The exclusion criteria were not completing the study protocol, injuries related or not to the study that could influence the outcome variables, and consuming dark chocolate, cocoa, and foods containing flavones on the days of the experiment. All the participants gave written informed consent to the study. The study was approved by the Institutional Ethics Committee and was conducted following the declaration of Helsinki. The study was approved by the Ethics Committee of Razi University Kermanshah, Iran (IR.RAZI.REC.1401.005) and registered in the Iranian Registry of Clinical Trials (IRCT id: IRCT20220417054556N1, Registration Date: 27/07/2022). The first participant was included on 07/08/2022, and the trial was terminated on 28/09/2022.
General experimental procedure
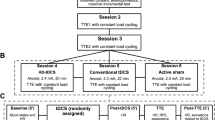
The participants visited the laboratory on six different occasions at one-week intervals. In the first session, participants underwent an anthropometric assessment and were familiarized with the whole experimental procedure. An individual not involved in the research team randomized the order of four experimental conditions using the Latin Square method. The participants and research team were blinded to the order of the experimental conditions throughout the study. In the second session, peak power output (PPO) was measured using an incremental cycling test in hypoxia (O2 = 13%). From the 3rd to 6th visits, the participants performed a time to exhaustion (TTE) cycling test with 60% of PPO in hypoxia under four different experimental conditions in a randomized order: (1) DC + anodal-tDCS, (2) white chocolate (WC) + anodal-tDCS, (3) DC + sham-tDCS, and (4) WC + sham-tDCS. Accordingly, two hours before coming to the lab for each experimental session, participants ingested either DC or WC. Upon arrival at the lab, participants first performed the choice reaction time (CRT) test as a measure of cognitive performance. Subsequently, the maximal isometric voluntary contraction (MIVC) test of knee extensor muscles was performed for normalizing the EMG data of that experimental session. Participants were then exposed to a hypoxic condition (O2 = 13%) while performing a color-word Stroop test (CWST) to induce mental fatigue for 30 min, followed by the CRT test. Following that, tDCS was applied to target areas for 20 min while still in hypoxia. Accordingly, each participant was under hypoxia for 50 min before performing the TTE. After tDCS, participants performed the TTE at 60% of PPO until voluntary exhaustion under the hypoxic condition. During the task, HR and SpO2 were measured constantly and, RPE, CRT, affective responses (Feeling Scale; FS), and perceived activation (Felt Arousal Scale; FAS) were measured every 3 min. Electromyographic activity (EMG) of the rectus femoris (RF), vastus lateralis (VL), and vastus medialis (VM) muscles were measured during TTE. After exhaustion, participants reported RPE, FS, and FAS, and then performed the CRT test under hypoxia. The whole experimental procedure is depicted in Fig. 4.
Study flowchart. PPO Peak Power Output; CRT Choice Reaction Time; tDCS Transcranial Direct Current Stimulation; TTE Time to Exhaustion; HR Heart Rate; SpO2 Blood Oxygen Saturation; RPE Rating of Perceived Exertion; EMG Electromyography; PS Pleasure Sensation (i.e., affective responses); FA Felt Arousal.
Maximal incremental test
In the second session, after 30 min being under hypoxia at rest, participants underwent the maximal incremental test (Astrand Test for Men) on a cycle ergometer (Cyclus 2, RBM Elektronik-Automation GmbH, Leipzig, Germany) to measure PPO under the hypoxic condition. The test was started with 100 watts and a cadence of 60 rpm for three minutes and 50 watts increments every three minutes83. The test was performed until volitional fatigue. During the test, participants reported their RPE. The test was considered maximum if two of the following criteria were achieved: (1) ≥ 90% of maximal predicted HR (220-age), (2) inability to maintain the pedal cadence of 60 rpm for more than 5 s (3) RPE ≥ 90. PPO was determined as follows: PPO = Wout + (t/180) * 50 [Wout: workload of the last completed stage; t: time in the final stage in seconds]45.
Dark chocolate supplementation
In the DC conditions (DC), 2 h before the experimental sessions participants consumed ~ 467 kcal of a typical commercial 70% cocoa product (Nestlé Noir 70%) containing the ingredients cocoa liquor, sugar, cocoa butter, milk fat, lecithin and vanilla84. In the control conditions (WC), participants consumed ~ 487 kcal of WC closely matched to DC in terms of CHO, fat, and calories. Importantly, WC was used as a control for DC because it contains no cocoa butter or cocoa liquor80, which are the components that could potentially generate the effects. Previous studies using WC as a placebo, used the method of alkalizing cocoa for coloring33,85. However, due to the technical unavailability of an alkalizing method, participants were not blinded concerning the chocolate supplementation condition (e.g., they knew they were consuming either DC or WC). This same method was used in previous studies80,85,86. It should be noted that participants were not given any hint regarding the superiority of either DC or WC in order to prevent possible “expectation effect” bias. Also, participants did not receive any feedback on their performance for any outcome measure. Only after the end of the study participants were fully debriefed concerning the study hypothesis and their respective performance.
Cognitive function—choice reaction time
The Visual Choice Reaction Time Apparatus (Model 63035A, Lafayette Instrument Company, Indiana, USA) with a four-choice compatible stimulus–response paradigm was used to measure cognitive performance. Three visual stimuli (lights turning on) were manually given to the participants, and they were instructed to respond as quickly as possible by pushing the corresponding button on the response panel. The reaction time in each stimulation was recorded and the mean value of 3 efforts was calculated. The CRT was performed at the beginning of each experimental session in normoxia, after the prolonged cognitive effort, and during and after TTE in hypoxia.
MIVC
At the beginning of each session, participants performed 3–5 s knee extension MIVC three times with a 150-s rest in between on a custom-made chair with knee and hip fixed at 90° as recommended for VL, VM, and RF muscles MIVC test87. The best performance in MIVCs was recorded for normalizing the EMG signals of that session.
EMG
The surface EMG signals were collected strictly according to the recommended standards88,89. In each session, surface wireless EMG sensors (Ultium Wireless EMG System, Noraxon, Inc., Scottsdale, AZ, USA) were fixed on the muscle belly of the VL, VM, and RF muscles of the dominant leg after skin preparation (shaving, abrading, and cleaning with alcohol). EMG signals were amplified (× 1.000), high-pass and low-pass filtered (10 and 500 Hz, respectively), and were sampled up to 4000 Hz with the common mode rejection ratio of < −100dB. EMG signals were then registered and analyzed using MyoRESEARCH 3 software (Noraxon, Inc., Scottsdale, AZ, USA) and EMG amplitude analysis instruction. To do so, EMG signals were normalized to the MIVC. The mean value of the EMG amplitude of the VL, VM, and RF muscles during the entire TTE under hypoxia was recorded.
Hypoxic exposure
The hypoxic condition (O2 = 13%; equivalent to an altitude of 3600 m) was induced using a hypoxic air generator equipped with a semipermeable filtration membrane (GO2 Altitude ERA II, Biomedtech, Melbourne, Australia). The generator constantly pumped the hypoxic air into two 120-L Douglas bags. Participants wore an inflatable air cushion mask with a non-rebreathing valve positioned and fixed comfortably using a rubber port full-face harness. The mask was connected to the Douglas bags. SpO2 was also recorded using a fingertip pulse oximeter.
Prolonged cognitive effort
Participants performed a modified computerized version of CWST (100% incongruent) for 30 min under hypoxia. The incongruent CWST consisted of four-color words (yellow, blue, green, and red) in different font inks (yellow, blue, green, and red) that were randomly displayed on a computer screen until the participant entered an answer and were followed by a 1500 ms interval. There were four colored buttons on the keyboard. The participants were instructed to press the button corresponding to the ink color displayed on the screen. There was an exception to this rule, however, if the word appeared in red ink, then the correct answer was pressing the button corresponding to the color word itself60. It is noteworthy that performing this modified CWST for 30 min has been shown to induce a state of mental fatigue57,58,59, including in professional road cyclists60.
Transcranial direct current electrical stimulation (tDCS)
Two-channel battery-driven stimulators (NeuroStim 2, Medina Tebgostar, Tehran, Iran) were used to apply tDCS over the target brain areas during the 3rd to 6th experimental sessions (two devices were used). Four carbon electrodes covered by saline-soaked surface sponges (NaCl 140 mmol dissolved in Milli-Q water) were used as anodes (5 × 4; 20 cm2) and cathodes (9 × 4; 36 cm2). The larger cathode electrodes were chosen to decrease the neuromodulatory effects of these electrodes. A 64-channel EEG cap with the international 10–20 EEG system positions was used to locate target areas over the scalp. The Unihemispheric Concurrent Dual-Site anodal tDCS (a-tDCSUHCDS) montage was used for simultaneously targeting M1 and DLPFC areas55,56. tDCS was applied offline (i.e., after the modified Stroop task) with 2 mA at each target for 20 min. One anode electrode was placed symmetrically over the Cz (2.5 cm on each side of the M1) targeting the representation of the lower limbs in M1 and the other anode electrode was placed vertically over F3 targeting the left DLPFC (Fig. 5A). The cathode electrodes were placed vertically over the supraorbital region, centered at AF4 and the other centered between Fpz and AFz (Fig. 5A). This montage was chosen based on recent studies showing that a-tDCSUHCDS is an effective strategy for simultaneous stimulation of M1 and DLPFC yielding higher and long-lasting corticospinal excitability compared to the isolated stimulation of M1 and DLPFC54,56.
Strength and radial component of the electric field induced by tDCS. Finite Element Models derived from Magnetic Resonance Imaging in a head model (MNI152) of the strength and radial (normal to the cortical surface) component of the electric field (EF) induced by tDCS. Electrode montage targeting the simultaneous stimulation with anodal tDCS of the representation of the lower limbs in the primary motor cortex and the left dorsolateral prefrontal cortex (A, B), with red electrodes representing the anodes (5 × 4 cm) and blue electrodes representing the cathodes (9 × 4 cm). The EF strength is presented in the color-coded figures (C–F), with hotter colors indicating stronger EF and colder colors indicating the opposite. The radial EF is presented in the color-coded figures (G–J), where red color represents the electric current flowing into the cortex (i.e., inducing excitatory effects) and blue color represents the electric current flowing out of the cortex (i.e., inducing inhibitory effects). The study montage has reached the target areas with enough electric current strength to induce a neuromodulatory effect, as shown in figures (E) and (F) (blue circles roughly representing the target areas). Furthermore, the target areas were stimulated with the desired polarity (i.e., anodal current) to induce excitatory effects in the target regions, as shown in panels (I) and (J) (blue circles roughly representing the target areas).
Two channels of the tDCS device were used for the simultaneous stimulation of these two regions. For anodal stimulation, the current was gradually ramped up for 30 s, maintained at 2 mA for 20 min, and then progressively ramped down for 30 s. In the sham condition (sh-tDCS), the same electrode position was applied, and the current was ramped up for 30 s, but the 2-mA current was only maintained for 30 s, and then ramped down for 30 s remaining off the rest of the time. This protocol has been shown to be adequate for blinding participants in tDCS studies90,91,92.
tDCS modeling
The brain current flow during tDCS was calculated using a finite element model (FEM) following the standard pipeline in SimNIBS 4.0.093. The magnetic resonance imaging (MRI) head model from the Montreal Neurologic Institute (MNI 152) available in the software was used for the simulation. MRI data were segmented into surfaces corresponding to the white matter (WM), gray matter (GM), cerebrospinal fluid (CSF), skull, and skin. The electrical conductivities of each segment were determined according to values previously established as follows: WM = 0.126 Siemens/meter (S/m), GM = 0.275 S/m, CSF = 1.654 S/m, bone = 0.010 S/m, and skin/scalp = 0.465 S/m94, rubber electrode = 29.4 S/m, and saline-soaked sponges = 1.000 S/m94. All information concerning the respective tDCS montages was entered into the software: current intensity = 2 mA for each anode; electrode position (+ F3 and + Cz/−Fpz and −AF4); electrode and sponge sizes (anodes 5 × 4 cm and cathodes 9 × 4 cm); electrode thickness = 1 mm; sponge thickness = 5 mm. The results of the simulations are presented in Fig. 5, in terms of the electric field strength (Fig. 5C–F) and radial (Fig. 5G–J) electric field (normal to the cortical surface), both of which are most important for neuromodulatory effects of tDCS95. As can be seen in Fig. 5C–F, the study montage has reached our target areas with enough electric current strength to induce a neuromodulatory effect (> 0.2–0.25 V/m)96. Furthermore, the target areas were stimulated with the desired polarity (i.e., anodal current) to induce excitatory effects in the target regions (Fig. 5G–J). Other areas such as the supplementary motor area, premotor cortex, and other prefrontal cortex were also stimulated in the current path from the anodal to the cathodal electrode. This spread electric current is a characteristic of tDCS applied with large rectangular pads (the so-called ‘conventional’ tDCS)43.
tDCS-induced sensations and blinding assessment
Participants completed a questionnaire provided by Fertonani et al.97 after each session, listing the sensations and level of intensity experienced during the stimulation to measure tDCS-induced sensations, similar to previous studies43,92,98. Itching, pain, burning, warmth/heat, pitching, metallic/iron taste, fatigue, and other sensations (open question) were all listed on the questionnaire. The degrees were none (zero), mild (one), moderate (two), considerable (three), and strong (four). Participants also indicated whether these sensations affected their ability to perform the exercise (0 = not at all; 1 = slightly; 2 = considerably; 3 = much; 4 = very much); when the discomfort started (1 = beginning; 2 = at about the middle; 3 = towards the end); and when it stopped (1 = stopped quickly; 2 = stopped in the middle; 3 = stopped at the end). An aggregate variable (referred to as "discomfort" generated by tDCS) was computed as the total of the strength scores recorded for all sensations so that the discomfort variable ranged from 0 (lack of discomfort) to 28 (maximum discomfort). According to recent literature, the end of the study corrects guess rates, which indicates the percentage of participants that successfully guessed their experimental condition, which might lead to a misleading interpretation of blinding effectiveness99,100. It has been suggested to report the “active stimulation guess rate”, which indicates the percentage of participants who guessed they received the active treatment100. Hence, despite we report both correct and active stimulation guess rates, we will consider the latter as the measure of blind effectiveness100.
Whole-body exhausting endurance task
Within the 3rd to 6th sessions, after 30 min performing CWST, followed by 20 min of tDCS, participants performed the TTE on a cycle ergometer (Cyclus 2, RBM Elektronik-Automation GmbH, Leipzig, Germany), all under hypoxia. Before TTE, participants warmed up cycling for 5 min at 45% PPO. After warming up, TTE started with a load of 60% of PPO and a 60 rpm cadence until exhaustion. The time between the beginning and interruption of the test was considered the TTE. HR and SpO2 were measured every minute during the task, and psychophysiological responses (i.e., RPE, FS, FAS) were reported every three minutes and at exhaustion. TTE was considered maximum if two of the following criteria were achieved: (1) ≥ 90% of maximal predicted HR (220-age), (2) inability to maintain the pedal cadence of ≥ 60 rpm for > 5 s, (3) RPE ≥ 90.
HR and SpO2
During the whole experimental session, HR was continuously monitored by the use of a chest strap (M430, Polar, Finland) connected to the cycle ergometer. SpO2 was also constantly measured and recorded by a fingertip pulse oximeter (Nonin Onyx II 9550, Nonin Medical, Plymouth, MN, USA).
Ratings of perceived exertion
The 0–100 Borg scale was used to measure RPE. Participants received the instruction to report their RPE at the end of each stage and the point of exhaustion in the incremental test, and every 3 min and upon reaching the point of exhaustion in the TTE in hypoxia101. All these responses were used to calculate the average RPE in each experimental condition and used for statistical analyses.
Affective responses and felt arousal
The affective responses were reported using the Feeling Scale (FS), which is a bipolar scale comprising eleven items ranging from − 5 (very bad) to + 5 (very good), validated by Hardy and Rejeski15. The positive numbers represent pleasure, the negative numbers represent displeasure, and the zero represents a neutral affective valence. To measure the arousal, the Felt Arousal Scale (FAS) consisting of 6 items scored on a continuum from 1 (low arousal) to 6 (high arousal) was used102,103. Participants reported their affective responses and FAS every 3 min during the task and at exhaustion. All these responses were used to calculate average responses for these measures in each experimental condition and used for statistical analyses.
Statistical analyses
The normal distribution of each data set was evaluated by the Shapiro–Wilk normality test. Values are presented as means and standard deviation (SD) or median and interquartile range (IQR) as stated. The Friedman test was used to compare tDCS-induced sensations, end-of-study guess rate accuracy, and active stimulation guess rate, followed by Wilcoxon signed-rank tests with a Bonferroni correction for pair-wise comparisons (0.05/6 = Bonferroni corrected p = 0.008), in case of significant differences (for tDCS-induced sensations, only).
One-way repeated measures ANOVA was performed to analyze the mean value of TTE, HR, SpO2, RPE, EMG amplitude, FS, and FAS during the TTE. Moreover, the mean value of CRT was analyzed using two-way repeated measures ANOVA (4 × 3 factorial design, 4 experimental conditions, and 3 time points). One-way repeated measures ANOVA was used for analyzing the simple main effect of condition and time when required. In case of a significant result, the post hoc test using Bonferroni correction for multiple comparisons was used for the pairwise comparisons. If the assumption of sphericity was violated, the Greenhouse–Geisser epsilon correction was applied. Partial eta squared (ɳ2p) was used as a measure of the effect size for the ANOVAs and interpreted as small (0.01–0.059), medium (0.06–0.139), or large (≥ 0.14). Cohen’s d was used as a measure of the effect size comparing all experimental conditions of interest (a-tDCS + DC, a-tDCS + WC, and sh-tDCS + DC) to the “complete” placebo (sh-tDCS + WC), and it was interpreted as small (0.20–0.49), medium (0.50–0.79), or large (≥ 0.80). The SPSS software version 26 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. The significance level for all tests was defined as p˂0.05.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Razi University Kermanshah, Iran (IRCT id: IRCT20220417054556N1, 2022-07-27). All participants provided written consent to participate in the study.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Sinex, J. A. & Chapman, R. F. Hypoxic training methods for improving endurance exercise performance. J Sport Health Sci. 4, 325–332 (2015).
Mira, J. et al. Neuromuscular fatigue of cycling exercise in hypoxia. Med. Sci. Sports Exerc. 52(9), 1888–1899 (2020).
Ulrich, S., Schneider, S. R. & Bloch, K. E. Effect of hypoxia and hyperoxia on exercise performance in healthy individuals and in patients with pulmonary hypertension: A systematic review. J. Appl. Physiol. 123(6), 1657–70 (2017).
Jeffries, O., Patterson, S. D. & Waldron, M. The effect of severe and moderate hypoxia on exercise at a fixed level of perceived exertion. Eur. J. Appl. Physiol. 119(5), 1213–1224 (2019).
Karayigit, R. et al. The acute effects of normobaric hypoxia on strength, muscular endurance and cognitive function: Influence of dose and sex. Biology 11(2), 309 (2022).
Amann, M. et al. AltitudeOmics: On the consequences of high-altitude acclimatization for the development of fatigue during locomotor exercise in humans. J. Appl. Physiol. 115(5), 634–42 (2013).
Mulliri, G. et al. Acute exercise with moderate hypoxia reduces arterial oxygen saturation and cerebral oxygenation without affecting hemodynamics in physically active males. Int. J. Environ. Res. Public Health 19(8), 4558 (2022).
Rupp, T. et al. CO2 clamping, peripheral and central fatigue during hypoxic knee extensions in men. Med. Sci. Sports Exerc. 47(12), 2513–2524 (2015).
Ando, S. et al. The interactive effects of acute exercise and hypoxia on cognitive performance: A narrative review. Scand. J. Med. Sci. Sports 30(3), 384–398 (2020).
Deb, S. K. et al. Quantifying the effects of acute hypoxic exposure on exercise performance and capacity: A systematic review and meta-regression. Eur. J. Sport Sci. 18(2), 243–256 (2018).
Komiyama, T. et al. Cognitive function during exercise under severe hypoxia. Sci. Rep. 7(1), 10000 (2017).
Pageaux, B. The psychobiological model of endurance performance: An effort-based decision-making theory to explain self-paced endurance performance. Sports Med. 44(9), 1319–1320 (2014).
Agrícola, P.M., da Silva Machado, D.G., de Farias Junior, L.F., do Nascimento Neto, L.I., Fonteles, A.I., da Silva, S.K., et al. 2017. Slow down and enjoy: The effects of cycling cadence on pleasure. Percept. Motor Skills, 124(1), 233–247.
Ramalho Oliveira, B. R., Viana, B. F., Pires, F. O., Junior Oliveira, M. & Santos, T. M. Prediction of affective responses in aerobic exercise sessions. CNS Neurol. Disord. Drug Targets. 14(9), 1214–1218 (2015).
Hardy, C. J. R. W. Not what, but how one feels: The measurement of affect during exercise. J. Sport Exer. Psychol. 11(3), 307–317 (1989).
Hartman, M. E., Ekkekakis, P., Dicks, N. D. & Pettitt, R. W. Dynamics of pleasure–displeasure at the limit of exercise tolerance: Conceptualizing the sense of exertional physical fatigue as an affective response. J. Exp. Biol. 222(3), jeb186585 (2019).
Jung, M. et al. Does exercise have a protective effect on cognitive function under hypoxia? A systematic review with meta-analysis. J. Sport Health Sci. 9(6), 562–577 (2020).
Limmer, M. & Platen, P. The influence of hypoxia and prolonged exercise on attentional performance at high and extreme altitudes: A pilot study. PLoS One. 13(10), e0205285 (2018).
Lefferts, W. K. et al. Effect of hypoxia on cerebrovascular and cognitive function during moderate intensity exercise. Physiol. Behav. 165, 108–118 (2016).
O’Keeffe, K., Raccuglia, G., Hodder, S. & Lloyd, A. Mental fatigue independent of boredom and sleepiness does not impact self-paced physical or cognitive performance in normoxia or hypoxia. J. Sports Sci. 39(15), 1687–1699 (2021).
O’Keeffe, K., Hodder, S. & Lloyd, A. A comparison of methods used for inducing mental fatigue in performance research: Individualised, dual-task and short duration cognitive tests are most effective. Ergonomics. 63(1), 1–12 (2020).
Pageaux, B., Marcora, S. M., Rozand, V. & Lepers, R. Mental fatigue induced by prolonged self-regulation does not exacerbate central fatigue during subsequent whole-body endurance exercise. Front. Hum. Neurosci. 9, 67 (2015).
Van Cutsem, J. et al. The effects of mental fatigue on physical performance: A systematic review. Sports Med. 47(8), 1569–1588 (2017).
Maughan, R. J. et al. IOC consensus statement: Dietary supplements and the high-performance athlete. Int. J. Sport. Nutr. Exerc. Metab. 28(2), 104–125 (2018).
Bell, L., Lamport, D. J., Butler, L. T. & Williams, C. M. A review of the cognitive effects observed in humans following acute supplementation with flavonoids, and their associated mechanisms of action. Nutrients. 7(12), 10290–10306 (2015).
Martín, M. A., Goya, L. & de Pascual-Teresa, S. Effect of cocoa and cocoa products on cognitive performance in young adults. Nutrients 12(12), 3691 (2020).
Medina-Remón, A. et al. Effects of total dietary polyphenols on plasma nitric oxide and blood pressure in a high cardiovascular risk cohort. The PREDIMED randomized trial. Nutr. Metab. Cardiovasc. Dis. 25(1), 60–67 (2015).
Myburgh, K. H. Polyphenol supplementation: Benefits for exercise performance or oxidative stress?. Sports Med. 44(1), S57-70 (2014).
Jalil, A. M. & Ismail, A. Polyphenols in cocoa and cocoa products: Is there a link between antioxidant properties and health?. Molecules. 13(9), 2190–2219 (2008).
Soares, T. F. & Oliveira, M. B. P. Cocoa by-products: Characterization of bioactive compounds and beneficial health effects. Molecules 27(5), 1625 (2022).
Samanta, S. et al. Dark chocolate: An overview of its biological activity, processing, and fortification approaches. Curr. Res. Food Sci. 5, 1916–1943 (2022).
Decroix, L. et al. One-week cocoa flavanol intake increases prefrontal cortex oxygenation at rest and during moderate-intensity exercise in normoxia and hypoxia. J. Appl. Physiol. 125(1), 8–18 (2018).
Shaw, K., Singh, J., Sirant, L., Neary, J. P. & Chilibeck, P. D. Effect of dark chocolate supplementation on tissue oxygenation, metabolism, and performance in trained cyclists at altitude. Int. J. Sport Nutr. Exer. Metab. 30(6), 420–426 (2020).
Decroix, L. et al. Acute cocoa flavanols intake improves cerebral hemodynamics while maintaining brain activity and cognitive performance in moderate hypoxia. Psychopharmacology. 235(9), 2597–2608 (2018).
Ammar, A. et al. Effects of polyphenol-rich interventions on cognition and brain health in healthy young and middle-aged adults: Systematic review and meta-analysis. J. Clin. Med. 9(5), 1598 (2020).
Lamport, D. J. & Williams, C. M. Polyphenols and cognition in humans: An overview of current evidence from recent systematic reviews and meta-analyses. Brain Plast. 6(2), 139–153 (2021).
Hepsomali, P., Greyling, A., Scholey, A. & Vauzour, D. Acute effects of polyphenols on human attentional processes: A systematic review and meta-analysis. Front. Neurosci. 15, 678769 (2021).
de Vries, K., Medawar, E., Korosi, A. & Witte, A. V. The effect of polyphenols on working and episodic memory in non-pathological and pathological aging: A systematic review and meta-analysis. Front. Nutr. 8, 720756 (2021).
Machado, D. et al. Effect of transcranial direct current stimulation on exercise performance: A systematic review and meta-analysis. Brain Stimul. 12(3), 593–605 (2019).
Brunoni, A. R. & Vanderhasselt, M. A. Working memory improvement with non-invasive brain stimulation of the dorsolateral prefrontal cortex: A systematic review and meta-analysis. Brain Cogn. 86, 1–9 (2014).
Dedoncker, J., Brunoni, A. R., Baeken, C. & Vanderhasselt, M. A. A systematic review and meta-analysis of the effects of transcranial direct current stimulation (tDCS) over the dorsolateral prefrontal cortex in healthy and neuropsychiatric samples: Influence of stimulation parameters. Brain Stimul. 9(4), 501–517 (2016).
Dedoncker, J., Brunoni, A. R., Baeken, C. & Vanderhasselt, M. A. The effect of the interval-between-sessions on prefrontal transcranial direct current stimulation (tDCS) on cognitive outcomes: A systematic review and meta-analysis. J. Neural. Transm. 123(10), 1159–1172 (2016).
da Silva Machado, D. G. et al. Acute effect of high-definition and conventional tDCS on exercise performance and psychophysiological responses in endurance athletes: A randomized controlled trial. Sci. Rep. 11(1), 13911 (2021).
Lattari, E. et al. Can transcranial direct current stimulation improve muscle power in individuals with advanced weight-training experience?. J. Strength Condition. Res. 34(1), 97–103 (2020).
Vitor-Costa, M. et al. Improving cycling performance: Transcranial direct current stimulation increases time to exhaustion in cycling. PloS One 10(12), e0144916 (2015).
Grospretre, S. et al. Effect of transcranial direct current stimulation on the psychomotor, cognitive, and motor performances of power athletes. Sci Rep. 11(1), 9731 (2021).
Alix-Fages, C. et al. Short-term effects of anodal transcranial direct current stimulation on endurance and maximal force production: A systematic review and meta-analysis. J. Clin. Med. 8(4), 536 (2019).
Angius, L., Pageaux, B., Hopker, J., Marcora, S. M. & Mauger, A. R. Transcranial direct current stimulation improves isometric time to exhaustion of the knee extensors. Neuroscience. 339, 363–375 (2016).
Angius, L., Santarnecchi, E., Pascual-Leone, A. & Marcora, S. M. Transcranial direct current stimulation over the left dorsolateral prefrontal cortex improves inhibitory control and endurance performance in healthy individuals. Neuroscience. 419, 34–45 (2019).
Radel, R., Tempest, G., Denis, G., Besson, P. & Zory, R. Extending the limits of force endurance: Stimulation of the motor or the frontal cortex?. Cortex. 97, 96–108 (2017).
Ouellet, J. et al. Enhancing decision-making and cognitive impulse control with transcranial direct current stimulation (tDCS) applied over the orbitofrontal cortex (OFC): A randomized and sham-controlled exploratory study. J Psychiatr Res. 69, 27–34 (2015).
Robertson, C. V. & Marino, F. E. A role for the prefrontal cortex in exercise tolerance and termination. J. Appl. Physiol. 120(4), 464–6 (2016).
Angius, L., Hopker, J. G., Marcora, S. M. & Mauger, A. R. The effect of transcranial direct current stimulation of the motor cortex on exercise-induced pain. Eur. J. Appl. Physiol. 115(11), 2311–2319 (2015).
Vaseghi, B., Zoghi, M. & Jaberzadeh, S. The effects of anodal-tDCS on corticospinal excitability enhancement and its after-effects: Conventional vs. unihemispheric concurrent dual-site stimulation. Front. Hum. Neurosci. 9, 533 (2015).
Gurdiel-Álvarez, F. et al. Effectiveness of unihemispheric concurrent dual-site stimulation over M1 and dorsolateral prefrontal cortex stimulation on pain processing: A triple blind cross-over control trial. Brain Sci. 11(2), 188 (2021).
Talimkhani, A. et al. Differential effects of unihemispheric concurrent dual-site and conventional tDCS on motor learning: A randomized Sham-Controlled study. Basic Clin. Neurosci. 10(1), 59–72 (2019).
Smith, M. R. et al. Mental fatigue impairs soccer-specific physical and technical performance. Med. Sci. Sports Exerc. 48(2), 267–276 (2016).
Rozand, V., Pageaux, B., Marcora, S. M., Papaxanthis, C. & Lepers, R. Does mental exertion alter maximal muscle activation?. Front. Hum. Neurosci. 8, 755 (2014).
Moreira, A. et al. Mental fatigue impairs technical performance and alters neuroendocrine and autonomic responses in elite young basketball players. Physiol. Behav. 196, 112–118 (2018).
Martin, K. et al. Superior inhibitory control and resistance to mental fatigue in professional road cyclists. PloS One. 11(7), e0159907 (2016).
McMorris, T., Hale, B.J., Barwood, M., Costello, J. & Corbett, J., 2019. Corrigendum to “Effect of acute hypoxia on cognition: A systematic review and meta-regression analysis” Neurosci. Biobehav. Rev. 74 (2017) 225–232. Neuroscience and biobehavioral reviews, 98:333.
Taylor, L., Watkins, S. L., Marshall, H., Dascombe, B. J. & Foster, J. The impact of different environmental conditions on cognitive function: A focused review. Frontiers in physiology. 6, 372 (2015).
McMorris, T., Barwood, M., Hale, B. J., Dicks, M. & Corbett, J. Cognitive fatigue effects on physical performance: A systematic review and meta-analysis. Physiol. Behav. 188, 103–107 (2018).
Pageaux, B. & Lepers, R. The effects of mental fatigue on sport-related performance. Progr. Brain Res. 240, 291–315 (2018).
Boolani, A., Lindheimer, J. B., Loy, B. D., Crozier, S. & O’Connor, P. J. Acute effects of brewed cocoa consumption on attention, motivation to perform cognitive work and feelings of anxiety, energy and fatigue: A randomized, placebo-controlled crossover experiment. BMC Nutr. 3(1), 1–11 (2017).
Lundberg, J. O., Weitzberg, E. & Gladwin, M. T. The nitrate–nitrite–nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 7(2), 156–167 (2008).
Muggeridge, D. J. et al. A single dose of beetroot juice enhances cycling performance in simulated altitude. Med. Sci. Sports Exer. 46(1), 143–150 (2014).
Masschelein, E. et al. Dietary nitrate improves muscle but not cerebral oxygenation status during exercise in hypoxia. J. Appl. Physiol. 113(5), 736–745 (2012).
Angius, L. et al. Bilateral extracephalic transcranial direct current stimulation improves endurance performance in healthy individuals. Brain Stimul. 11(1), 108–117 (2018).
Holgado, D. et al. Transcranial direct current stimulation (tDCS) over the left prefrontal cortex does not affect time-trial self-paced cycling performance: Evidence from oscillatory brain activity and power output. PloS one. 14(2), e0210873 (2019).
Etemadi, M. et al. Anodal tDCS over the left DLPFC but not M1 increases muscle activity and improves psychophysiological responses, cognitive function, and endurance performance in normobaric hypoxia: a randomized controlled trial. BMC Neurosci. 24, 25. https://doi.org/10.1186/s12868-023-00794-4 (2023).
Massaro, M., Scoditti, E., Carluccio, M. A., Kaltsatou, A. & Cicchella, A. Effect of cocoa products and its polyphenolic constituents on exercise performance and exercise-induced muscle damage and inflammation: A review of clinical trials. Nutrients. 11(7), 1471 (2019).
Workman, C. D., Fietsam, A. C. & Rudroff, T. Different effects of 2 mA and 4 mA transcranial direct current stimulation on muscle activity and torque in a maximal isokinetic fatigue task. Front. Hum. Neurosci. 14, 240 (2020).
Tergau, F. et al. Motor cortex fatigue in sports measured by transcranial magnetic double stimulation. Med. Sci. Sports Exerc. 32(11), 1942–1948 (2000).
Sidhu SK, Bentley DJ, Carroll TJ. Locomotor exercise induces long-lasting impairments in the capacity of the human motor cortex to voluntarily activate knee extensor muscles. Journal of applied physiology (Bethesda, Md : 1985). 2009;106(2):556–65.
Yamaguchi, T., Fujiwara, T., Liu, W. & Liu, M. Effects of pedaling exercise on the intracortical inhibition of cortical leg area. Exp. Brain Res. 218(3), 401–406 (2012).
da Silva, J. C. et al. Quadriceps and hamstring muscle activity during cycling as measured with intramuscular electromyography. Eur. J. Appl. Physiol. 116(9), 1807–1817 (2016).
Patel, R. K., Brouner, J., Allgrove, J. E. & Spendiff, O. The influence of different concentrations of flavanol chocolate bars under acute supplement conditions on exercise and performance. Eur. J. Appl. Physiol. 120(9), 2075–2082 (2020).
Allgrove, J., Farrell, E., Gleeson, M., Williamson, G. & Cooper, K. Regular dark chocolate consumption’s reduction of oxidative stress and increase of free-fatty-acid mobilization in response to prolonged cycling. Int. J. Sport Nutr. Exerc. Metab. 21(2), 113–123 (2011).
Stellingwerff, T. et al. The effect of acute dark chocolate consumption on carbohydrate metabolism and performance during rest and exercise. Appl. Physiol. Nutr. Metab. 39(2), 173–182 (2014).
Filipas, L., Gallo, G., Meloni, A., Luzi, L. & Codella, R. Effects of bilateral dorsolateral prefrontal cortex high-definition transcranial direct-current stimulation on time-trial performance in cyclists with type 1 diabetes mellitus. Brain Stimul. 15(5), 1292–1299 (2022).
Gallo, G. et al. Effects of bilateral dorsolateral prefrontal cortex high-definition transcranial direct-current stimulation on physiological and performance responses at severe-intensity exercise domain in elite road cyclists. Int. J. Sports Physiol. Perform. 17(7), 1085–1093 (2022).
Heyward, V. H, & Gibson, A. L. (2014) Advanced fitness assessment and exercise prescription, Seventh edition. Human Kinetics. xiv, 537 pages p.
Davison, G., Callister, R., Williamson, G., Cooper, K. A. & Gleeson, M. The effect of acute pre-exercise dark chocolate consumption on plasma antioxidant status, oxidative stress and immunoendocrine responses to prolonged exercise. Eur. J. Nutr. 51(1), 69–79 (2012).
Sathyapalan, T., Beckett, S., Rigby, A. S., Mellor, D. D. & Atkin, S. L. High cocoa polyphenol rich chocolate may reduce the burden of the symptoms in chronic fatigue syndrome. Nutr. J. 9, 55 (2010).
Rostami, A. et al. High-cocoa polyphenol-rich chocolate improves blood pressure in patients with diabetes and hypertension. ARYA Atheroscler. 11(1), 21–29 (2015).
Rouffet, D. M. & Hautier, C. A. EMG normalization to study muscle activation in cycling. J. Electromyogr. Kinesiol. 18(5), 866–878 (2008).
Tankisi, H. et al. Standards of instrumentation of EMG. Clin. Neurophysiol. 131(1), 243–258 (2020).
Verdugo, R. J. & Matamala, J. M. Clinical neurophysiology standards of EMG instrumentation: Twenty years of changes. Clin. Neurophysiol. 131(1), 235–236 (2020).
Gandiga, P. C., Hummel, F. C. & Cohen, L. G. Transcranial DC stimulation (tDCS): A tool for double-blind sham-controlled clinical studies in brain stimulation. Clin. Neurophysiol. 117(4), 845–850 (2006).
Moreira, A. et al. Effect of tDCS on well-being and autonomic function in professional male players after official soccer matches. Physiol. Behav. 233, 113351 (2021).
Moreira, A. et al. Effect of transcranial direct current stimulation on professional female soccer players’ recovery following official matches. Percept. Mot. Skills 128(4), 1504–1529 (2021).
Thielscher, A., Antunes, A. & Saturnino, G. B. Field modeling for transcranial magnetic stimulation: A useful tool to understand the physiological effects of TMS?. Annu Int Conf IEEE Eng Med Biol Soc. 2015, 222–225 (2015).
Opitz, A., Paulus, W., Will, S., Antunes, A. & Thielscher, A. Determinants of the electric field during transcranial direct current stimulation. Neuroimage. 109, 140–150 (2015).
Rahman, A. et al. Cellular effects of acute direct current stimulation: Somatic and synaptic terminal effects. J. Physiol. 591(10), 2563–2578 (2013).
Kronberg, G., Bridi, M., Abel, T., Bikson, M. & Parra, L. C. Direct current stimulation modulates LTP and LTD: Activity dependence and dendritic effects. Brain Stimul. 10(1), 51–58 (2017).
Fertonani, A., Ferrari, C. & Miniussi, C. What do you feel if I apply transcranial electric stimulation? Safety, sensations and secondary induced effects. Clin. Neurophysiol. 126(11), 2181–2188 (2015).
Moreira, A. et al. Effect of tDCS on well-being and autonomic function in professional male players after official soccer matches. Physiol Behav 233, 113351 (2021).
Fassi L KR. Is it all in our head? When sunjective beliefs about receiving an intervention are better predictors of experimental results than the intervention itself. 2020.
Fassi, L. & Cohen, K. R. Letter to the editor: How some brain stimulation studies fail to evaluate blinding adequately. J Psychiatr Res. 137, 452–453 (2021).
Okano, A. H. et al. Brain stimulation modulates the autonomic nervous system, rating of perceived exertion and performance during maximal exercise. Br J Sports Med 49(18), 1213–8 (2015).
Rodrigues, G. M. et al. Effects of anodal transcranial direct current stimulation on training volume and pleasure responses in the back squat exercise following a bench press. J Strength Condition Res 36(11), 3048–3055 (2022).
Kong, Z. et al. Affective and enjoyment responses to sprint interval exercise at different hypoxia levels. Int. J. Environ. Res. Public Health. 18(15), 8171 (2021).
Acknowledgements
The authors would like to thank Iman Talebi-Rasa and Matin Etemadi for their help in data acquisition. The authors also express their sincere gratitude to the participants for their commitment to the experimental procedure. This research was conducted in the Exercise Metabolism and Performance Lab (EMPL), Faculty of Sport Sciences, Razi University, Kermanshah, Iran.
Funding
This research was conducted in the Exercise Metabolism and Performance Lab (EMPL), Faculty of Sport Sciences, Razi University, Kermanshah, Iran.
Author information
Authors and Affiliations
Contributions
P.B., V.T., E.A., and D.M. conceptualized and designed the study. P.B., V.T., and E.A. conducted the experiments. P.B., V.T., E.A., and D.M. participated in the formal analysis. P.B. wrote the original draft of the manuscript. V.T., E.A., and D.M. reviewed and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Banaei, P., Tadibi, V., Amiri, E. et al. Concomitant dual-site tDCS and dark chocolate improve cognitive and endurance performance following cognitive effort under hypoxia: a randomized controlled trial. Sci Rep 13, 16473 (2023). https://doi.org/10.1038/s41598-023-43568-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-43568-y
- Springer Nature Limited